Abstract
The enzyme aromatase (which converts androgens into oestrogens) is expressed throughout the brain in zebra finches. Aromatase is enzymatically active in both cell bodies and synaptic terminals of neurones of the songbird brain, particularly within forebrain motor and auditory networks. Aromatisation within synaptic terminals could thus provide localised and acute modulatory oestrogens within the forebrain during singing and/or audition. In male zebra finches, we tested the hypothesis that forebrain aromatase activity is elevated during singing behaviour and/or hearing male song. This study reports that aromatase activity is elevated in males that were singing for 30 min as compared to non-singing males, and that this elevation occurs only within the cellular compartment that contains synaptic terminals. In a separate experiment, males that heard acoustic playback of song for 30 min exhibited no differences in aromatase activity or in aromatase mRNA levels as revealed by quantitative PCR analysis. Therefore, these results indicate that activation of the motor pathway for song production is linked to local elevations in synaptic aromatase activity within the forebrain of male zebra finches. Future experiments could assess whether elevated synaptic aromatase activity during song is dependent on acute regulation of the aromatase protein.
Introduction
The enzyme aromatase converts androgens into oestrogens, and aromatase is expressed in endocrine glands and within the central nervous system. In most vertebrates, aromatase is expressed and biologically active within the preoptic area/hypothalamus, a primary locus of reproductive behaviour (1-3). Among individuals, the enzymatic activity of hypothalamic aromatase is related to the intensity of aggressive behaviour in mammals and birds (4-6) and is inversely related to aggression in fish (7, reviewed in 8). Hypothalamic aromatase activity is also acutely regulated during copulatory behaviour, in birds and mammals (9, 10), and pharmacological inhibition of aromatase suppresses reproductive behaviour in male mice and fish (11, 12). Therefore, the regulation of reproductive behaviour by hypothalamic aromatase activity has emerged as a conserved feature of the vertebrate forebrain (3, 13).
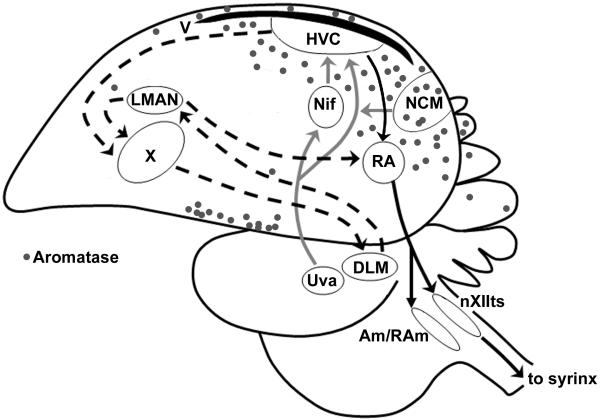
Non-mammalian vertebrates exhibit robust aromatase expression in forebrain regions outside the preoptic area/hypothalamus. Songbirds and teleost fishes exhibit abundant aromatase expression throughout the forebrain (3, 14), although the function of this extra-hypothalamic aromatase is not yet clear. One proposed functional explanation is that forebrain aromatase provides a local source of oestrogen that modulates circuits involved in complex behaviours. In songbirds, the discrete caudal forebrain network of nuclei involved in song production is surrounded by robust aromatase expression (see Fig. 1). In zebra finches, injection of oestrogens or aromatisable androgens increases the expression of singing behaviour in males (15) and in adult white-crowned sparrows, local forebrain aromatisation is responsible for seasonal plasticity of neuronal firing properties within the song network (16). These and other findings raise the possibility that acute, local aromatase activity in the forebrain song network is associated with singing behaviour in songbirds, but this possibility is currently untested.
Figure 1.
A saggital view of the zebra finch brain, showing primary motor and auditory circuits, along with aromatase expression (grey dots). The motor network contains two pathways: a posterior forebrain pathway (HVC, RA, nXIIts) involved in song production, and an anterior forebrain pathway (HVC, Area X, DLM, lMAN) involved in song learning. An ascending auditory network contains a series of midbrain (Uva) and forebrain (Nif, NCM) nuclei that project to HVC. The enzyme aromatase (grey dots) is expressed throughout the posterior forebrain, in particular within NCM and surrounding the nucleus HVC. Figure adapted from (49). Abbreviations: HVC, proper name also known as ‘high vocal centre’; RA, robust nucleus of the arcopallium; nXIIts, tracheo-syringeal nucleus of the 12th nerve; DLM, medial dorsolateral nucleus of the thalamus; lMAN, lateral magnocellular nucleus of the anterior nidopallium; Uva, nucleus uvaeformis; NIf, nucleus interface of the nidopallium; NCM, caudomedial nidopallium.
In addition to cell bodies, aromatase is expressed in synaptic terminals in the mammalian hypothalamus (17) and hippocampus (18), though the function of this neuronal compartmentalisation is not clear. In zebra finch brain, aromatase has been localised to axonal fibres (14) and synaptic terminals (19) within forebrain nuclei involved in auditory-motor integration (HVC and caudomedial nidopallium; NCM; see Fig. 1). The subcellular compartments enriched with the aromatase enzyme can be separated via differential centrifugation into a microsomal fraction (somal aromatase) and a synaptosomal fraction (synaptic aromatase) for biochemical analysis of enzyme activity (20). Within the purified synaptosomal fraction from posterior telencephalon of adult zebra finches, aromatase activity is higher in males than in females (21). Since only males sing, this raises the hypothesis that synaptic aromatase is associated with singing behaviour and/or auditory processing in male zebra finches. Furthermore, acute changes in aromatase activity within synaptic terminals could provide a proximate source for local fluctuations in forebrain oestrogen levels during social interactions, as observed recently using in vivo microdialysis (22).
Here we test the hypothesis that forebrain aromatase activity is linked to the expression of singing behaviour and auditory activation in male zebra finches. Aromatase activity could be linked to the production of song, the auditory processing of song, or both. We first examine aromatase activity in forebrain homogenates and then within subcellular compartments by comparing aromatase activity in singing vs. non-singing males. Next, we test whether playback of conspecific song alters aromatase activity within subcellular compartments and/or the expression of aromatase mRNA in the forebrain of male zebra finches.
