Abstract
The molecular events linking lipid accumulation in atherosclerotic plaques to complications such as aneurysm formation and plaque disruption are poorly understood. Balb-Apoe−/− mice bearing a null mutation in the Npc1 gene display prominent medial erosion and athero-thrombosis, while their macrophages accumulate free cholesterol in late endosomes and show increased cathepsin K (Ctsk) expression. We now show increased cathespin K immunostaining and increased SH-proteinase activity using near infrared fluorescence imaging over proximal aortas of Apoe−/−, Npc1−/− mice. In mechanistic studies, cholesterol loading of macrophage plasma membranes (cyclodextrin-cholesterol) or endosomal system (AcLDL+U18666A or Npc1 null mutation) activated Toll-like receptor signaling, leading to sustained phosphorylation of p38 MAP kinase, and induction of p38 targets, including Ctsk, S100a8, Mmp8, and Mmp14. Studies in macrophages from knock-out mice showed major roles for TLR4, following plasma membrane cholesterol loading, and for TLR3, after late endosomal loading. TLR signaling via p38 led to phosphorylation and activation of the transcription factor MITF, acting at E-box elements in the Ctsk promoter. These studies suggest that free cholesterol enrichment of either plasma or endosomal membranes in macrophages leads to activation of signaling via various TLRs, prolonged p38 MAP kinase activation and induction of Mmps, Ctsk, and S100a8, potentially contributing to plaque complications.
Keywords: cathepsin K, p38, Toll-like receptor
Introduction
Clinical complications of atherosclerosis usually result from the formation of thrombus on a ruptured or eroded atherosclerotic plaque 1. While early fatty streak formation involves lipid accumulation in macrophage foam cells in arteries, the growth of plaques and their complications are thought to involve a modified inflammatory response comprising both innate and acquired immune systems 2. Nonetheless, LDL cholesterol lowering remains the cornerstone for the treatment of established atherosclerosis and accumulation of cholesterol and oxidized lipids likely continue to drive the inflammatory response even in advanced plaques. The molecular mechanisms linking cellular lipid accumulation and inflammatory gene expression remain poorly understood.
Murine models have been useful for analyzing the role of different genes in plaque development 3, and recently there has been intense interest in the use of Apoe−/− mice to monitor plaque complications such as intra-plaque hemorrhage, or apparent rupture involving the brachio-cephalic artery 4. We observed a high frequency of medial degradation and athero-thrombosis associated with lesions in the proximal aorta of Apoe−/−, Npc1−/− mice 5, and the incidence was higher in mice in the Balbc compared with C57BL6 genetic background. The Npc1 mutation results in prominent accumulation of unesterified cholesterol in late endosomes 6 and peritoneal macrophages from chow-fed Apoe−/−, Npc1−/− mice displayed marked accumulation of free cholesterol (FC) and to a lesser extent cholesteryl ester5. Using microarrays, we discovered that Apoe−/−, Npc1−/− peritoneal macrophages have increased expression of cathepsin K (Ctsk) as well as several matrix metalloproteases genes (Mmps). Cathepsin and MMPs have important roles in plaque development, medial breakdown and aneurysm formation 7, 8. Cathepsin K protein (CATK) is a potent elastase and Ctsk−/−, Apoe−/− mice show reduced lesion area, increased intimal collagen accumulation and decreased medial elastin fiber disruption compared to Apoe−/− controls 9, 10, suggesting an important role of Ctsk in plaque growth and complications. The present study was undertaken to elucidate the mechanisms linking macrophage cholesterol accumulation to increased expression of Ctsk and various Mmps.
Material and Methods
Reagents
Aggregated LDL (AggLDL) and moderately oxidized LDL (OxLDL) 11, 12, and cyclodextrin-cholesterol complex (CD-chol) 13 were prepared as described (online supplements).
Mice
BALB/cNctr-Npc1m1N/+/J, C3H/HeJ, Tlr4del (strain C57BL/10ScNJ), Tlr4+/+ (strain C57BL/10ScSnJ), Tlr3−/− (strain 129S1/Sv/C57BL/6), Tlr3+/+ (strain B6129SF1/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Apoe−/−, Npc1−/− mice on BALB/cJ background were bred as described 5. Macrophage p38 deficient mice 14, Myd88/Trif−/− mice in the C57BL/6J background (kindly provided by Dr. Shizuo Akira 15) and Mitfmi/mi mice 16 have been described (online supplements).
Cell culture
Peritoneal and bone-marrow (BM)-derived macrophages were cultured as described 17. Human THP-1 and mouse RAW264.7 cells were purchased (ATCC). Spleen-derived macrophages were derived from myeloid precursor cells from spleen (see online).
RNA Analysis
Total RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA). Real-time quantitative polymerase chain reaction assays were performed as described 5.
siRNA knockdown
RANK and scrambled siRNAs were purchased from Applied Biosystems; TLR3, 4, 7 and 8 from Invitrogen. siRNA was transfected into macrophages with Oligofectamine (Invitrogen).
Immunofluorescent staining of aortic cross-sections
Paraffin-embedded sections of the proximal aorta were immunostained with affinity-purified rabbit polyclonal CATK antibody or mouse monoclonal α-actin antibody as described 18 (see online).
Molecular imaging
Ex vivo fluorescence reflectance imaging was performed using Image Station 4000MM Pro and Molecular Imaging Software (Kodak) as described 19.
Promoter luciferase assay
Human CTSK promoter (−928 to +2 bp) was cloned into a pGL3 vector (Promega) and mutated as described 20. CTSK promoter-luciferase and TK-Renila luciferase were transfected into RAW264.7 cells with Lipofectamine 2000 (Invitrogen). Cells were collected and assayed 24 hrs after loading using the Dual-luciferase reporter assay system and a TD20/20 luminometer (Promega).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed as described {Sharma, 2007 #100(see online).
Results
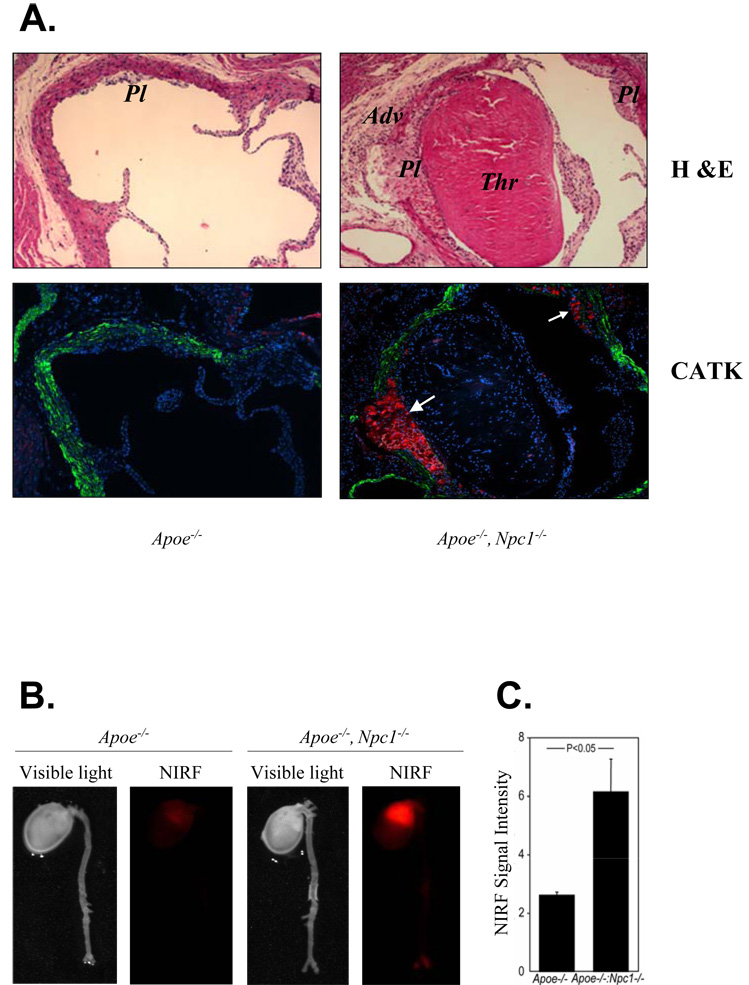
We previously found increased Ctsk expression and secretion in Npc1−/− macrophages and increased elastase activity in aortic homogenates of Apoe−/−, Npc1−/− mice 5. To confirm in vivo relevance, we used an affinity-purified antibody 18 and found strong CATK immunostaining in the macrophage-rich regions of atherosclerotic plaques in Apoe−/−, Npc1−/− mice (Fig 1A, white arrows), but not in Apoe−/− controls. To confirm an increase in CATK activity, we performed near-infrared fluorescence (NIRF) imaging using peptide substrates that fluoresce following cleavage by cysteinyl proteinases. This procedure revealed a marked increase in signal over the proximal aortic region of Apoe−/−, Npc1−/− mice compared to Apoe−/− controls (Fig 1B, C).
