Abstract
GABAergic neurotransmitter systems are important for many cognitive processes, including learning and memory. We identified a single neuron in each hemisphere of the Drosophila brain - the anterior paired lateral (APL) neuron - as a GABAergic neuron that broadly innervated the mushroom bodies. Reducing GABA synthesis in the APL neuron enhanced olfactory learning, suggesting that APL suppressed learning by releasing the inhibitory neurotransmitter GABA. Functional optical imaging experiments revealed that APL responded to both odor and electric shock stimuli presented to the fly with increases of intracellular calcium and released neurotransmitter. More importantly, a memory trace formed in the APL neuron by pairing odor with electric shock. This trace was detected as a reduced calcium response in APL after conditioning specifically to the trained odor. These results demonstrated a mutual suppression between the GABAergic APL neuron and olfactory learning, and functional neuroplasticity of the GABAergic system due to learning.
In both mammals and insects, the neurotransmitter γ-amino-butyric acid (GABA) plays important roles in learning and memory. For instance, the mammalian hippocampus is important for many types of learning and it is heavily innervated by GABAergic interneurons1. GABAAα5 is a GABAA receptor subunit highly expressed in the hippocampus and GABAAα5 knockout mice display enhanced performance in a match-to-place version of the water maze test2. In Drosophila, the GABAA receptor RDL is preferentially expressed in the mushroom bodies, a brain structure essential for olfactory learning3,4. Over expression of RDL in the mushroom bodies impairs olfactory learning while a knock down of RDL enhances learning. In addition, the level of RDL expression is inversely related to the calcium influx into mushroom body neurons that occurs when a fly is exposed to an odor4. These neuroanatomical, behavioral, and physiological observations indicate that the neuronal circuits mediating learning and memory are modulated by the inhibitory neurotransmitter GABA.
Other than the importance of the RDL receptor noted above, limited information exists about the GABAergic systems that modulate Drosophila mushroom bodies and learning. Electron microscopic experiments have shown that GABAergic processes make synaptic contact with both the antennal lobe projection neuron terminals as well as dendrites of the mushroom body intrinsic cells, the Kenyon cells. These contacts were observed in the mushroom body calyx, an area of the brain that contains the mushroom body dendrites and presynaptic terminals5. The critical questions that ensue from these observations include: (1) Where are the neuronal cell bodies of the GABAergic fibers that innervate the mushroom body neuropil? (2) Is there GABAergic input to the neuropil housing the axons of the mushroom bodies (the lobes) as well as the dendrites? (3) Are the GABAergic neurons responsive to the cues presented during learning? (4) Does learning alter the response properties of these neurons? If the response properties of the GABAergic neurons were blunted by learning, then this would indicate that learning may occur by inhibiting the inhibitory inputs to mushroom body neurons.
We have identified, in this study, the first GABAergic neuron that projected to and innervated the mushroom bodies and we have shown that this neuron innervated both the calyx and the lobes of the mushroom bodies. We showed that this neuron normally suppressed olfactory learning and that its activity was also suppressed by olfactory learning, thus indicating a mutually antagonistic relationship between this GABAergic neuron and the process of olfactory learning and memory.
RESULTS
APL is a GABAergic neuron innervating the mushroom bodies
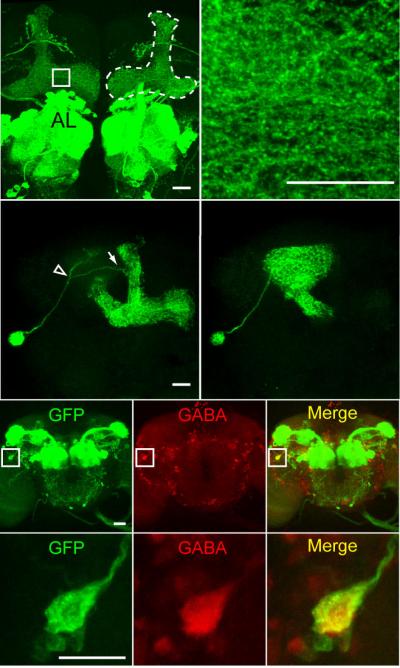
We started by carefully examining the expression pattern of a specific Gal4 driver line, GH146-Gal4. Although widely used as a Gal4 line with expression specific to the antennal lobe projection neurons 6-8, the GH146-Gal4 line also exhibited significant expression in the mushroom bodies (Fig. 1a), which has been previously reported8-10. The innervation of the mushroom bodies by neurons identified by GH146-Gal4 was observed as a complex reticulum throughout the lobes (Fig. 1b), which did not resemble the tightly packed bundles characteristic of Kenyon cells, suggesting a non-Kenyon cell origin. We performed clonal analysis8 using GH146-Gal4 to identify the origin of these neuronal processes. We crossed the GH146-Gal4 line to a heat shock FLP-out line to generate progeny carrying GH146-Gal4, UAS >CD2,y+ >CD8-GFP, and Hs-flp transgenes. A heat shock of proper duration and developmental timing will cause the excision of the >CD2,y+> cassette randomly in single neurons, such that the CD8-GFP construct becomes linked directly to the UAS sequence8, thus labeling different individual GH146-positive neurons in different animals. In total, we prepared and analyzed over 300 brains potentially containing a single cell GH146-Gal4 clone. Thirteen of the brains showed a single neuron in either hemisphere as the source of the ipsilateral mushroom body innervation. The cell body for this neuron was located lateral to the mushroom body calyx and near the lateral horn (Fig. 1c, d). This neuron was recently identified by two additional Gal4 lines from another study and named the mushroom body anterior paired lateral (MB-APL) neuron10. Although the APL neuron process was previously reported to enter the mushroom bodies through the calyx region10, our single cell clone analysis revealed that the APL neuron sent one projection dorsomedially towards the mushroom bodies, which bifurcated into two branches. One branch entered the mushroom bodies at the waist of the vertical lobes (Fig. 1c), and the other branch entered through the calyx (Fig. 1d). Since the APL processes formed a continuum of innervation throughout the mushroom body neuropil, we were unable to determine where these two branches met.