Methods
Subjects
All experiments were approved by the UCLA Chancellor’s Committee on Animal Care and Use. Animals were adult zebra finches > 120 days of age taken from the Schlinger laboratory breeding colony at UCLA. Males were taken from a mixed-sex aviary room that provided them visual and acoustic contact with females and other males. Males were therefore likely to have been sexually experienced with females. Males were placed individually in acoustic isolation chambers for 24 hrs prior to each experiment trial. All trials were carried out within 1 hr of lights-on to minimise circadian variation. Songs were recorded using a Shure-SM57 microphone connected to a computer and digitised with Syrinx software.
Singing experiment 1
We asked whether an acute period of singing behaviour (30 min duration, consisting of intermittent song bouts characteristic of male zebra finches) is linked to forebrain aromatase activity. The 30-min sampling time was chosen, in part, to match the rapidity of observed changes in forebrain immediate-early gene expression associated with the production of song in male zebra finches (23, 24). For each of 12 males (one male only per trial), two female zebra finches were added to a cage adjacent to the focal male’s cage inside an acoustic isolation chamber for a 30 min period. Two females were used because pilot testing showed that some males preferentially sang to one female of a pair only. During each trial, the focal male was monitored and scored by 1-2 observers through a one-way glass partition for the occurrences of courtship songs, chirps, beak wipes, flights, preening, feeding, and drinking. According to their within-trial behaviours, males were separated into two groups for comparison: 1) males that sang at least 1 song in the presence of females (‘singers’; n = 8; mean song bouts ± S.D. = 18.75 ± 10.66), and 2) males that did not sing at all in the presence of females (‘non-singers’, n = 4). The proportion of non-singers observed here is similar to previous studies in which 20-30% of male zebra finches do not sing courtship song when individually presented with females for brief trials (e.g., 25). As also shown previously, these non-singer males are considered capable of song, but did not exhibit song when presented with our stimulus females. At the end of trials, males were caught and immediately euthanised by rapid decapitation (< 1 min after initial disturbance). Trunk blood was collected, centrifuged, and plasma stored at -80°C. Brains were immediately dissected on ice into major brain regions following established protocols (21, 26): anterior hypothalamus-preoptic area (AH-POA; separated from optic tecta and posterior hypothalamus), ventral forebrain containing nucleus taeniae (NT), anterior telencephalon (AT; separated from NT and hippocampus, containing song nuclei Area X and lMAN), and posterior telecephalon (PT; separated from NT and hippocampus, containing song nuclei HVC, RA, and auditory nuclei NCM, Field L, CMM and Nif, see also Fig. 1). Aromatase mRNA and protein within the ventral forebrain region that contains nucleus taeniae is restricted to the bounds of nucleus taeniae (14, 27), and our measures of biochemical activity of aromatase from this region is therefore presented as ‘NT’. Dissected brain regions were immediately frozen on dry ice and stored at -80°C until the aromatase activity assay.
Singing experiment 2
In experiment 2, we sought to determine the subcellular compartment (microsomes vs. synaptosomes) that accounts for differences in aromatase activity between singers and non-singers. All experimental procedures were as outlined above for the singing experiment 1 for a new set of 12 birds (8 singers, 4 non-singers). As in experiment 1, all singers sang at least 1 song in the presence of females (mean song bouts ± S.D. = 10.50 ± 10.18), while non-singers did not produce a single bout of song when presented with females. Immediately following brain region dissection, AT and PT samples were processed for cellular subfractionation. We focused here on telencephalic tissue since singing was associated with differences in aromatase activity only in posterior telencephalon in Experiment 1 (see Results). Brain subfractions were prepared according to established protocols with some modifications (20, 21, 28). Briefly, AT and PT samples were homogenised in 2 mL of ice-cold sucrose phosphate buffer (ph = 7.2 - 7.6; on ice) in 3 × 5 sec intervals with an electric homogeniser (Tissue Tearor, Biospec Products, Bartelsville, OK). Homogenates were then centrifuged for 15 min at 1046 x g at 4°C to separate whole cell debris, nuclei, and dense organelles (P1 pellet), from mitochondria, synaptosomes, cytosol, and microsomes (S1 supernatant). The resulting S1 supernatant was decanted and centrifuged for 30 min at 10,900 x g at 4°C to separate synaptosomes and mitochondria (P2 pellet) from microsomes and cytosol (S2 supernatant). The S2 supernatant was collected and frozen at -80°C until the aromatase assay (‘S2 microsomes’, see below). The P2 pellet was resuspended in 2 mL of fresh sucrose phosphate buffer and gently shaken before being centrifuged again for 30 min at 10,900 x g at 4°C to purify the synaptosomal/mitochondria sample. The resulting purified P2 pellet was collected and frozen at -80°C until the aromatase activity assay (‘synaptosomal fraction’) and the supernatant was discarded. We report here on aromatase activity in the synaptosomal fraction (P2) which contains both purified synaptosomes and mitochondria, and in the microsomal fraction (S2), which contains both microsomes and cytosol. Aromatase activity is not found in cytosol or mitochondria (20), so this study reports P2 aromatase activity as ‘synaptosomal’ and S2 aromatase activity as ‘S2 microsomal’.
Playback experiment
We reasoned that the differences in posterior telencephalon aromatase activity we observed between singers vs. non-singers (see Results) could be due to activation of the auditory pathway (i.e., self-stimulation) and not singing behaviour per se. To test this hypothesis for aromatase activity, a separate set of 19 males were individually housed in acoustic isolation chambers for 24 hrs prior to experimental trials. For 13 males (one male per trial only), conspecific male song (1 min recording of a single song motif sequence from each of 3 individual males, looped 30 x) was broadcast inside the acoustic chamber for 30 min. For 6 other males, pulsed white noise (1 min intermittent white noise looped 30 x) was broadcast inside the acoustic chamber for 30 min. All sound stimuli were band-pass filtered (1 kHz-12 kHz, CoolEdit Pro), and standardised for peak amplitude < 70 dB (after 29). At the end of trials, males were caught and immediately euthanised by rapid decapitation (mean = 39 ± 11 sec after initial disturbance). Trunk blood was collected and brains dissected as above. The left hemisphere AT and PT samples were homogenised and processed for subfractionation as above. The right hemisphere AT and PT samples were immediately frozen on dry ice and processed for quantitative PCR for aromatase mRNA expression (see below). We focused here once again on telencephalic tissue since singing was associated with differences in aromatase activity only in posterior telencephalon in Experiments 1 and 2 (see Results). Three males in the ‘male song’ group responded to song playback by singing at least 1 song bout. Samples from these three singing birds were not included in the biochemistry assay with the 16 non-singing birds (resultant n = 10 ‘male song’ birds, n = 6 ‘white noise’ birds). None of the birds in the ‘white noise’ treatment group sang during the 30 min white noise playback period. Samples analysed for the quantitative PCR of aromatase mRNA included a subset of n = 6 males from the ‘male song’ group, n = 6 males from the ‘white noise’ group, and n = 3 males from the ‘male song’ group who sang during the playback experiment (‘MS Singers’).