Fig. 1. CATK immunostaining and Cathespin activity are induced in atherosclerotic lesion-containing aortas of Apoe−/−, Npc1−/− mice compared to Apoe−/− controls.
A. Immunofluorescent staining of aortic cross-sections using specific antibodies against CATK (red, white arrows) and α-actin (green). Blue is hoechts staining. M, media; Pl, plaque; Thr, thrombus. 10X magnification. Mice were chow-fed and 10-wk-old. B. Ex vivo fluorescence reflectance imaging of mouse aortas using a probe that reports on cysteinyl protease activity. A visible light image indicates formation of early atherosclerotic changes in the aortas of Apoe−/− mice. A NIRF channel detected no substantial red signal in control. Npc1 mutation increased NIRF signal intensity in the aortas, particularly at the level of aortic root. C. Quantification of NIRF signal intensities (the target-to-background ratio, n=3).
Cholesterol loading of endosomes and plasma membrane induces Ctsk expression
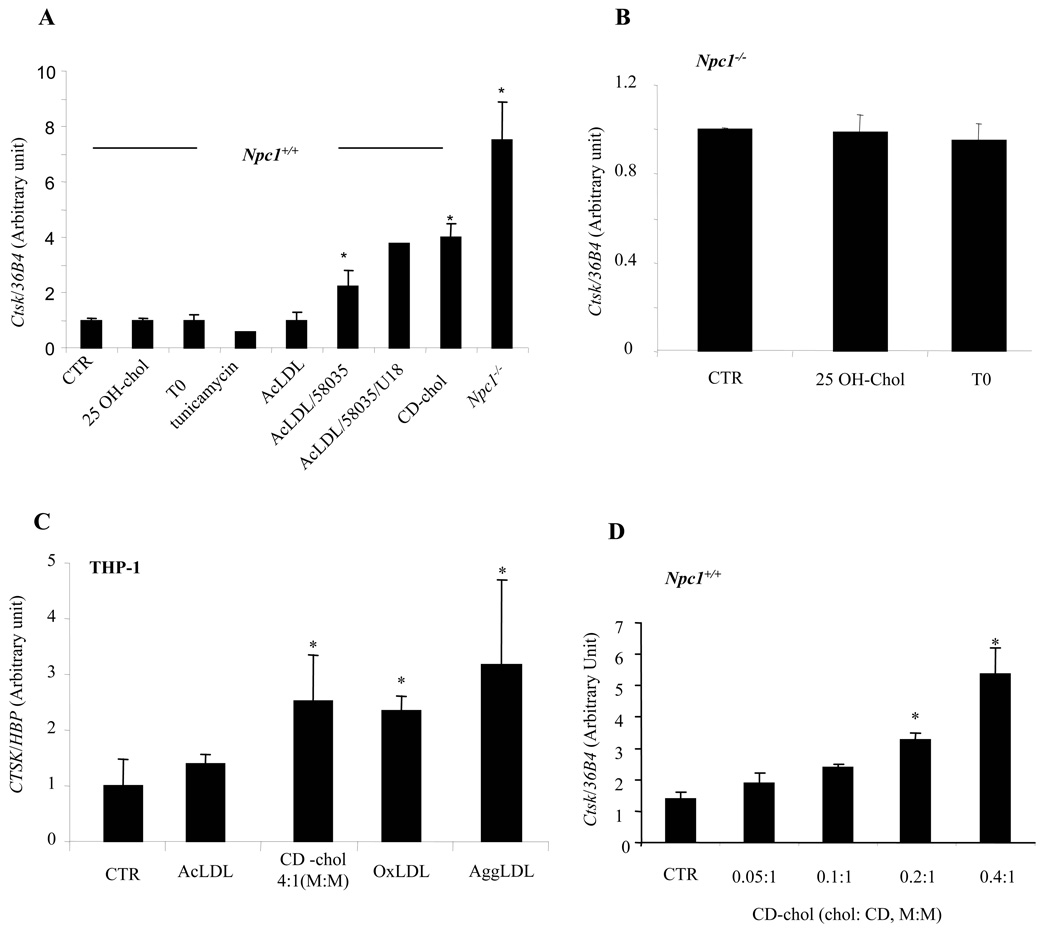
Npc1−/− macrophages have a defect in the trafficking of cholesterol from late endosomes to the plasma membrane and endoplasmic reticulum (ER) and accumulate FC in late endosomes 21. We used different methods of cholesterol loading in concanavalin A (ConA)-elicited Npc1+/+ peritoneal macrophages to determine if increased Ctsk expression resulted from decreased trafficking of cholesterol to the ER, or from cholesterol accumulation in late endosomes. When Npc1−/− cells take up LDL or AcLDL, there is defective suppression of LDL receptors and HMG-CoA reductase; however, these SREBP target genes are effectively repressed by 25-OH cholesterol (online Fig IA) 22. 25-OH-chol treatment of Npc1+/+ or Npc1−/− macrophages (Fig 2A, B), did not reduce Ctsk expression levels suggesting that induction does not result from defective SREBP function. LXR target genes are poorly regulated in Npc1−/− cells following cholesterol loading of the endosomal system 23. However, LXR activation with compound T0901317 (T0) in macrophages did not affect Ctsk expression. AcLDL loading of Npc1+/+ macrophages, which results in uptake into lysosomes and re-esterification of lipoprotein-derived cholesterol in the ER 24, had no effect on Ctsk expression. However, FC loading of Npc1+/+ macrophages by treatment with AcLDL+ACAT inhibitor 58035 resulted in a 2-fold induction of Ctsk mRNA. Addition of U18666A, which causes a block in the exit of cholesterol from late endosomes and thus mimics many aspects of the Npc1−/− phenotype 25, caused a further increase in Ctsk in FC-loaded cells, indicating that effects of FC loading reflect endosomal rather than ER cholesterol accumulation. Since AcLDL+58035 treatment results in FC loading of the ER and induces the unfolded protein response (UPR) 14, we also tested the effects of UPR inducers thapsigargin and tunicamycin to ensure that FC-induced ER stress was not involved in Ctsk induction. These treatments did not increase Ctsk expression (shown for tunicamycin, Fig 2A) although CHOP protein was induced (online Fig IB). These experiments suggested that Ctsk induction results from FC loading of late endosomes rather than altered sterol-induced ER regulatory events.