Figure 1.
The APL neuron innervating the mushroom body neuropil is GABAergic. (a) The GH146-Gal4 driver promotes expression of membranelocalized mCD8-GFP in the antennal lobes and the mushroom body lobes (outlined by dashed line for the right hemisphere). (b) Higher magnification image of the area marked by a square in (a) illustrating a complex punctate and reticular pattern of the innervation. (c, d) One example of a single cell clone showing the morphology of the APL neuron, as viewed from two different focal planes. The primary process of the APL neuron bifurcates (empty arrow head) prior to reaching the mushroom body neuropil into a branch that enters the vertical lobes (arrow) as shown in (c) and a branch that enters the mushroom body calyx as shown in (d). (e-g) Anti-GFP (e), anti-GABA (f) staining, and the merge (g) of GH146-Gal4 driving mCD8-GFP expression. (h-j) Higher magnification images of the areas marked by squares in (e-g) showing the cell body of the APL neuron. The only process observed from the APL cell body is the one that exits with a trajectory towards the mushroom body neuropil. Scale bars represent 20 μm in a, c, e, h and 10 μm in b.
The APL neuron projected to all compartments of the mushroom body neuropil, including the lobes, the peduncle, and the calces. No innervation of the Kenyon cell bodies was observed. This pattern of innervation matched precisely the expression pattern of the GABAA receptor RDL in the mushroom bodies4. In addition, the ramification pattern of the APL neuron in the calyx resembled the previously reported reticular anti-GABA staining pattern in the calyx5, identifying the APL neuron as a candidate for a GABAergic neuron that innervates the mushroom bodies. Immunohistochemical experiments using anti-GABA antibodies confirmed that the cell body of the APL neuron exhibited strong GABA immunoreactivity (Fig. 1e-j). Therefore, we concluded that the APL neuron was a GABAergic neuron that innervated the mushroom body neuropil.
Knocking down GABA synthesis in the APL enhances learning
Since the APL neuron innervated the mushroom bodies, we wondered whether this neuron might influence olfactory learning and memory. Of the three Gal4 lines known to exhibit expression in the APL neuron10, GH146-Gal4 is the most specific. Therefore, we focused our attention on this line for reducing GABAergic input into the mushroom bodies. We decided to use GH146-Gal4 to disrupt genes specific to GABA synthesis, since APL was a GABAergic neuron. Although GH146-Gal4 drives expression in a large number of antennal lobe projection neurons (Fig. 1a), these neurons are generally cholinergic7,11. This strategy should allow the relatively specific reduction of GABAergic inputs into the mushroom bodies from the APL neurons with minimal effects on other neurons that also express Gal4 in GH146-Gal4. Other options like expressing the temperature-sensitive Shibire12,13 with GH146-Gal4 would clearly have broad effects not specific to the APL neuron. We chose to knock down the expression of the key enzyme for GABA synthesis: glutamic acid decarboxylase (Gad)14 using RNA interference15.