Biochemistry assay
Aromatase activity was measured using standard biochemical techniques, as reported previously for zebra finches and quail (20, 21). Tissue homogenates (either whole homogenate in 180 μl ice-cold sucrose-phosphate buffer, a synaptosomal pellet homogenised in 180 μl ice-cold sucrose-phosphate buffer, or 180 μl of ice-cold S2 fraction) were incubated with 100 nM [1,2,6,7-3H] AE (androstenedione; New England Nuclear, Waltham MA) and cofactor mix (20 μl) for a final volume of 200 μl. We did not isolate a purified P3 (microsomal) pellet (e.g., 20, 21), but assayed aromatase activity in the S2 supernatant which contains microsomes and cytosol. To confirm that microsomes within the S2 cytosolic fraction exhibited aromatase activity, we conducted pilot time course experiments on a separate set of S2 samples in which the concentration of [1,2,6,7-3H] AE was varied from 20, 50, and 100 nM, and the time course ranged from 5-30 min. Based on this validation, we chose a 10-min incubation time and 100 nM [1,2,6,7-3H] AE starting concentration for all S2 samples containing microsomes. We assessed the validity of this approach by confirming that an excess of radio-isotopic substrate ([1,2,6,7-3H] AE) was present during and after the aromatase reaction (data not shown), since high activity of cytosolic enzymes (such as 5β-reductase) in the S2 supernatant could shunt substrate away from the aromatase reaction. The regenerating cofactor mix for all experiments consisted of 22.2 mM NAD-P, 22.2 mM βNAD, 103.8 mM glucose-6-phosphate, 22.2 mM ATP, and 20 IU/mL glucose-6-phosphate dehydrogenase. Whole brain homogenate and synaptosomal samples were incubated for 5 min, while S2 microsomal samples were incubated for 10 min at 40°C, each tube was then snap frozen in a dry ice/methanol bath to stop the reaction. Steroids were extracted with diethyl ether (3x), and the aqueous phase stored at -20°C for a Bradford protein assay. The organic phase was dried in a warm water bath, and resuspended with CH2Cl2/methanol. Samples were air-dried and oestrogens were separated from androgens by phenolic partition using carbon tetrachloride and 0.1 M sodium hydroxide (20). All synaptosomal samples were processed in parallel for activity of aromatase and the androgen-metabolic enzymes 5αreductase, 5β-reductase, and 17β-hydroxysteroid dehydrogenase (20). Radioinert steroids were added to each tube as markers for visualisation (E1 and E2 for aromatase; 5α-androstanedione, 5β-androstanedione, androstenedione and testosterone for other androgen-metabolic pathways), and samples were separated using thin-layer chromatography with ether:hexane (3:1) as a mobile phase. Bands containing oestrogens were visualised using iodine, and bands containing androgens were visualised under UV-radiation after primulin spray (Sigma). All bands were scraped, and steroids eluted from silica using methanol. Radioisotope decay counts were measured on a liquid scintillation counter (E1 and E2 counts were combined for total oestrogen content to reflect aromatase activity). Protein content was estimated for each sample using the Bradford method. To account for procedural losses (recovery), separate tubes containing known quantities of [1,2,6,7-3H] AE and [2,4,6,7-3H] E1 were processed in parallel through the entire assay.
Quantitative PCR
Differences in the activity of the aromatase protein between individuals could arise from constitutive or acute differences in expression of aromatase mRNA levels, or from post-transcriptional modifications. To test the former hypothesis, we isolated RNA from the right hemisphere from birds in the playback experiment using quantitative real-time PCR. Total RNA was extracted from brain tissue using the TRIzol method, following the manufacturer’s instructions (Invitrogen, Carlsbad). Tissue was suspended in TRIzol and homogenised with an electric homogeniser for 20 seconds on ice. Following homogenisation, tubes were manually shaken for 45 seconds, and samples were separated with choloroform. The aqueous phase was pipetted into a new set of tubes and washed with isopropanol. Following 10 min centrifugation (12 g at 4°C), pelleted RNA was washed with 75 % ethanol, redissolved to final stock volume with DEPC water, and incubated for 10 min at 60°C. Isolated RNA was verified via gel electrophoresis, and the final concentration was determined via spectrophotometer at 260 nm. Isolated RNA was stored at -80°C until PCR.
RNA was treated for contamination using DNase (Promega, Madison, WI), and 1μg of total RNA was reverse transcribed using Superscript II Reverse Transcriptase (Invitrogen) on a thermal cycler (MJ Research, Waltham MA) for 50 min at 42°C followed by 15 min at 70°C. The resulting cDNA was analysed by quantitative PCR for aromatase mRNA using specific zebra finch aromatase primers: Forward: 5′ GGATGAGCACATGGATTTTGC; Reverse 5′ GCAGTCAGATCCCCTCTGTTC. GAPDH was used as internal control, primers: Forward 5′ CCATCAGCAGCAGCCTTCA; Reverse 5′ GACCTGCCGTCTGGAAAA. Reactions were carried out an ABI 7300 sequence detection system (Applied Biosystems) with Sybr Green. Dissociation curves of PCR products were checked to verify the absence of DNA contamination, and all samples were run in duplicate. The delta Ct method was used for quantification.
Hormone levels
Plasma oestradiol was quantified via enzyme immunoassay (Cayman Chemical, Assay Designs, respectively), according to previously established protocols (22). Assay sensitivity was 5.40 pg/ml.
Statistics
Enzyme activity results (fmol/mg protein/minute) for each experiment were analysed using Statview 4.5 (Abacus, Berkeley, CA), and tested for normality using JMP 5.0.1 (SAS, Cary, NC). For all experiments, biochemistry results did not fit normal distributions, and common transformations did not improve normality (see Results below), so Mann-Whitney nonparametric U-tests were performed on all biochemistry data with two-tailed probability distributions. When multiple comparisons were performed for a given experimental run (i.e. anterior vs. posterior telencephalon), the Bonferroni correction was used to correct p-values. For quantitative PCR, delta Ct values were log-transformed to achieve normality and analysed via two-way ANOVA. Plasma steroid levels were analysed by unpaired t-tests.