Fig. 2. Induction of Ctsk mRNA by cellular cholesterol loading.
Ctsk mRNA levels measured by quantitative PCR and normalized to ribosomal 36B4 (mouse) or HBP (human). A., B. ConA-elicited mouse peritoneal macrophages with different treatments: 10 uM 25-OH-chol, 3 uM T0, 2 ug/ml tunicamycin, 50 ug/ml AcLDL, or 5 mM CD-chol (2.5:1, M:M) for 24 hrs; 50 ug/ml AcLDL+10 ug/ml ACAT inhibitor 58035±200 nMU18666A for 18 hrs. C. Differentiated human THP-1 cells treated with 100 ug/ml AcLDL, 100 ug/ml OxLDL, or 75 ug/ml AggLDL for 3 days; 5 mM CD-chol (4:1, M:M) for 24 hrs; or untreated. D. ConA-elicited mouse peritoneal macrophages treated with 5 mM CD-chol at increasing cholesterol:CD ratio for 24 hrs. CTR, control. *p<0.05 compared to CTR.
Prolonged loading of human THP-1 macrophages with aggregated LDL or OxLDL causes accumulation of FC in late endosomes/lysosomes resembling the phenotype of Npc1−/− cells 11, 12. This effect is not observed in mouse peritoneal macrophages 11. Loading of human THP-1 macrophages with aggregated LDL or OxLDL led to a 2–3-fold increase in Ctsk expression (Fig 2C). These findings further support the hypothesis that cholesterol loading of late endosomes leads to Ctsk induction and show relevance in human macrophages.
Since the Npc1 mutation results in defective trafficking of both cholesterol and glycolipids 6, and aggregated LDL and OxLDL are complex preparations containing many different bioactive lipids, we tested whether cholesterol itself could lead to induction of Ctsk. CD-chol loading of Npc1+/+ macrophages resulted in a robust, dose-related induction of Ctsk (Fig 2A, D). Ctsk was also induced in CD-chol loaded THP-1 macrophages (Fig 2C) but not 293 cells (not shown). CD-chol loading similarly induced Ctsk in the presence of 58035. Thus, Ctsk induction can be directly related to the effects of FC loading. Since CD-chol loading enriches the plasma membrane with FC 21, while AcLDL+U18666A treatment loads late endosomes, it appears that cholesterol enrichment of either endosomal or plasma membranes can induce Ctsk.
A role of p38 MAP kinase in the induction of Ctsk
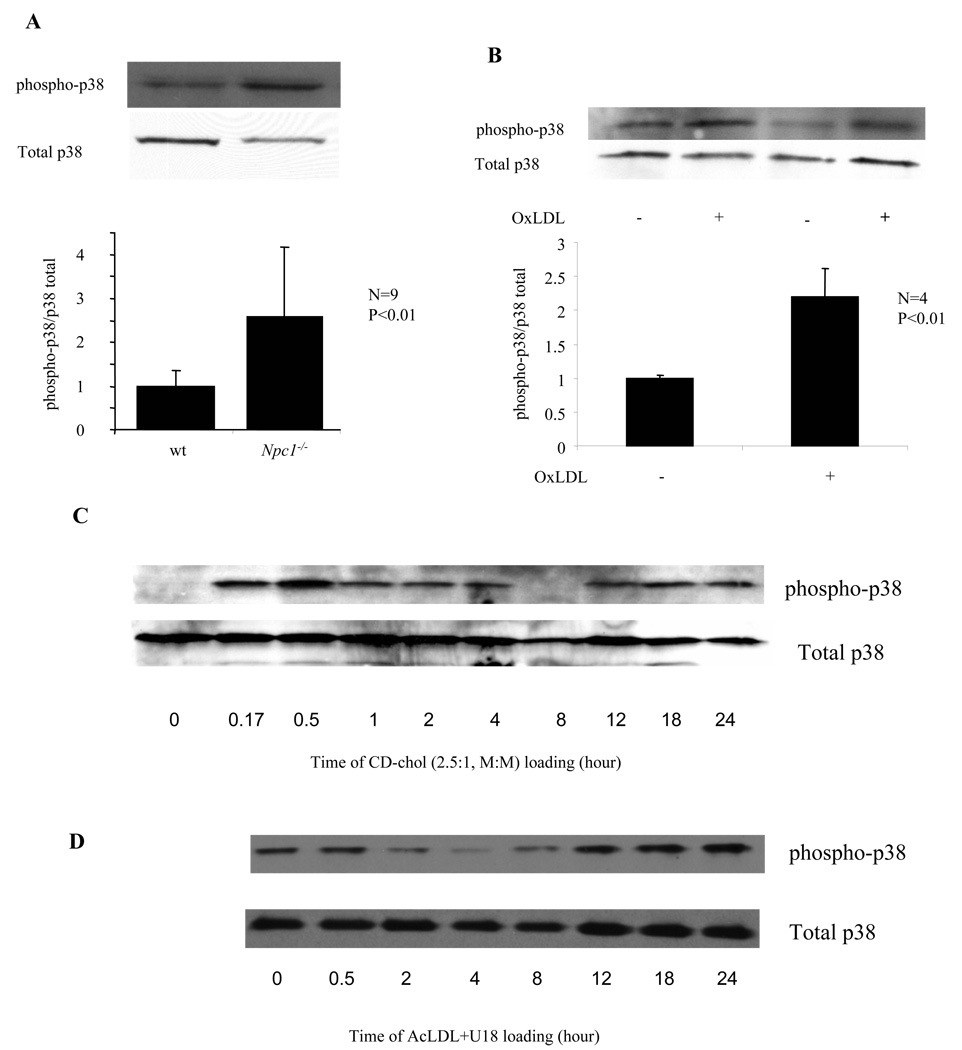
We repeated microarray experiments using Affymetrix arrays with more complete coverage of the genome. This revealed additional genes upregulated in Npc1−/− cells. We confirmed the upregulation of newly discovered genes and Ctsk with real-time PCR in peritoneal macrophages elicited in various ways and in bone marrow derived cells. Several genes upregulated in Npc1−/− cells are known p38 targets: Ctsk, S100a8 and Mmp8 (online table). Activation of p38 was confirmed by detection of increased phospho-p38 levels in Npc1−/− macrophages (Fig 3A). Elevated phospho-p38 levels were also observed in OxLDL loaded THP-1 cells (Fig 3B) and CD-chol loaded mouse peritoneal macrophages (Fig 3C). In a time-course study, CD-chol loading led to an early peak (~0.5 hr), a variable decrease and then a prolonged late-phase (>8 hr) of phospho-p38 induction. Interestingly, AcLDL+U18666A loading of macrophages caused predominantly late-phase p38 phosphorylation (Fig 3D), possibly due to a delay in hydrolysis of AcLDL CE.
Fig. 3. Npc1 mutation or cellular cholesterol loading induces p38 MAP kinase activation.