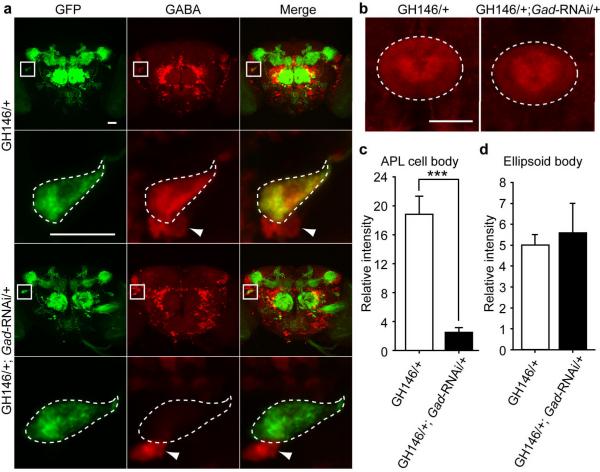
To confirm the tissue-specific knock down of GABA synthesis, we performed immunohistochemistry experiments to quantify the level of GABA immunoreactivity in the knock down and control animals. While the control flies with the GH146-Gal4 driver alone showed strong anti-GABA staining in the APL cell body, the GH146-Gal4 driving Gad-RNAi flies showed a significant reduction of GABA immunoreactivity in the same region (Fig. 2a). The smaller GABAergic cell bodies near the APL neuron were not affected, illustrating the cell specificity of the knock down (Fig. 2a). Since the locations, shapes and sizes of the smaller GABAergic neurons in the vicinity varied significantly among different samples, they were valuable only as a qualitative internal control. Thus we selected the GABAergic ellipsoid body16 region as a quantitative internal control (Fig. 2b). Quantification confirmed that Gad-RNAi driven by GH146-Gal4 decreased the GABA immunoreactivity in the APL cell body by 4-5 fold, but had no significant effect on GABA immunoreactivity observed in the ellipsoid body (Fig. 2c, d), indicating a relatively cell-type specific knock down of GABA synthesis.
Figure 2.
Tissue-specific knock down of GABA synthesis by driving a Gad-RNAi transgene. (a) Anti-GFP (left column), anti-GABA (middle column) staining, and merge of the two (right column) for brains of flies carrying the GH146-Gal4 driver alone (top two rows) or GH146-Gal4 driving an RNAi construct against glutamic acid decarboxylase (Gad) (bottom two rows). Rows 2 and 4 show higher magnification images of the APL cell body areas marked by squares highlighted in rows 1 and 3, respectively. The cell bodies of APL neurons are outlined by dashed lines in rows 2 and 4. Arrowheads indicate the cell bodies of nearby smaller GABAergic neurons that are not identified by the GH146-Gal4 driver. Some of the red fluorescence shown within the areas marked by squares in row 3 is not observable in row 4 because it is outside of the thinner average projection images of row 4 that encompass only the thickness of the APL cell body. (b) Anti-GABA staining of the ellipsoid body (dashed oval) of flies carrying the GH146-Gal4 driver alone (left) or the GH146-Gal4 driving Gad-RNAi (right). (c) Quantification of anti-GABA staining of the APL cell body, n = 16 samples per group. (d) Quantification of anti-GABA staining of the ellipsoid body, n = 8 samples per group. Means ± s.e.m.; ***P<0.001 (Student's t-test). Although not indicated on the figure, all flies depicted here carried one copy of UAS-spH (a pH sensitive GFP) on the second chromosome for visualizing the APL cell body and its processes. Scale bars represent 20 μm.
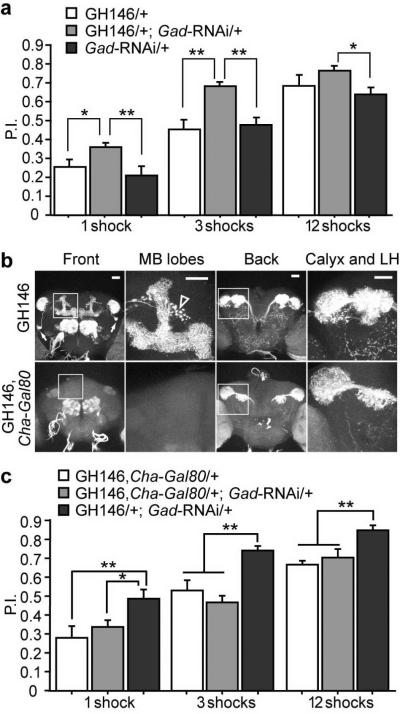
Flies expressing Gad-RNAi under the control of the GH146-Gal4 exhibited a significant enhancement of performance after olfactory classical conditioning (Fig. 3a). This result is consistent with our prior observation that knocking down the GABAA receptor RDL in the mushroom bodies enhances olfactory learning4, which supports the model that the APL neuron supplies the presynaptic GABA to the RDL receptor expressed on the mushroom body neurons. Control experiments showed no difference between the genotypes in their naïve responses to either the odors or electric shock used for training (Table 1). Since the antennal lobe projection neurons are predominantly cholinergic11, we reasoned that expression of the Gal80 gene, a suppressor of Gal4, under the control of a cholinergic neuron specific promoter should remove the projection neuron expression in the GH146-Gal4 line, so that we could potentially access the function of the APL neuron more directly. We combined the GH146-Gal4 driver line with three independent lines carrying Gal80 driven by the promoter of the choline acetyltransferase (Cha) gene13,17,18. Unexpectedly, instead of removing the reporter expression by the cholinergic projection neurons and leaving APL expression intact, all three Cha-Gal80 lines removed expression by the APL neuron together with a variable numbers of projection neurons (Supplementary