Results
Singing experiment 1
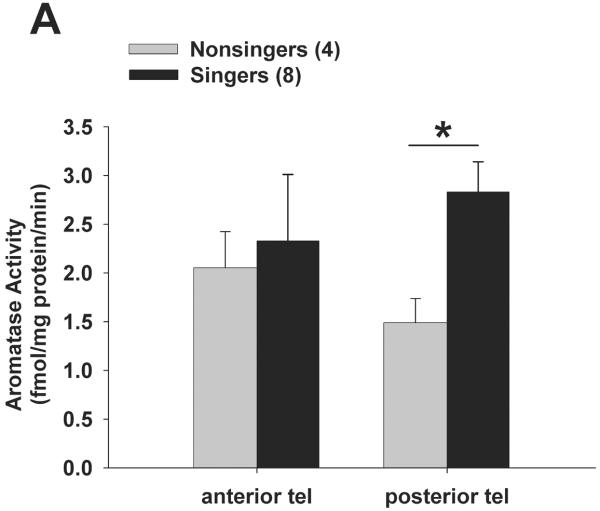
We asked whether a brief (30 min) bout of singing behaviour was linked to aromatase activity in the forebrain of male zebra finches. Results (including log-transformed data) did not fit normal distributions (Shapiro-Wilk p < 0.0001), so results were analysed using nonparametric statistics. Aromatase activity was significantly higher in singers than non-singers in posterior telencephalon, but not anterior telencephalon. Mann-Whitney U-tests showed that aromatase activity (Fig. 2A-B) was significantly higher in singers vs. non-singers in posterior telencephalon (U = 30; Bonferroni corrected p = 0.032) but not in anterior telencephalon (p = 0.49). Mann-Whitney U-tests also showed there were no differences between singers and non-singers in AH-POA (p = 0.39), or nucleus taeniae (p = 0.73). Despite higher mean PT aromatase activity levels in singers vs. non-singers, there was no significant correlation between the number of songs sung by each male during the trial and PT aromatase activity in singers (R2 = 0.28; Spearman rank z = 1.705; p = 0.088). Other than singing behaviour (see Methods), all other scored behaviours were not significantly different between singers vs. non-singers (data not shown; chirps p = 0.80; beak wipes p = 0.44; flights p = 0.29; preening p = 0.71; drinking p = 0.23; feeding p = 0.33). There was no significant difference in plasma levels of oestradiol between singers (58.18 ± 23.23 pg/ml) and non-singers (27.47 ± 11.58; p = 0.29). There was also no significant correlation between plasma oestradiol and the number of songs each male sang (R2 = 0.104; F = 0.581; p = 0.48), or between plasma oestradiol and either PT aromatase activity or AT aromatase activity (all p > 0.54).
Figure 2.
Aromatase activity is elevated in the posterior telencephalon of singing male zebra finches. All birds were exposed to females for 30 min, and aromatase activity was measured in whole brain homogenate in birds that sang at least one song (‘singers’) vs. birds that did not sing (‘non-singers’). Aromatase activity was significantly elevated (* p = 0.032) in singers within the posterior telencephalon only (A), and was not different in anterior telencephalon (A), nucleus taeniae (B), or anterior hypothalamus (B). Data are presented as mean + SEM, sample sizes are in parentheses. All biochemical data were analysed by nonparametric Mann-Whitney U-tests.
Singing experiment 2
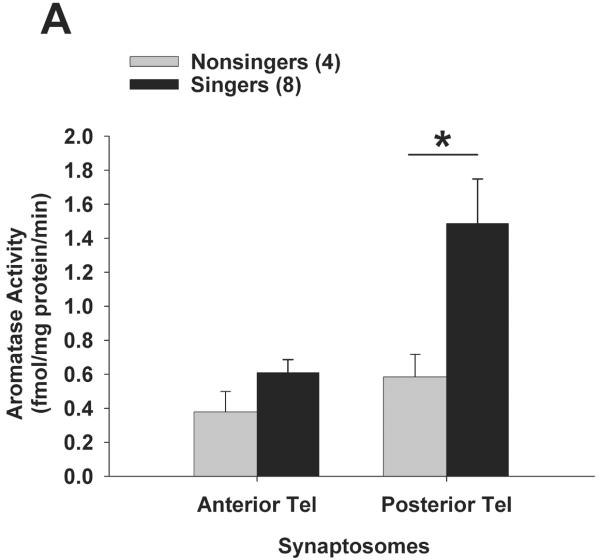
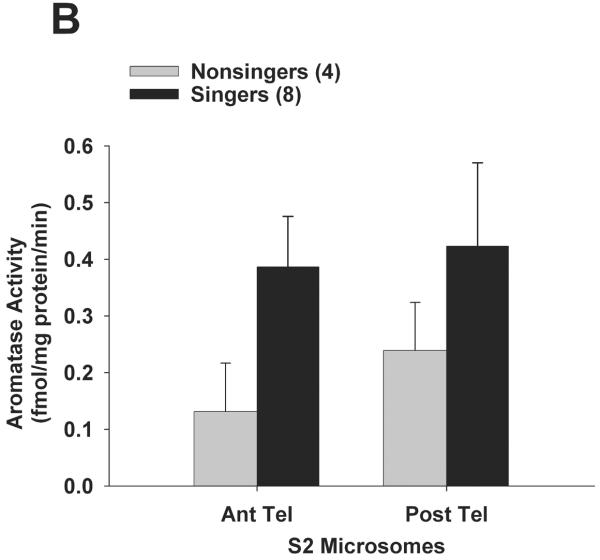
In experiment 2, for a separate group of birds we sought to determine the subcellular compartment (S2 microsomes vs. synaptosomes) that accounted for differences in aromatase activity between singers and non-singers. Results (including log-transformed data) did not fit normal distributions (Shapiro-Wilk p < 0.0002), so results were analysed using nonparametric statistics. Once again, singers had higher aromatase activity than non-singers in posterior telencephalon. Mann-Whitney U-tests showed that synaptosomal aromatase activity (Fig. 3A) was significantly higher in singers vs. non-singers in posterior telencephalon (U = 32; Bonferroni-corrected p = 0.008), but not in anterior telencephalon (p = 0.24). Aromatase activity in the S2 fraction (microsomes; Fig. 3B) was not significantly different in singers vs. non-singers for either posterior (p = 0.93) or anterior (p = 0. 26) telencephalon. Similar to experiment 1, there was no significant correlation between synaptosomal aromatase activity from posterior telencephalon and the number of songs each male sang (R2 = 0.104; Spearman rank z = 1.68; p = 0.094).