Detection of total and phosphorylated p38 in protein lysates using specific antibodies. A. ConA-elicited peritoneal macrophages. B. Differentiated THP-1 cells incubated with or without 100 ug/ml OxLDL for 3 days. C, D. ConA-elicited wild-type macrophages treated with 5 mM CD-chol (2.5:1, M:M) (C) or 50 ug/ml AcLDL+400 nM U18666A (D) for indicated time.
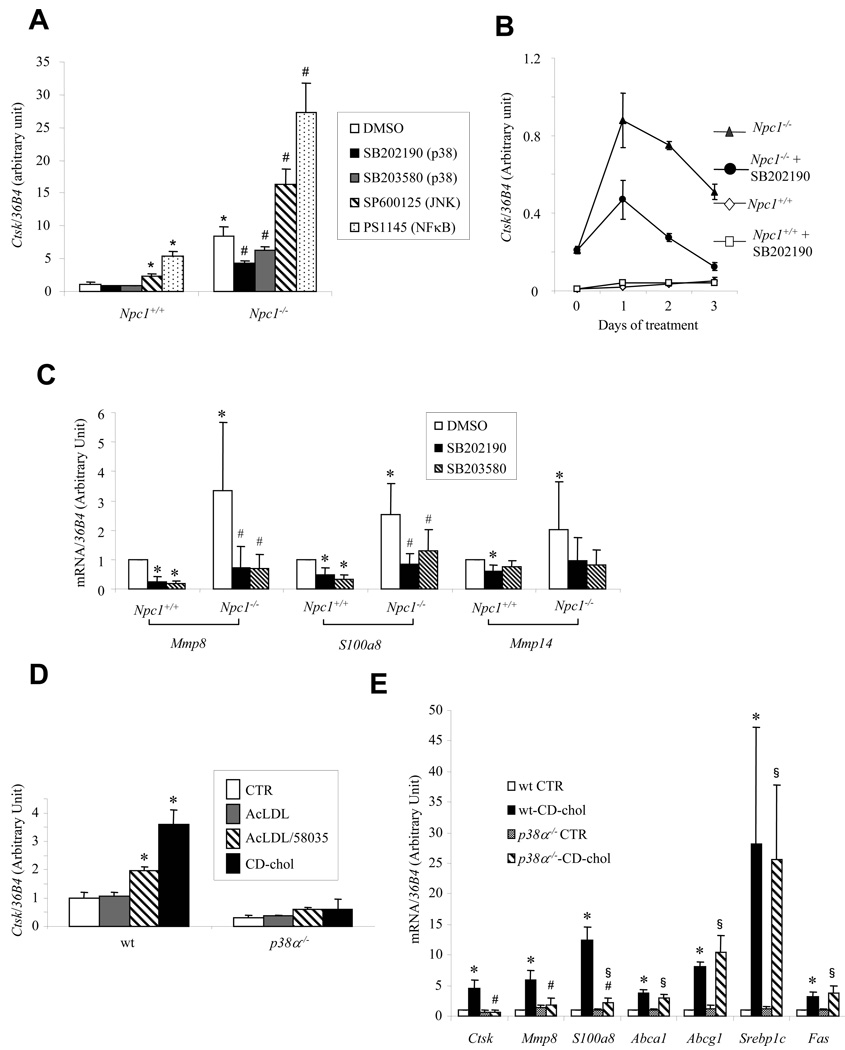
We next assessed the role of p38 in Npc1−/−- and cholesterol-induced Ctsk expression. Treatment of Npc1−/− cells with two different p38 inhibitors reduced Ctsk expression (Fig 4A). In contrast, treatment with c-Jun NH2-terminal kinase (JNK) or NFκB inhibitors increased Ctsk mRNA levels (Fig 4A, online Figure II), while ERK inhibitors had no effect (not shown). Prolonged treatment with the p38 inhibitor SB202190 reduced Ctsk mRNA in Npc1−/− cells by more than 50% (Fig 4B). As shown by Western blotting in macrophage cell lysates, Npc1−/−- or CD-chol-induced CATK protein levels were also greatly reduced by the p38 inhibitors (online Fig III). Mmp8, S100a8 and Mmp14 mRNA levels were all increased in Npc1−/− cells, and reduced by treatment with the p38 inhibitors (Fig 4C). The cells treated with inhibitors appeared healthy by microscopy and the p38 inhibitors did not affect p38 phosphorylation as expected.
Fig. 4. Npc1 mutation- or cholesterol-mediated Ctsk induction is inhibited by chemical p38 inhibition or genetic p38 deletion.
mRNA levels measured in ConA-elicited peritoneal macrophages by quantitative PCR and normalized to ribosomal 36B4. A, C. Cells treated with different inhibitors (10 uM) or DMSO for 24 hrs. *P<0.05 vs. wt/DMSO, #P<0.05 vs. Npc1−/−/DMSO. B. Cells treated with 10 uM SB202190 for indicated time. D, E. wt or p38α−/− cells treated with 50 ug/ml AcLDL±10 ug/ml ACAT inhibitor 58035 for 18 hrs, 5 mM CD-chol (2.5:1, M:M) for 24 hrs, or untreated (CTR). *P<0.05 vs. wt CTR, #P<0.05 vs. wt-CD-chol, §P<0.05 vs. p38α−/− CTR.
To confirm a role of p38 in Ctsk induction, we used p38α-deficient macrophages obtained from p38flox/flox X LysMCre mice. Basal levels of Ctsk were reduced in these macrophages, and the effects of FC loading (AcLDL+58035 or CD-chol) on Ctsk expression were abolished (Fig 4D). CD-chol loading also induced the expression of Mmp8 and S100a8 and these effects were abolished in p38−/− macrophages (Fig 4E). To determine the specificity of these p38-dependent responses, we measured transcript levels of other sterol-regulated genes (Fig 4E). Apart from a modest reduction of Abca1 mRNA in p38−/− macrophages, also previously observed in TNF-treated macrophages 17, the cholesterol-mediated responses of these genes were not affected by p38 deficiency. These findings indicate that a novel signaling pathway can be initiated by cholesterol accumulation in plasma or endosomal membranes, leading to activation of p38 and induction of Ctsk, Mmp8, S100a8 and Mmp14.