Fig. 1). This indicated that all three Cha-Gal80 lines exhibited expression in some non-cholinergic neurons. We selected the line that removed the least projection neurons expression (Fig. 3b and Supplementary Fig. 1) and used it as a negative control for the behavioral tests. We found that the Gad-RNAi driven by the GH146-Gal4 alone enhanced learning, reproducing the observations shown in Fig. 3a, but that the Gad-RNAi driven by the combined GH146-Gal4, Cha-Gal80 driver, which eliminated APL neuron GAL4 activity, failed to enhance learning (Fig. 3c). These observations suggested that the knock down of Gad in the APL neuron was responsible for the enhanced learning. Two additional GABAergic neurons have been reported in the antennal lobes within the expression domain of the GH146-Gal4 driver7, and GAL4 activity in these neurons was also eliminated by Cha-Gal80 (data not shown. Therefore, we cannot completely rule out the less-likely possibility that these GABAergic neurons contributed to the phenotype. Our combined behavioral data were most consistent with the model that the APL neuron suppressed olfactory learning by releasing the inhibitory neurotransmitter GABA, which activated the GABAA receptor RDL postsynaptically in the mushroom bodies.
Figure 3.
Reducing GABA synthesis in the APL neuron enhances olfactory learning. (a) The flies carrying the GH146-Gal4 driver and the UAS-Gad-RNAi transgene exhibited an enhanced performance index (P.I.) after olfactory conditioning using 1, 3, or 12 electric shock pulses presented within a 1 min exposure to the conditioned odor. The P.I. of the Gad knock down group was not significantly higher than one control group, GH146/+, after 12 shocks of training. This was due to a ceiling effect as the P.I.s approached 1.0. (b) Expression pattern of mCD8-GFP driven by GH146-Gal4 alone (top row) or by the GH146-Gal4, Cha-Gal80 combined driver (bottom row). Both front and back views of the brains are shown, together with higher magnification images of the areas marked by squares in both views, illustrating the mushroom body lobes or the mushroom body calyx and the lateral horn (LH). The APL neuron cell bodies are marked by the arrows in the first panel of row 1. The punctate arc of fluorescence that is posterior to the vertical lobes shown in the second panel of row 1 (marked by the empty arrow head) is from an antennal lobe projection neuron previously characterized10,34. Note the loss of APL and mushroom body neuropil fluorescence by the introduction of Cha-Gal80. (c) Knock down of Gad by the combined GH146-Gal4, Cha-Gal80 driver failed to enhance learning, while knock down by GH146-Gal4 alone reproduced the enhanced learning shown in (a). n = 6 for each group under each condition. Means ± s.e.m.; *P<0.05; **P<0.01 (Fisher's PLSD). Scale bars represent 20 μm.
Table 1.
Normal naïve response of Gad knock down flies towards odors and electric shock
| Genotype | MCH avoidance | OCT avoidance | Shock avoidance |
|---|---|---|---|
| GH146/+ | 0.621±0.068 | 0.616±0.039 | 0.682±0.091 |
| Gad-RNAi/+ | 0.644±0.081 | 0.701±0.059 | 0.722±0.069 |
| GH146/+; Gad-RNAi/+ | 0.686±0.049 | 0.633±0.052 | 0.690±0.066 |
There were no statistically significant differences among any groups for each experiment. Means ± s.e.m.; n = 8 for each experiment.
APL responds to both odor and electrical shock stimuli
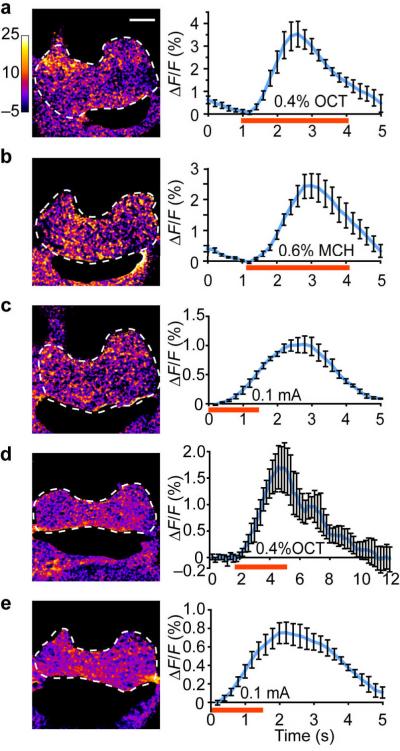
To probe the physiological role of the APL neuron during olfactory learning, we studied the APL neuron's response properties using functional optical imaging towards odors and electric shock used for conditioning. We expressed a calcium sensitive fluorescence reporter, G-CaPM1.619, using the GH146-Gal4 driver and recorded the response from the APL ramifications in the mushroom body horizontal lobes. The APL neuron showed an increased calcium response during the presentation of both odors (methylcyclohexanol [MCH] and 3-octanol [OCT]) at the concentrations used for the behavioral tests (Fig. 4a, b). We have also detected similar responses when we delivered electric shock stimuli to the abdomen of the flies (Fig. 4c).