Figure 3.
Aromatase activity within synaptosomes is elevated in the posterior telencephalon of singing male zebra finches. A new set of birds was exposed to females for 30 min, and aromatase activity was measured in subcellular compartments that contain synaptic terminals (synaptosomes) or an S2 fraction containing microsomes in singers vs. non-singers. Aromatase activity was significantly elevated (* p = 0.008) in singers within the synaptosomal fraction of posterior telencephalon only (A), and not within anterior telencephalon (A) or within S2 microsomes from either telencephalic region (B). Data are presented as mean + SEM, sample sizes are presented in parentheses.
Unlike aromatase, the activity of synaptosomal androgen-conversion enzymes was not different between singers and non-singers, for either brain region (data not shown). Results (including log-transformed data) did not fit normal distributions (Shapiro-Wilk p < 0.0001), so results were analysed using nonparametric statistics. Mann-Whitney U-tests showed no significant differences between singers and non-singers for synaptosomal 5α-reductase activity (posterior p = 0.99; anterior p = 0.99), 5β-reductase activity (posterior p = 0.18; anterior p = 0.86), and 17β-hydroxysteroid dehydrogenase activity (posterior p = 0.10; anterior p = 0.24). Other than singing behaviour itself, all other scored behaviours were again not significantly different between singers vs. non-singers (data not shown; chirps p = 0.70; beak wipes p = 0.27; flights p = 0.55; preening p = 0.83; drinking p = 0.07; feeding p = 0.11). There was again no significant difference (p = 0.20) between plasma oestradiol in singers (51.79 ± 20.83 pg/ml) vs. non-singers (21.61 ± 8.19). There was also no significant correlation between plasma oestradiol and the number of songs each male sang (R2 = 0.07; F = 0.754; p = 0.40). Also, there were no significant correlations between plasma oestradiol and PT aromatase activity or AT aromatase activity in either subcellular fraction (all p > 0.23).
Playback experiment
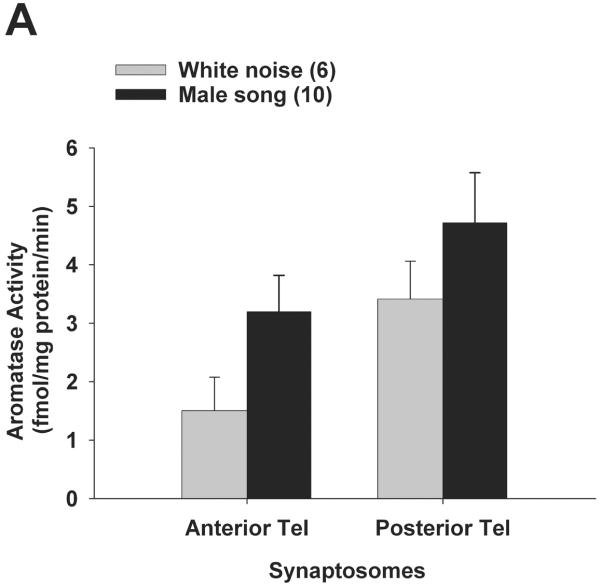
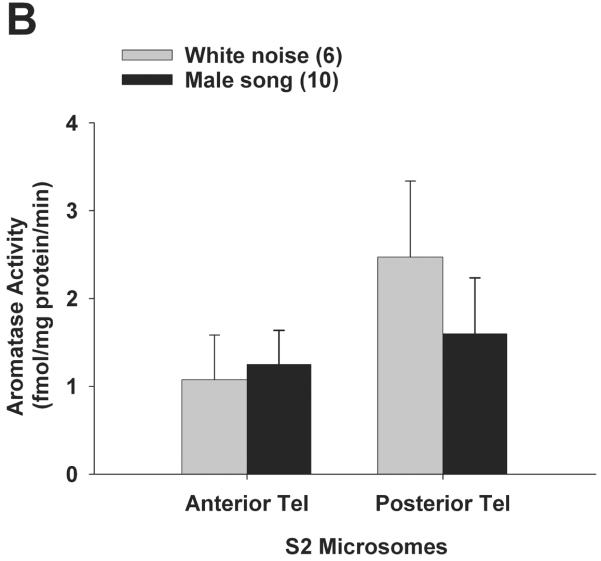
We reasoned that the differences in PT aromatase activity levels between singers vs. non-singers could be due to self-stimulated activation of the forebrain auditory circuit rather than singing behaviour per se. Results (including log-transformed data) did not fit normal distributions (Shapiro-Wilk p < 0.0000), so results were analysed using nonparametric statistics. There were no differential effects of playback stimuli on telencephalic aromatase activity, for either cellular compartment. Mann-Whitney U-tests tests showed that synaptosomal aromatase activity (Fig. 4A) was not significantly different between male song vs. white noise treatments in posterior telencephalon (p = 0.33) or anterior telencephalon (p = 0.12). Similarly, Mann-Whitney U-tests showed that S2 microsomal aromatase activity (Fig. 4B) was also not significantly different between male song vs. white noise treatments in posterior telencephalon (p = 0.45) or anterior telencephalon (p = 0.74). The qualitatively higher aromatase activity levels in posterior vs. anterior telencephalon are consistent with earlier reports for elevated aromatase expression (14) as well as aromatase activity (21) in posterior vs. anterior telencephalon of zebra finches.
Figure 4.
Aromatase activity does not change in either subcellular compartment in response to acoustic playback. All birds were exposed playback of male song or white noise for 30 min, and aromatase activity was measured in synaptosomes (A) and S2 microsomes (B). Data are presented as mean + SEM, sample sizes are presented in parentheses.