Cholesterol-mediated induction of Ctsk requires Microphthalmia transcription factor (MITF)
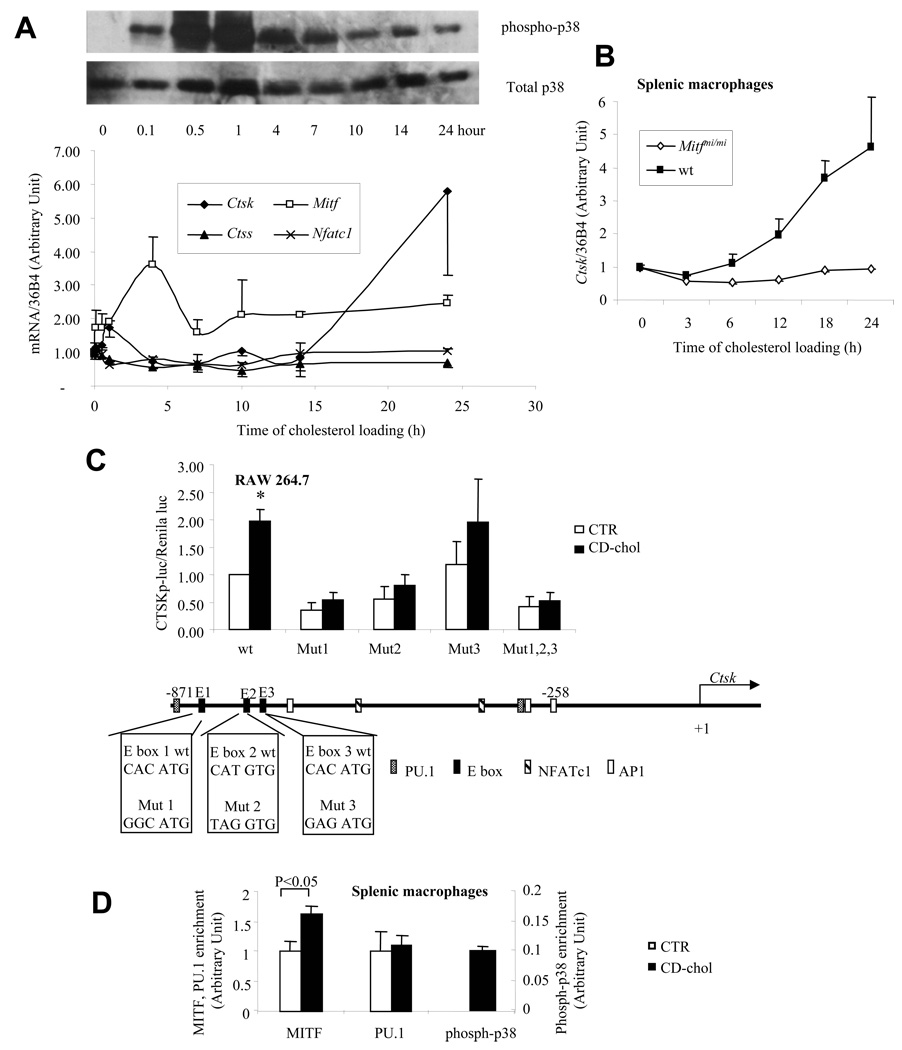
Ctsk is highly induced during macrophage differentiation into multi-nucleated osteoclasts. MITF and NFATc1 are transcription factors necessary for osteoclast differentiation, and are transcriptionally-induced and regulated by p38-mediated phosphorylation 26. We measured the expression of Mitf, Nfatc1 and Ctsk following CD-chol loading over time and related these changes to p38 phosphorylation (Fig 5A). Nfatc1 and cathepsin S (Ctss) were not induced by cholesterol loading. There was an early peak of Mitf induction. The induction of Ctsk was initiated after Mitf induction and during the late-phase p38 phosphorylation. Addition of p38 inhibitor following 7 hours of CD-chol loading resulted in substantial inhibition of Ctsk induction (not shown), suggesting that prolonged p38 activation was required for Ctsk induction. Interestingly, time course studies showed that NFκB and JNK activation accompanied the early phase of p38 activation (<5 h) but were not sustained during the late phase (not shown). Thus, NFκB and JNK repression of Ctsk (Fig 4A) may explain the delay in Ctsk induction by CD-chol.
Fig. 5. Cholesterol-mediated Ctsk induction requires the transcription factor MITF.
A. CD-chol induces the expression of Mitf and Ctsk, as well as p38 MAP kinase activation. ConA-elicited macrophages were treated with 5 mM CD-cho (2.5:1, M:M) for indicated time. mRNA levels were measured by Taqman real-time PCR. Phospho- and total p38 protein were detected with specific antibodies. B. Inducation of Ctsk by CD-chol was abolished by Mitf dominant negative mutation. Splenic macrophages from wt or Mitfmi/mi mice were treated with 5 mM CD-chol (2.5:1, M:M) for indicated time. Ctsk mRNA level in untreated condition was set as 1. C. Disruption of three upstream E boxes on human CTSK promoter blunted the induction by CD-chol loading. RAW cells were transfected with indicated promoter-luciferase constructs and TK-Renilla luciferase. Transfection media was changed to DMEM/10%FBS (control) or 5 mM CD-chol (2.5:1, M:M) in DMEM/10%FBS (CD-chol) 6 hours after transfection. Cells were collected for luciferase assay 28 hours later. A schematic representation of human Ctsk promoter was shown at the bottom. The sequences of wild type and mutated E boxes used were shown in the boxes. D. CD-chol loading enriched MITF and phospho-p38 on Ctsk promoter. Splenic macrophages from wt mice were loaded with 5 mM CD-chol (2.5:1, M:M) for 18 hours. ChIP assay was performed in duplicates to determine the MITF, PU.1 and phospho-p38 on Ctsk promoter. N=2.
To assess the role of MITF in Ctsk induction, we carried out cholesterol loading of splenic macrophages from mice carrying a dominant negative mutation of Mitf (mi/mi). The mi/mi mutation abolished the response to CD-chol loading (Fig 5B). Three E boxes in the human CTSK promoter are responsible for MITF transactivation during osteoclast differentiation 20. Transfection of a human CTSK promoter-luciferase construct into RAW macrophages resulted in significant induction of gene expression in response to CD-chol loading, and mutations in any of the three E box elements abolished the induction (Fig 5C). These experiments indicate an essential role for MITF in cholesterol-mediated CTSK induction, and suggest that regulation occurs via binding of E box elements in the promoter 20. This was confirmed by ChIP analysis 16, showing a modest increase in binding of MITF, no change in binding of PU.1, and a marked increase in binding of phospho-p38 to the Ctsk promoter in mouse splenic macrophages (Fig 5D; note binding of p38 was only detected after CD-chol loading). These findings are similar to previous studies in which receptor activator of NF-κB ligand (RANKL) was shown to induce Ctsk expression via p38-mediated phosphorylation of MITF on the Ctsk promoter 16.
RANK or Small GTPase Rab do not mediate Ctsk induction
We next carried out experiments to elucidate the signaling pathways acting upstream of p38/MITF-mediated induction of Ctsk. RANKL and CSF-1 are cytokines that commit myeloid precursor cells to the osteoclast lineage 27. Another secreted protein, osteoprotegerin (OPG), competes with RANK for RANKL binding and blocks RANK/RANKL signaling in cells. We treated BM-derived macrophages from Npc+/+ and Npc1−/− mice with OPG to assess the potential role of RANK/RANKL signaling in cholesterol-mediated Ctsk induction. While OPG abolished Ctsk induction by exogenous RANKL and CSF-1 in both Npc1+/+ and Npc1−/− cells, there was no effect of OPG in untreated Npc1−/− cells (online Fig IVA). Also, OPG treatment did not affect CD-chol-induced Ctsk expression in Npc1+/+ macrophages, and injection of OPG into the peritoneal cavity of ConA-injected Npc1−/− mice did not affect Ctsk induction (not shown). Moreover, a 70% decrease in RANK mRNA by siRNA knock-down did not reduce Ctsk mRNA (online Fig IVB). These experiments suggested that the RANK-RANKL signaling pathway is unlikely to be involved in Npc1−/−- or CD-chol-induced Ctsk expression. Athough Rabs have been suggested to rescue the lipid trafficking defect in Npc1−/− cells28, overexpression of either wild-type or dominant negative forms of rab7, rab9 and recycling endosome-associated rab11 did not affect Ctsk promoter activity in CD-chol-loaded RAW macrophages (not shown). We also assessed the possible involvements of autocrine or paracrine factors in the induction of Ctsk with a media transfer experiment. However, levels of Ctsk in bone marrow derived cells from either Npc1+/+ or Npc1−/− mice were not affected by conditioned media (online Figure V).