Figure 4.
Calcium responses and neurotransmitter release of the APL neuron during odor or electric shock stimulation. Optical recordings were made from the APL innervation in the horizontal lobes of the mushroom bodies (circled by dashed lines). (a-c) Representative pseudo-color images taken at the peak response (average of 5 frames) and percent fluorescence change of group data across time during the presentation of 3-octanol (a), methylcyclohexanol (b) or electric shock (c), detected as calcium responses with G-CaMP driven by the GH146-Gal4 driver. (d, e) Representative pseudo-color images taken at the peak response (average of five frames) and percent fluorescence changes of group data across time during the presentation of 3-octanol (d) or electric shock (e), detected as neurotransmitter release using synapto-pHluorin (spH) driven by the GH146-Gal4 driver. The red bar under x-axis indicates the stimulation period. n = 5-8 trials. Means ± s.e.m. Scale bars represent 20 μm.
To determine whether this increase of intercellular calcium level translated into an increased firing rate of the APL neuron, we expressed a second reporter, synapto-pHluorin (spH), using the same GH146-Gal4 driver. SpH is a pH sensitive fluorescence reporter that has a synaptic vesicle targeting sequence and increased fluorescence during synaptic vesicle release20,21. Using the spH reporter, we were also able to detect responses during odor or shock presentation in the processes of the APL neuron (Fig. 4d, e). These results suggested that the APL neuron responded to both odors and electric shock by increasing intercellular calcium levels and releasing the neurotransmitter GABA.
APL exhibits a decreased response to trained odors
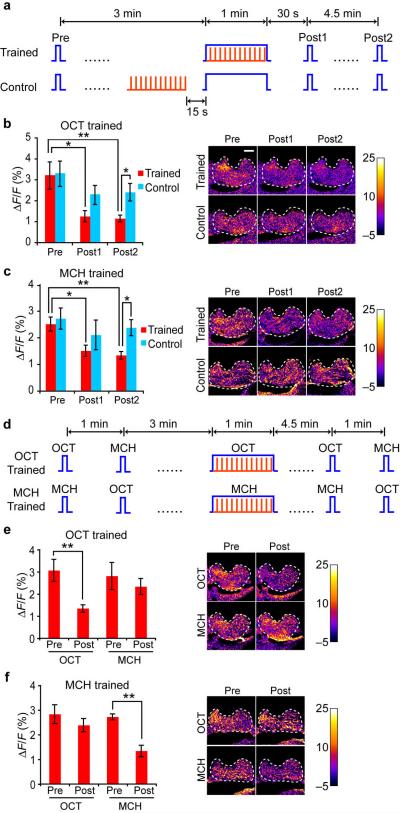
Since the APL neuron responded to both the odor and electric shock stimuli used for conditioning, an important question was whether the APL neuron showed any training-induced plasticity. In other words, did the APL neuron form a memory trace by changing its response properties towards the CS stimulus due to conditioning? We trained single flies under the microscope with odors and electric shock and measured the calcium response in the APL neuron before and after training (Fig. 5a). When we trained the flies with 1 min of OCT presented simultaneously with 12 electric shock pulses, the APL neuron showed a significantly reduced response to OCT at 30 s and 5 min after training. This decrease was due to the temporal pairing of the odor and shock stimuli rather than simple exposure to these stimuli, since the same odor and shock delivered 15 s apart failed to cause this decrease (Fig. 5b). We obtained a similar result using MCH as the odor (Fig. 5c), indicating that the decreased response was not specific to OCT as the trained odor.
Figure 5.