Similar to aromatase, the activity of synaptosomal androgen-conversion enzymes was not different among playback groups, for either brain region (data not shown). Results (including log-transformed data) did not fit normal distributions (Shapiro-Wilk p < 0.0001), so results were analysed using nonparametric statistics. Mann-Whitney U-tests showed no significant differences between male song and white noise treatment for synaptosomal 5α-reductase activity (posterior p = 0.66; anterior p = 0.89), 5β-reductase activity (posterior p = 0.16; anterior p = 0.66), and 17β-hydroxysteroid dehydrogenase activity (posterior p = 0.16; anterior p = 0.93). For birds in experiment 3, observed behaviour scores were not significantly different between male song vs. white noise treatments (data not shown; chirps p = 0.12; beak wipes p = 0.71; flights p = 0.55; preening p = 0.41; drinking p = 0.55; feeding p = 0.99). Plasma oestradiol levels for this experiment were as reported in an earlier study (22); there were no differences between playback groups (male song: 41.96 ± 10.16; white noise: 51.24 ± 24.65; p = 0.70).
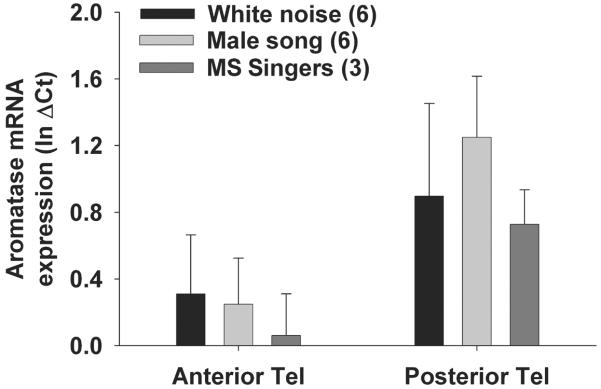
Quantitative PCR analysis showed no effect of playback treatment on aromatase mRNA expression in either anterior or posterior telencephalon (Fig. 5). Delta Ct values were log-transformed to achieve normality (Shapiro-Wilk p > 0.05) and analysed via two-way ANOVA. In the two-way factorial ANOVA there was no significant effect of playback treatment on aromatase mRNA expression (F = 0.307; p = 0.74), but there was a significant effect of brain region on aromatase mRNA expression (F = 4.59; p = 0.042). Tukey’s post hoc tests showed that aromatase mRNA expression was elevated in posterior telencephalon vs. anterior telencephalon in all three experimental groups (p < 0.03 for ‘male song’, ‘white noise’, and ‘male song - singers’). Interestingly, there were no significant correlations between telencephalic aromatase activity and quantitative mRNA expression level, (synaptosomes: anterior telencephalon R2 = 0.02; F = 0.22; p = 0.65, posterior telecephalon R2 = 0.05; F = 2.14; p = 0.16, S2 microsomes: anterior telencephalon R2 = 0.02; F = 0.001; p = 0.99, posterior telecephalon R2 = 0.029; F = 0.29; p = 0.59).
Figure 5.
Aromatase mRNA expression level, as measured by quantitative PCR, does not change in response to acoustic playbacks. All birds were exposed playback of male song or white noise for 30 min, and three males in the ‘male song’ group sang at least once (‘MS-Singers’). Aromatase mRNA levels are significantly elevated in posterior vs. anterior telencephalon within all three treatment groups (p < 0.03), but do not significantly differ among treatments (e.g., white noise vs. male song). Data are presented as mean + SEM, sample sizes are presented in parentheses. Data were analysed by factorial ANOVA following log-transformation.
Discussion
We observed elevated aromatase activity in the posterior telencephalon of singing male zebra finches as compared to non-singing males. Importantly, singers have elevated aromatase activity in the posterior telencephalon that is localised to the subcellular fraction that contains synaptosomes. This indicates that aromatase in synaptic terminals within the posterior telencephalon (19, 21) has a functional role in association with song production. A series of recent findings complimentary to the current results show that social interactions with females increases local oestradiol levels within the caudal forebrain in zebra finches, as measured by in vivo microdialysis (22). Local forebrain oestradiol and testosterone levels determined by this method also change rapidly in response to infusion of the neurotransmitters glutamate and GABA. Collectively, these results are consistent with the hypothesis that local oestrogen synthesis within the caudal forebrain is regulated at the presynaptic level by acute (e.g. neurotransmitter-dependent) mechanisms. This study emphasises that the neural circuits that underlie song production and auditory processing in zebra finches have the capacity for acute modulation by local steroidal microenvironments.
The elevated synaptosomal aromatase activity levels in singing vs. non-singing males observed here could be due to either fast upregulation of aromatase activity during the period of singing itself, or a ‘prior condition’ of constitutively elevated aromatase activity in the telencephalon of singers. There is experimental precedent for rapid (within 5 min) regulation of brain aromatase activity in birds. In quail hypothalamus, aromatase activity is rapidly regulated in males during copulation (9), and glutamate is a potent modulator of aromatase activity in in vitro hypothalamic explants (30). Future experiments could assess whether similarly rapid changes in aromatase activity occur within the zebra finch forebrain within time periods of less than 30 min. Complementary tests of this hypothesis could include short-term administration of aromatase inhibitors such as fadrozole, which has been shown to reduce song and other courtship behaviours following long-term (days-weeks) implantation in a wide range of vertebrates (11, 31-34).
A rapid increase in synaptic aromatase activity within the posterior telencephalon of singing males would be associated with similarly rapid oestrogen actions. Synaptic aromatase activity is most likely regulated by acute interactions between steroids and neurotransmitters, since aromatase is colocalised with NMDA-receptors (35), and forebrain oestradiol levels are regulated by glutamate (22). There is now strong evidence for a multitude of rapid downstream oestrogen effects on neuronal activity and intracellular second messengers (36, 37), some mediated via membrane oestrogen receptors (38, 39). Although no evidence exists to date for rapid (seconds-to-minutes) oestrogen actions in the avian song system, oestradiol rapidly affects the motor circuit that patterns vocalisations in a teleost fish, the plainfin midshipman (40, 41). Therefore, rapid steroid actions on vocal pathways may be conserved among vertebrates. Alternatively, changing forebrain oestrogens could also influence the activity of other steroidogenic enzymes (e.g., 42), leading to secondary modulatory effects. Lastly, oestrogens could interact with neurotransmitter/neuropeptides systems in the song circuit, such as catecholamines like dopamine (43, 44), to acutely modulate song production.