Toll-like receptor (TLR) signaling mediates cholesterol-induced Ctsk expression
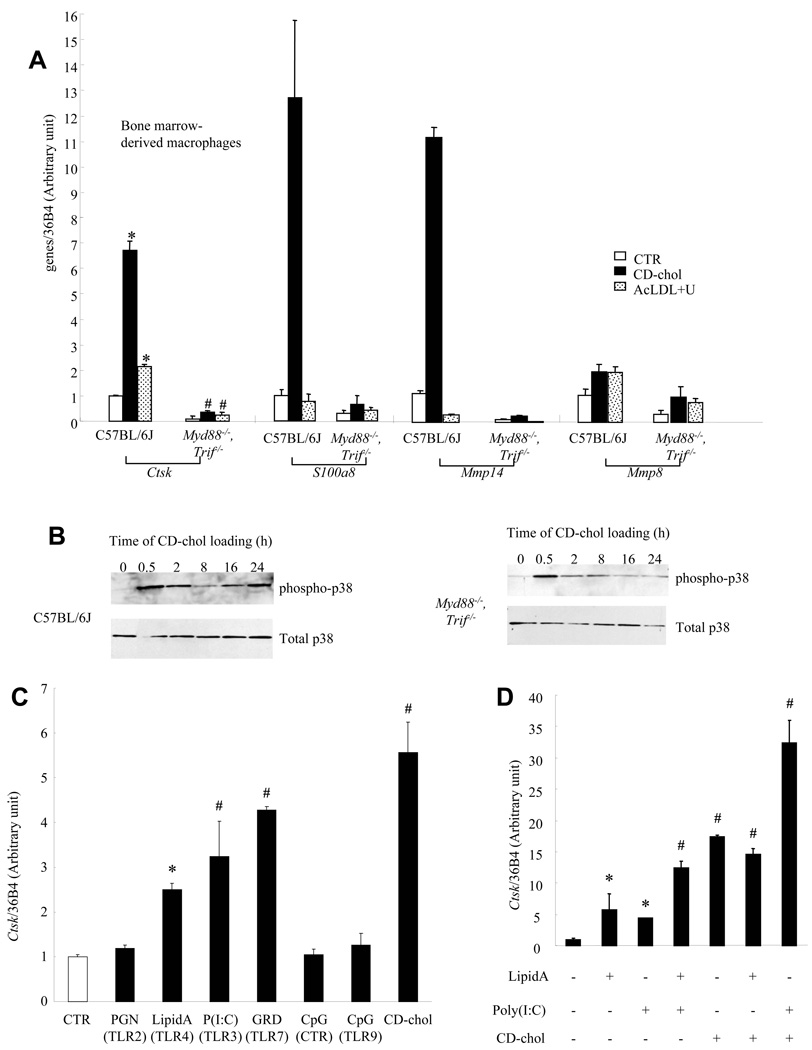
Multiple TLRs can activate p38 signaling 29. MyD88 and TRIF are adaptor proteins that link TLR activation to downstream MAP kinase signaling 29. To evaluate the role of TLRs in cholesterol-mediated Ctsk induction, we assessed Ctsk expression in bone marrow derived Myd88−/−, Trif−/− macrophages and wt controls. The expression of Ctsk and other p38 targets was reduced in untreated Myd88−/−, Trif−/− cells compared to controls, and the cholesterol-mediated induction was abolished (Fig 6A). In addition, late-phase p38 phosphorylation was abolished in Myd88−/−, Trif−/− macrophages (Fig 6B). These data indicate that TLRs or IL-1 receptor initiate cholesterol-induced signaling leading to sustained p38 phosphorylation and Ctsk induction. IL-1 (5ng/ml) treatment did not induce Ctsk in mouse peritoneal macrophages. To determine if TLR activation was sufficient for Ctsk induction, we treated mouse peritoneal macrophages with activators of various TLRs (Fig 6C). TLR3 ((poly (I:C), 2.5 ug/ml), TLR4 (lipid A, the active component of lipopolysaccharide, 100 ng/ml) and TLR7 (gardiquimod, 10 ug/ml) ligands increased Ctsk mRNA by 2–4 fold while TLR2 (PGN) and TLR9 (CpG) ligands did not. Lipid A and Poly (I:C) had additive effects on Ctsk mRNA induction (Fig 6D). While lipid A did not have any additional effect on CD-chol-induced Ctsk expression, the combination of Poly (I:C) and CD-chol synergistically increased Ctsk mRNA (Fig 6D). Importantly, the CD-chol preparation contained <0.125 endotoxin units/ml. Moreover, CD-chol showed greater induction of Ctsk than lipid A, while the induction of inflammatory genes such as IL-6 or TNF by CD-chol was only about 1/10th of that observed with lipid A treatment, indicating that LPS contamination could not explain the Ctsk response.
Fig. 6. TLR signaling mediates cholesterol-induced Ctsk expression.
A. Reduced expression of Ctsk and other genes in basal and CD-chol-loaded bone marrow derived macrophages from Myd88−/−, Trif−/− mice. B. Inhibition of late-phase p38 phosphorylation in Myd88−/−, Trif−/− cells compared to wild-type (C57BL/6J) controls. C. Induction of Ctsk expression by TLR3, 4 and 7 ligands in wild-type peritoneal macrophages. D. Synergistic effect of Poly (I:C) and CD-chol, but not LipidA, on Ctsk expression. *P<0.05 vs CTR, #P<0.004 vs C57BL/6J, same treatment. All treatments were for 24 hrs.
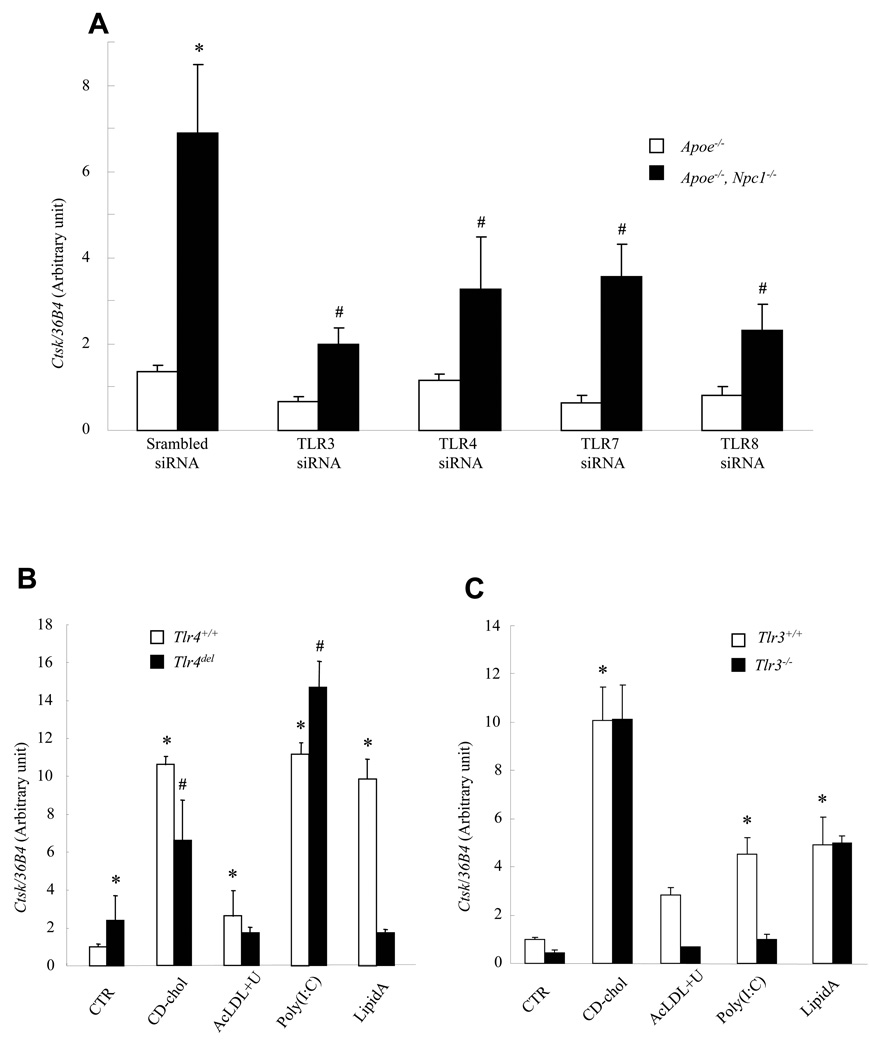
To assess the role of various TLRs in Ctsk induction in Npc1−/− macrophages, we used siRNA knock-downs (Fig 7A). As determined by real-time PCR, the knock-downs were effective (63 to 79%, Fig legend) and specific for each TLR. Knock-down of TLR3 had the largest effect on Ctsk expression. However, knock-down of all TLRs localized to the endosomal system (TLR3, 7 and 8) 30 as well as TLR4 led to some reduction in Ctsk, indicating activation of signaling via multiple TLR family members in Npc1−/− cells.