The APL neuron forms a memory trace of reduced calcium response for the conditioned odor after training. (a) Flies were exposed to 1 min odor simultaneously with 12 pulses of electric shock (Trained) or the same odor and shock but separated by 15 s (Control). The G-CaMP response for the odor was probed 3 min before (Pre), 30 s after (Post1), and 5 min after (Post2) the 1 min odor presentation. Each probe test lasted for 3 s. (b, c) Quantitative summary of group data and representative pseudo-color images of the groups treated as in (a) using 3-octanol (b) or methylcyclohexanol (c). Planned comparisons were made between each group at different time points, and between the two groups at each time point, with all statistically significant differences labeled. (d) Flies were differentially trained using one odor paired with shock and the responses to both odors used in the assay were tested at 3-4 min before (Pre) or 4.5-5.5 min after (Post) training. Tests to the alternative odors were separated by 1 min. (e, f) Quantitative summary and representative pseudo-color images of groups treated in (d) using 3-octanol (e) or methylcyclohexanol (f) as the conditioned odor. For (b) and (c), n = 7 in each group. Means ± s.e.m.; *P<0.05; **P<0.01 (paired Student's t-tests for within group, unpaired Student's t-tests for between group). For (e) and (f), n = 9 in each group. Means ± s.e.m.; **P<0.01 (paired Student's t-test). Scale bars represent 20 μm.
To study whether this decrease was specific to an odor paired with shock or whether it was generalized to a second odor presented during conditioning, we trained flies with one odor paired with electric shock and tested the APL responses to both the trained odor and the control odor, both before and after training (Fig. 5d). The APL neuron showed a significant decrease in response towards the trained odor, but not the control odor (Fig. 5e, f). Thus the APL neuron formed a memory trace manifested by a decreased response specifically towards the trained odor.
DISCUSSION
Using single neuron labeling techniques and immunohistochemistry, we identified the APL neuron within the GH146-Gal4 expression domain as the first GABAergic neuron that innervated the mushroom bodies of Drosophila. The innervation was surprisingly broad, with this single neuron accounting for GABAergic processes that extend across the complete three-dimensional volume of the calyx, peduncle, and lobes. Knocking down GABA synthesis in the APL neuron enhanced olfactory learning, indicating that the role of APL was to suppress olfactory learning by releasing the inhibitory neurotransmitter GABA. Functional optical imaging revealed that the APL neuron responded to both CS and US stimuli used for training. We further demonstrated that a memory trace registered as a reduced response specifically to the trained odor formed in the APL neuron after conditioning, suggesting that olfactory learning somehow suppressed the activity of this inhibitory neuron.
These observations meshed well with our prior observations made from altering the expression level of the RDL receptor in the mushroom bodies4. We discovered that over expression of RDL in the mushroom bodies inhibits learning, whereas reducing RDL expression in the mushroom bodies enhances learning, similar to the effect of reducing GABA synthesis in the APL neuron. Furthermore, the calcium responses to odor observed in the mushroom body neurons of flies that over express RDL are reduced, whereas the responses observed in flies with reduced expression of RDL are increased. Thus, increased learning is observed by either reducing RDL expression in the mushroom body neurons, or by decreasing GABA synthesis in the APL neuron that innervates the mushroom body neuropil. The logical conclusion is that the APL neuron provides the GABAergic input to the RDL receptor expressed on the mushroom body neurons, and that this neurotransmitter:receptor dynamic establishes the probability for learning to occur.
GABAergic feedback neurons projecting to the mushroom bodies have been reported in the honeybees22. The morphology of these feedback neurons and their innervation patterns in the mushroom bodies are similar to the Drosophila APL neuron described here. Pairing an odor with a sucrose reward induces a decreased spike activity in the GABAergic feedback neurons towards the trained odor shortly after training23, similar to the decreased response observed by optical imaging in the APL neuron after training. These observations suggest that the APL neuron in Drosophila might be the equivalent of the honeybee GABAergic feedback neurons. The processes of the GABAergic feedback neurons in the mushroom body lobes of the honeybee are considered to be postsynaptic and their processes in the mushroom body calyces are considered to be presynaptic. However, the processes of the APL neuron in the mushroom body lobes of Drosophila clearly contained presynaptic specializations, since synaptic vesicle release was observed from these processes by functional imaging (Fig. 4d, e). Thus, the functional relationship between the Drosophila APL neuron and the Apis GABAergic feedback neurons remains uncertain.
Functional optical imaging experiments have revealed multiple memory traces formed after olfactory conditioning in different areas of the Drosophila brain21,24-29. The APL neuron memory trace was unique compared to previously described traces, since it was registered as a decrease rather than an increase of neuronal activity. This is not surprising given that the APL neuron releases the inhibitory neurotransmitter GABA. However, an important issue is raised by the combined observations. Is the increased activity in the mushroom bodies after training inducing the decreased activity in the APL neuron, or is the later serving as a permissive event for the former to take place? Temporally, the APL memory trace we observed here forms within a similar time window as the early memory trace recently reported to form in the α'/β' mushroom body neurons25, so these two scenarios remain equally possible. Another more complicated scenario is that these memory traces could form synergistically and in parallel rather than sequentially, since many insect neurons have mixed axons and dendrites and communicate bi-directionally with connected neurons30.