Support for the interpretation of a ‘prior condition’ of brain aromatase activity comes from two previous observations. In rats, medial preoptic aromatase activity levels are higher in copulating vs. non-copulating males (10), and these differences are also region-specific (i.e., no differences were reported for the BNST, MeA, and VMN). Similarly, quail hypothalamic aromatase activity is elevated in highly-aggressive males, and the stability of aggression propensity is linked to the ‘prior condition’ of high vs. low hypothalamic aromatase activity (4). Aromatase activity is positively correlated with behavioural score in rats and quail (copulatory or aggressive events, respectively), whereas, in contrast, the current findings report that song bouts and aromatase activity in posterior telencephalon are not directly linked. Therefore, it remains possible that a threshold relationship exists between telencephalic aromatase activity and the onset of singing behaviour in zebra finches, or that aromatase activity is related to auditory processing (i.e. self stimulation or song memory retreival) within the posterior telencephalon. Despite these differences, these studies are among a growing number which indicate that aromatisation provides a localised mechanism within the brain to allow plasticity in reproductive behaviours.
Aromatase activity in the posterior telencephalon is higher in male vs. female zebra finches, and this sex difference is most pronounced in synaptosomes (21). The current study suggests that sex differences in synaptosomal aromatase activity are linked to the sex-specific production of song in male zebra finches. A comprehensive review showed that telencephalic aromatase activity is elevated in bird species that produce learned song vs. non-learners (45). This study predicts that aromatase activity is particularly pronounced within forebrain synaptic terminals of song learning species, and that synaptic aromatase is acutely regulated during the production of learned song.
We observed that aromatase activity does not differ between males that heard conspecific song playback vs. white noise in both anterior and posterior telencephalon, in either subcellular compartment. This is in contrast to a recent observation in which song playback (and not white noise) causes acute elevations in local oestradiol levels within auditory forebrain, as measured by in vivo microdialysis (22). The evidence for laterality of auditory processing in the zebra finch telencephalon emphasises the left NCM (46, 47), and the previous microdialysis experiments and current biochemical experiments analysed the left telencephalon. The discrepancy in previous microdialysis vs. current biochemical results could be due to one of the following: 1) Changes in oestradiol levels within NCM as measured by microdialysis are localised and do not occur in adjacent telencephalic regions, and they therefore reflect localised increases in aromatase activity. Thus, homogenisation of the posterior telencephalon in the current study may not yield sufficient spatial resolution to detect such changes in aromatase activity during auditory activation. 2) Local changes in oestradiol could also be the result of changing levels of androgen precursors, which are then aromatised to result in increased oestradiol levels, without active changes in aromatase activity. 3) The current methods may have missed the peak in aromatase activity by sampling after 30 min of playback, while microdialysis experiments collect oestradiol for the entire 30 min period and reveal a two-fold surge in oestradiol levels. Microdialysis methods may therefore be more sensitive to recent neuroendocrine events, while biochemical measurement of aromatase activity (which determines enzymatic capacity) could be predictive of future brain steroid levels. Similarly, the utilisation (i.e., half-life) of local oestrogens in vivo may be considerably different as compared to in vitro, leading to differences in detection by these two methods.
Quantitative PCR analysis showed that aromatase mRNA expression was elevated in the posterior vs. anterior telencephalon of males, consistent with immunohistochemical results for zebra finch aromatase protein (14) and aromatase biochemical activity (21). The current study also reported no effect of playback stimulus (male song vs. white noise) on quantified aromatase mRNA expression levels in either anterior or posterior telencephalon. Furthermore, aromatase mRNA expression was not elevated in birds that sang during male song playback compared to birds that did not sing. Therefore, this study reports that singing male zebra finches have elevated aromatase activity in the posterior telencephalon within synaptic terminals, and provides limited evidence that telencephalic aromatase mRNA levels are not similarly elevated during singing. Interestingly, there was no strict correlation between telencephalic aromatase mRNA levels and biochemical activity, indicating that post-transcriptional regulation of aromatase (e.g., protein phosphorylation, see 48) could be a primary mechanism for controlling aromatase (and thereby local oestrogen levels) in zebra finch telencephalon. Together with a lack of evidence for the regulation of androgen conversion enzymes during singing and audition, this study focuses attention for future work on the acute changes in the biochemical activity of the aromatase protein that are associated with singing behaviour in male songbirds.
Acknowledgments
We thank Ni Feng, Amnon Katz and Nicole Reamey for technical assistance, Stephanie White, Austin Hilliard and Mark Konishi for logistical support, and Art Arnold for access to the ABI 7300. Funding was provided by NINDS NRSA F32NS058009 and NIMH 061994.