Fig. 7. Ctsk induction by cholesterol loading is regulated by various TLRs.
Ctsk mRNA levels measured in ConA-elicited mouse peritoneal macrophages by quantitative PCR and normalized to ribosomal 36B4. A. Knockdown of TLR 3 (66%), 4 (62%), 7(79%) or 8 (64%) by siRNA reduced Ctsk expression in Apoe−/−, Npc1−/− macrophages. *P<0.0001 vs CTR/Apoe−/−, #P<0.0001 vs CTR/Apoe−/−, Npc1−/−. B. Ctsk expression induced by cholesterol loading was blunted in Tlr4−/− macrophages. C. Ctsk expression induced by endosomal cholesterol loading was blocked in Tlr3−/− macrophages. Cells were treated for 24 hrs. *P<0.05 vs. CTR/Tlr4+/+ or Tlr3+/+, # P<0.005 vs. CTR/Tlr4del.
To determine if TLR4 is necessary for cholesterol-induced Ctsk expression, we loaded peritoneal macrophages from Tlr4del mice with CD-chol or AcLDL+U18666A. The response to lipid A was abolished while the response to CD-chol loading was significantly but only partially reduced by the Tlr4 mutation (32–44% in different experiments) (Fig 7B). Although baseline Ctsk expression was higher in Tlr4del macrophages (possibly reflecting decreased NFκB and JNK expression), AcLDL+U18666A resulted in no further increase in Ctsk expression, suggesting that TLR4 was also involved in the response to endosomal cholesterol loading. Similar results were obtained using macrophages from C3H/HeJ mice that carry a spontaneous mutation in Tlr4 (not shown). CD-chol-induction of Ctsk was unchanged but poly (I:C) and AcLDL+U18666A-mediated expression were abolished in Tlr3−/− macrophages (Fig 7C). These data suggest that Ctsk induction by CD-chol loading of plasma membrane depends partially on TLR4, while AcLDL+U18666A loading of the endosomal compartment leads to TLR3 and TLR4 signaling.
Discussion
Our studies have elucidated a novel signaling pathway in macrophages, initiated by free cholesterol accumulation in plasma or endosomal membranes and leading to activation of TLRs, notably TLR3 and 4, and sustained phosphorylation of p38 MAP kinase. Several p38 targets that were induced by cholesterol loading, such as Ctsk 9, 10, various Mmps 8 and S100a8 31, participate in atherogenesis or its complications, suggesting the relevance of this signaling pathway to the mechanisms of accelerated, complicated atherosclerosis of Apoe−/−, Npc1−/− mice 5.
Although the Npc1 mutation is rare, our findings may be relevant to the common forms of chronic atherosclerosis that involve overloading of macrophages with FC. For example, chronic loading of human macrophages with AggLDL or OxLDL, a situation that is likely to be physiologically relevant to human atherogenesis 32, also leads to accumulation of unesterified cholesterol in late endosomes 11, 12, resulting in p38 activation and Ctsk induction (Fig 3A, 3B). Moreover, the enrichment of plasma membrane cholesterol using CD-chol led to marked activation of p38 signaling and Ctsk induction. This may simulate the effects of cellular membrane enrichment occurring as a result of contact between cells and atherogenic plasma lipoproteins with a high cholesterol/phospholipid ratio 32. Although CD-chol loading also leads to increased sterol in the endocytic recycling compartment 33, reversal of CD-chol induction of Ctsk by mutant forms of ABCA1 that are localized to plasma membrane 34 suggest that the plasmalemma is the relevant compartment (unpublished studies).
Previous studies have indicated an important role of TLRs in atherogenesis, providing a link between hyperlipidemia and expression of a variety of inflammatory and chemokine genes 35. Deficiency of Myd88 in Apoe−/− mice led to a major reduction in atherosclerosis and inflammatory gene expression. Similarly, deficiency of TLR4 in Apoe−/− mice led to reduced atherosclerosis but the effect was much smaller than that of MyD88 deficiency, suggesting involvement of different TLRs upstream of MyD88. TLR2 has also been implicated in atherogenesis 35. Interestingly, our studies indicate an important role of TLR3 in the response to endosomal cholesterol loading with AcLDL+U18666A as well as the Npc1−/− mutation. In fact, multiple TLRs that reside in endosomes appeared to be involved in the induction of Ctsk in Npc1−/− cells, i.e. TLR3, 7 and 8, as well as TLR4. Endosomes play a critical role in sorting and silencing signaling receptors such as TLRs. Inhibition of the endosomal pathway blocks post-endosomal cholesterol sorting and increases TLR4 signaling 36 and mislocalization of late endosomes results in the sustained activation of MAP kinases 37. In Npc1−/− fibroblasts, TLR4 signaling fails to shut off, and active TLR4 accumulates in endosomes and increases cytokine secretion 38.
Our studies suggest spontaneous activation of TLR signaling by cholesterol loading of endosomes, but indicate that cholesterol accumulation may also enhance the responses to exogenous TLR ligands (Fig 6D). A variety of endogenous ligands of TLR4 as well as LPS may be involved in atherogenesis 39. Although TLR3 or 7 have not yet been implicated in atherogenesis, necrotic cells release endogenous RNA and heat shock protein-70 that stimulate TLR3 and 7 40, 41. Poly(I:C) and endosomal cholesterol loading synergistically induced Ctsk (Fig 6D), suggesting that macrophage phagocytosis of virally infected apoptotic cells may also result in a similar synergy between RNA-mediated and cholesterol-induced responses.