The APL neuron exhibited a depression in activity after training that was specific to the trained odor compared to a control odor. The mechanism underlying this specificity is unclear. One of the simpler possibilities is that the APL neuron is both pre- and post-synaptic to mushroom body neurons, similar to models proposed for the dorsal paired medial (DPM) neuron29. Training may produce a synaptic depression at the synapses between mushroom body neurons conveying the information about the trained odor and the postsynaptic APL neuron, but not at synapses between mushroom body neurons conveying information about other odors and the postsynaptic APL neuron. Such depression would reduce the activity of the APL neuron specifically to the trained odor. This depression of APL activity to the trained odor would also be registered as increased activity in the mushroom body neurons representing the trained odor, since the mushroom body neurons would then receive reduced inhibitory signals from the APL neuron acting presyaptically. A second possibility is that the increased activity of the mushroom body neurons conveying information about the trained odor might induce retrograde signaling causing a depression in specific APL presynaptic, inhibitory fibers. Recent studies of endocannabinoid-mediated hippocampal metaplasticity have revealed that focal stimulation of CA1 pyramidal neurons triggers a long-term depression at inhibitory synapses (I-LTD) restricted to a very small dendritic area (~10 μm), mediated by the postsynaptic release of endocannabinoid that binds to the presynaptic CB-1 receptor on the inhibitory neuron presynaptic terminals31. It remains unknown whether a similar retrograde signaling system exists in flies to mediate a similar effect, although a Ca2+ and synaptotagmin 4 dependent retrograde signaling mechanism has been discovered at the Drosophila neuromuscular junction that functions in a synapse-specific fashion32. If selective suppression of inhibitory inputs exists in the central nervous system of Drosophila, then it may serve as a novel mechanism to code and store information in the brain.
METHODS
Fly lines
We cultured flies on standard medium at 25 °C, 60% relative humidity and a 12-hour light/dark cycle. We out-crossed flies used for behavior tests to the w(CS10) (Canton-S flies carrying the w1118 mutation) background, which was used as a wild-type control. The FLP-out line used in the clonal analysis was a gift from R. Axel8. The Gad-RNAi line was originally from the Vienna Drosophila RNAi Center (VDRC)15, Transformant ID 32344. We used three Cha-Gal80 lines, #117, #218, and #313 in our studies, all of which were generated by fusing the 3.3 kb upstream promoter region of Cha with the Gal80 coding region. The three transgenes are inserted at different chromosomal locations. The UAS-G-CaMP1.6 line was a gift from D. Reiff19. The UAS-spH line was a gift from G. Miesenböck20.
Clonal analysis
We crossed the GH146-Gal4 line to a FLP-out line to generate progeny carrying GH146-Gal4, UAS >CD2,y+ >CD8-GFP and Hs-flp. We heat shocked a mixed population of third instar larvae and early pupae at 32 °C for 20 min to label single neuronal clones with CD8-GFP.
Immunohistochemistry
We dissected and fixed fly brains and incubated them at 4 °C overnight with primary antibody. We obtained antibodies from the following suppliers and used at these dilutions: 1:100 for rabbit anti-GFP (Molecular Probes, A11122), 1:100 for rat anti-mCD8 (CALTAG Laboratories, MCD0800), 1:50 for rabbit anti-GABA (Sigma, A2052) and 1:100 for mouse anti-GFP (Molecular Probes, A11120).
Olfactory conditioning
Drosophila olfactory conditioning followed an olfactory classical conditioning paradigm33. Briefly, we exposed flies sequentially to two odors (methylcyclohexanol [MCH] and 3-octanol [OCT]) for 1 min each. Only the first odor (CS+) was paired with electric shock pulses (US). Immediately after this training, we loaded the flies into a T-maze where they made a choice between two arms, each containing one of the two odors. The flies' avoidance of the odor previously paired with shock was calculated as the performance index (P.I.), which was the number of flies that responded correctly minus the number of flies that responded incorrectly, divided by total number of the flies. To eliminate naïve odor bias, each trial was composed of two simultaneous half trials, where we trained one group to associate MCH with shock and the other to associate OCT with shock, and the complete P.I. was the average of these two half P.I.s. We varied the number of shocks used in the training as previously described4 to measure the memory strength as a function of different training intensities.