References
- 1.Callard GV, Petro Z, Ryan KJ. Phylogenetic distribution of aromatase and other androgen-converting enzymes in central nervous-system. Endocrinology. 1978;103(6):2283–2290. doi: 10.1210/endo-103-6-2283. [DOI] [PubMed] [Google Scholar]
- 2.De Vries GJ, Simerly RB. Anatomy, development and function of sexually dimorphic circuits in the mammalian brain. In: Pfaff DW, editor. Hormones, brain and behavior. Elsevier; Amsterdam: 2002. [Google Scholar]
- 3.Forlano PM, Schlinger BA, Bass AH. Brain aromatase: New lessons from non-mammalian model systems. Frontiers in Neuroendocrinology. 2006;27(3):247–274. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Schlinger BA, Callard GV. Aromatase-activity in quail brain - correlation with aggressiveness. Endocrinology. 1989;124(1):437–443. doi: 10.1210/endo-124-1-437. [DOI] [PubMed] [Google Scholar]
- 5.Silverin B, Baillien M, Balthazart J. Territorial aggression, circulating levels of testosterone, and brain aromatase activity in free-living pied flycatchers. Horm. Behav. 2004;45(4):225–234. doi: 10.1016/j.yhbeh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Compaan JC, Wozniak A, Deruiter AJH, Koolhaas JM, Hutchison JB. Aromatase-activity in the preoptic area differs between aggressive and nonaggressive male house mice. Brain Res. Bull. 1994;35(1):1–7. doi: 10.1016/0361-9230(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 7.Black MP, Balthazart J, Baillien M, Grober MS. Socially induced and rapid increases in aggression are inversely related to brain aromatase activity in a sex-changing fish, lythrypnus dalli. P Roy Soc Lond B Bio. 2005;272(1579):2337–2344. doi: 10.1098/rspb.2005.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: How interactions between aromatase and the environment modulate aggression. Frontiers in Neuroendocrinology. 2006;27(2):170–179. doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146(9):3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portillo W, Castillo CG, Retana-Marquez S, Roselli CE, Paredes RG. Neuronal activity of aromatase enzyme in non-copulating male rats. J Neuroendocrinol. 2007;19(2):139–141. doi: 10.1111/j.1365-2826.2006.01513.x. [DOI] [PubMed] [Google Scholar]
- 11.Hallgren SLE, Linderoth M, Olsen KH. Inhibition of cytochrome p450 brain aromatase reduces two male specific sexual behaviours in the male endler guppy (poecilia reticulata) Gen. Comp. Endocrinol. 2006;147(3):323–328. doi: 10.1016/j.ygcen.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J. Neurosci. 2007;27(24):6563–6572. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nature Neuroscience. 2004;7(10):1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 14.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J. Comp. Neurol. 2000;423(4):619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Walters M, Collade D, Harding C. Oestrogenic modulation of singing in male zebra finches: Differential effects in directed and undirected songs. Anim. Behav. 1991;42:445–452. [Google Scholar]
- 16.Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J. Neurosci. 2007;27(44):12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain - an immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63(2):149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 18.Hojo Y, Hattori T, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WGM, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes p45017 alpha and p450 aromatase localized in neurons. Proc. Natl. Acad. Sci. U. S. A. 2004;101(3):865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings of the Royal Society B-Biological Sciences. 2005;272(1576):2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (coturnix-coturnix-japonica) brain. Neuroendocrinology. 1989;49(4):434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- 21.Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67(1):1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- 22.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nature Neuroscience. 2008;11(11):1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc. Natl. Acad. Sci. U. S. A. 1997;94(8):4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mello CV, Ribeiro S. Zenk protein regulation by song in the brain of songbirds. J. Comp. Neurol. 1998;393(4):426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Forstmeier W. Female resistance to male seduction in zebra finches. Anim. Behav. 2004;68:1005–1015. [Google Scholar]
- 26.Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase in adult zebra finch brain: Sex difference and rapid effect of stress. Endocrinology. 2004;145(4):1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- 27.Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase messenger-rna expression in the zebra finch brain. J. Comp. Neurol. 1995;360(1):172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- 28.Schlinger BA, Arnold AP. Plasma sex steroids and tissue aromatization in hatchling zebra finches - implications for the sexual-differentiation of singing behavior. Endocrinology. 1992;130(1):289–299. doi: 10.1210/endo.130.1.1727704. [DOI] [PubMed] [Google Scholar]
- 29.Doupe AJ, Konishi M. Song-selective auditory circuits in the vocal control-system of the zebra finch. Proc. Natl. Acad. Sci. U. S. A. 1991;88(24):11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147(1):359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 31.Van Duyse E, Pinxten R, Snoeijs T, Eens M. Simultaneous treatment with an aromatase inhibitor and an anti-androgen decreases the likelihood of dawn song in free-living male great tits, parus major. Horm. Behav. 2005;48(2):243–251. doi: 10.1016/j.yhbeh.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Soma KK, Sullivan KA, Tramontin AD, Saldanha CJ, Schlinger BA, Wingfield JC. Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. J. Comp. Physiol. A-Sens. Neural Behav. Physiol. 2000;186(7-8):759–769. doi: 10.1007/s003590000129. [DOI] [PubMed] [Google Scholar]
- 33.Soma KK, Tramontin AD, Wingfield JC. Oestrogen regulates male aggression in the non-breeding season. P Roy Soc Lond B Bio. 2000;267(1448):1089–1096. doi: 10.1098/rspb.2000.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huddleston GG, Paisley JC, Clancy AN. Effects of estrogen in the male rat medial amygdala: Infusion of an aromatase inhibitor lowers mating and bovine serum albumin-conjugated estradiol implants do not promote mating. Neuroendocrinology. 2006;83(2):106–116. doi: 10.1159/000094400. [DOI] [PubMed] [Google Scholar]
- 35.Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL. Presynaptic n-methyl-d-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J. Comp. Neurol. 2004;469(4):522–534. doi: 10.1002/cne.11035. [DOI] [PubMed] [Google Scholar]
- 36.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 37.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J. Neurosci. 2007;27(35):9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ers) originate from a single transcript: Studies of er alpha and er beta expressed in chinese hamster ovary cells. Mol. Endocrinol. 1999;13(2):307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 39.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a g protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 40.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J. Neurosci. 2004;24(26):5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J. Neurosci. 2007;27(5):1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pradhan DS, Yu Y, Soma KK. Rapid estrogen regulation of dhea metabolism in the male and female songbird brain. J Neurochem. 2008;104(1):244–253. doi: 10.1111/j.1471-4159.2007.04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: Multiple sites of action of sex steroid hormones. Frontiers in Neuroendocrinology. 2002;23(2):137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- 44.Barclay SR, Harding CF, Waterman SA. Central dsp-4 treatment decreases norepinephrine levels and courtship behavior in male zebra finches. Pharmacol Biochem Be. 1996;53(1):213–220. doi: 10.1016/0091-3057(95)00183-2. [DOI] [PubMed] [Google Scholar]
- 45.Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mrna in the forebrain of songbirds and nonsongbirds. J. Comp. Neurol. 1999;407(1):115–129. [PubMed] [Google Scholar]
- 46.George I, Hara E, Hessler NA. Behavioral and neural lateralization of vision in courtship singing of the zebra finch. J. Neurobiol. 2006;66(10):1164–1173. doi: 10.1002/neu.20273. [DOI] [PubMed] [Google Scholar]
- 47.Avey MT, Phillmore LS, MacDougall-Shackleton SA. Immediate early gene expression following exposure to acoustic and visual components of courtship in zebra finches. Behav. Brain Res. 2005;165(2):247–253. doi: 10.1016/j.bbr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Balthazart J, Baillien M, Ball GF. Interactions between kinases and phosphatases in the rapid control of brain aromatase. J Neuroendocrinol. 2005;17(9):553–559. doi: 10.1111/j.1365-2826.2005.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlinger B, Brenowitz EA. Neural and hormonal control of birdsong. In: Pfaff DW, editor. Hormones, brain and behavior. Elsevier; 2002. pp. 799–838. [Google Scholar]