In summary, our study provides new insights into the mechanisms linking macrophage cholesterol accumulation and inflammatory responses mediated by p38 activation. Our findings add to the growing evidence for involvement of TLRs and implicate TLR3 as well as other TLRs in mediating signaling from cholesterol-loaded endosomes. There is increasing evidence for involvement of MMPs and cathepsins in aneurysm formation and possibly plaque destabilization 7, 8. While there is likely to be redundancy amongst individual MMPs or cathepsins, our studies suggest a common underlying mechanism involving p38 activation in plaque complications.
Supplementary Material
Acknowledgments
Sources of Funding
This work has been funded by NIH grant HL22682 (A.T.).
Footnotes
Cholesterol Induced Macrophage Signaling
Subject codes: 138, 142
Disclosures
None.
References
- 1.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001;103:1718–1720. doi: 10.1161/01.cir.103.13.1718. [DOI] [PubMed] [Google Scholar]
- 3.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 4.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch CL, Sun Y, Arey BJ, Lemaitre V, Sharma N, Ishibashi M, Sayers S, Li R, Gorelik A, Pleskac N, Collins-Fletcher K, Yasuda Y, Bromme D, D'Armiento JM, Ogletree ML, Tall AR. Spontaneous atherothrombosis and medial degradation in Apoe−/−, Npc1−/− mice. Circulation. 2007;116:2444–2452. doi: 10.1161/CIRCULATIONAHA.107.701276. [DOI] [PubMed] [Google Scholar]
- 6.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O'Neill RR, van Diggeen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1359–1366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 8.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutgens E, Lutgens SP, Faber BC, Heeneman S, Gijbels MM, de Winther MP, Frederik P, van der Made I, Daugherty A, Sijbers AM, Fisher A, Long CJ, Saftig P, Black D, Daemen MJ, Cleutjens KB. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 10.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancey PG, Jerome WG. Lysosomal sequestration of free and esterified cholesterol from oxidized low density lipoprotein in macrophages of different species. J Lipid Res. 1998;39:1349–1361. [PubMed] [Google Scholar]
- 12.Griffin EE, Ullery JC, Cox BE, Jerome WG. Aggregated LDL and lipid dispersions induce lysosomal cholesteryl ester accumulation in macrophage foam cells. J Lipid Res. 2005;46:2052–2060. doi: 10.1194/jlr.M500059-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Sun Y, Welch C, Gorelik A, Leventhal AR, Tabas I, Tall AR. Preferential ATP-binding cassette transporter A1-mediated cholesterol efflux from late endosomes/lysosomes. J Biol Chem. 2001;276:43564–43569. doi: 10.1074/jbc.M107938200. [DOI] [PubMed] [Google Scholar]
- 14.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 16.Sharma SM, Bronisz A, Hu R, Patel K, Mansky KC, Sif S, Ostrowski MC. MITF and PU.1 recruit p38 MAPK and NFATc1 to target genes during osteoclast differentiation. J Biol Chem. 2007;282:15921–15929. doi: 10.1074/jbc.M609723200. [DOI] [PubMed] [Google Scholar]
- 17.Gerbod-Giannone MC, Li Y, Holleboom A, Han S, Hsu LC, Tabas I, Tall AR. TNFalpha induces ABCA1 through NF-kappaB in macrophages and in phagocytes ingesting apoptotic cells. Proc Natl Acad Sci U S A. 2006;103:3112–3117. doi: 10.1073/pnas.0510345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samokhin AO, Wong A, Saftig P, Bromme D. Role of cathepsin K in structural changes in brachiocephalic artery during progression of atherosclerosis in apoE-deficient mice. Atherosclerosis. 2008;200:58–68. doi: 10.1016/j.atherosclerosis.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 19.Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114:55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 20.Motyckova G, Weilbaecher KN, Horstmann M, Rieman DJ, Fisher DZ, Fisher DE. Linking osteopetrosis and pycnodysostosis: regulation of cathepsin K expression by the microphthalmia transcription factor family. Proc Natl Acad Sci U S A. 2001;98:5798–5803. doi: 10.1073/pnas.091479298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz JC, Sugii S, Yu C, Chang TY. Role of Niemann-Pick type C1 protein in intracellular trafficking of low density lipoprotein-derived cholesterol. J Biol Chem. 2000;275:4013–4021. doi: 10.1074/jbc.275.6.4013. [DOI] [PubMed] [Google Scholar]
- 22.Dahl NK, Reed KL, Daunais MA, Faust JR, Liscum L. Isolation and characterization of Chinese hamster ovary cells defective in the intracellular metabolism of low density lipoprotein-derived cholesterol. J Biol Chem. 1992;267:4889–4896. [PubMed] [Google Scholar]
- 23.Choi HY, Karten B, Chan T, Vance JE, Greer WL, Heidenreich RA, Garver WS, Francis GA. Impaired ABCA1-dependent lipid efflux and hypoalphalipoproteinemia in human Niemann-Pick type C disease. J Biol Chem. 2003;278:32569–32577. doi: 10.1074/jbc.M304553200. [DOI] [PubMed] [Google Scholar]
- 24.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 25.Liscum L, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-beta-[2-(diethylamino)ethoxy]androst-5-en-17-one. J Biol Chem. 1989;264:11796–11806. [PubMed] [Google Scholar]
- 26.Matsumoto M, Kogawa M, Wada S, Takayanagi H, Tsujimoto M, Katayama S, Hisatake K, Nogi Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem. 2004;279:45969–45979. doi: 10.1074/jbc.M408795200. [DOI] [PubMed] [Google Scholar]
- 27.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 28.Choudhury A, Dominguez M, Puri V, Sharma DK, Narita K, Wheatley CL, Marks DL, Pagano RE. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J Clin Invest. 2002;109:1541–1550. doi: 10.1172/JCI15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 30.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 33.Wustner D, Mondal M, Tabas I, Maxfield FR. Direct observation of rapid internalization and intracellular transport of sterol by macrophage foam cells. Traffic. 2005;6:396–412. doi: 10.1111/j.1600-0854.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Wang N, Tall AR. A PEST deletion mutant of ABCA1 shows impaired internalization and defective cholesterol efflux from late endosomes. J Biol Chem. 2005;280:29277–29281. doi: 10.1074/jbc.M505566200. [DOI] [PubMed] [Google Scholar]
- 35.Tobias PS, Curtiss LK. Toll-like receptors in atherosclerosis. Biochem Soc Trans. 2007;35:1453–1455. doi: 10.1042/BST0351453. [DOI] [PubMed] [Google Scholar]
- 36.Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, Brech A, Latz E, Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taub N, Teis D, Ebner HL, Hess MW, Huber LA. Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogenactivated protein kinase signaling. Mol Biol Cell. 2007;18:4698–4710. doi: 10.1091/mbc.E07-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki M, Sugimoto Y, Ohsaki Y, Ueno M, Kato S, Kitamura Y, Hosokawa H, Davies JP, Ioannou YA, Vanier MT, Ohno K, Ninomiya H. Endosomal accumulation of Toll-like receptor 4 causes constitutive secretion of cytokines and activation of signal transducers and activators of transcription in Niemann-Pick disease type C (NPC) fibroblasts: a potential basis for glial cell activation in the NPC brain. J Neurosci. 2007;27:1879–1891. doi: 10.1523/JNEUROSCI.5282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 40.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Tolllike receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R, Town T, Gokarn V, Flavell RA, Chandawarkar RY. HSP70 enhances macrophage phagocytosis by interaction with lipid raft-associated TLR-7 and upregulating p38 MAPK and PI3K pathways. J Surg Res. 2006;136:58–69. doi: 10.1016/j.jss.2006.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.