Functional optical imaging
We performed functional optical imaging as previously described4. Briefly, we immobilized a fly in a pipette tip and cut a window from the cuticle of the fly head and covered it with a piece of transparent plastic wrap to expose the dorsal brain for imaging. We performed imaging under a Leica TCS SP5 confocal microscope using a 20× objective. We dissolved odorants in mineral oil and delivered them using a computerized controller, which switched between delivering an air stream mixed with air wafted over either mineral oil alone (baseline control) or mineral oil containing odorants. The flow rate was 100 ml/min. We delivered constant AC current of 0.1 mA to the fly abdomen using a custom-made platinum electrode at 1.25 s/pulse and 12 pulse/min. We took an imaging time series at a rate of about 0.2 s/frame, and then temporally smoothed it using a sliding window of size 5 to bin consecutive frames. We calculated the baseline fluorescence (F0) by averaging the 3-5 bins just prior to odor or shock delivery. We divided the change of fluorescence (ΔF) for each time point by F0 to calculate the percentage fluorescence change (ΔF/F). We prepared pseudo-color images according to the percentage change on a pixel-by-pixel basis. We performed all of these analyses using a custom-made plug-in (Response View) for the NIH ImageJ software.
Statistical analysis
We performed statistical analyses using StatView software (SAS Institute Inc.). For comparisons among multiple groups, we performed oneway ANOVA followed by planned pairwise comparisons between the relevant groups with Fisher's PLSD test. For comparison between two groups, we used paired Student's t-tests for the same animals before and after treatment, and unpaired Student's t-tests for comparisons between different groups.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Axel, D. Reiff, G. Miesenböck, A. Ferrús, and H. Nash for various fly stocks, L. Griffith for constructive suggestions, and W. Krause for assistance in functional optical imaging. This work was supported by NIH grant NS19904 to R.L.D. and the R. P. Doherty-Welch Chair in Science at the Baylor College of Medicine.
REFERENCES
- 1.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Collinson N, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J. Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison JB, et al. Immunocytochemical mapping of a C-terminus antipeptide antibody to the GABA receptor subunit, RDL in the nervous system in Drosophila melanogaster. Cell Tissue Res. 1996;284:269–278. doi: 10.1007/s004410050587. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Krause WC, Davis RL. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron. 2007;56:1090–1102. doi: 10.1016/j.neuron.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuyama K, Meinertzhagen IA, Schürmann FW. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J. Comp. Neurol. 2002;445:211–226. doi: 10.1002/cne.10155. [DOI] [PubMed] [Google Scholar]
- 6.Schwaerzel M, Heisenberg M, Zars T. Extinction antagonizes olfactory memory at the subcellular level. Neuron. 2002;35:951–960. doi: 10.1016/s0896-6273(02)00832-2. [DOI] [PubMed] [Google Scholar]
- 7.Jefferis GS, et al. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 9.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J. Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J. Comp. Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 11.Yasuyama K, Meinertzhagen IA, Schürmann FW. Synaptic connections of cholinergic antennal lobe relay neurons innervating the lateral horn neuropile in the brain of Drosophila melanogaster. J. Comp. Neurol. 2003;466:299–315. doi: 10.1002/cne.10867. [DOI] [PubMed] [Google Scholar]
- 12.McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- 13.Kitamoto T. Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. Proc. Natl. Acad. Sci. USA. 2002;99:13232–13237. doi: 10.1073/pnas.202489099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Küppers B, Sánchez-Soriano N, Letzkus J, Technau GM, Prokop A. In developing Drosophila neurones the production of gamma-amino butyric acid is tightly regulated downstream of glutamate decarboxylase translation and can be influenced by calcium. J. Neurochem. 2003;84:939–951. doi: 10.1046/j.1471-4159.2003.01554.x. [DOI] [PubMed] [Google Scholar]
- 15.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 16.Hanesch U, Fischbach K-F, Heisenberg M. Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res. 1998;257:343–366. [Google Scholar]
- 17.Acebes A, Grosjean Y, Everaerts C, Ferveur JF. Cholinergic control of synchronized seminal emissions in Drosophila. Curr. Biol. 2004;14:704–710. doi: 10.1016/j.cub.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Keene AC, et al. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Reiff DF, et al. In vivo performance of genetically encoded indicators of neural activity in flies. J. Neurosci. 2005;25:4766–4778. doi: 10.1523/JNEUROSCI.4900-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 21.Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- 22.Grünewald B. Morphology of feedback neurons in the mushroom body of the honeybee, Apis mellifera. J. Comp. Neurol. 1999;404:114–126. doi: 10.1002/(sici)1096-9861(19990201)404:1<114::aid-cne9>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Grünewald B. Physiological properties and response modulations of mushroom body feedback neurons during olfactory learning in the honeybee, Apis mellifera. J. Comp. Physiol. A. 1999;185:565–576. [Google Scholar]
- 24.Liu X, Davis RL. Insect olfactory memory in time and space. Curr. Opin. Neurobiol. 2006;16:679–685. doi: 10.1016/j.conb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Mamiya A, Chiang AS, Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J. Neurosci. 2008;28:4368–4376. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry J, Krause WC, Davis RL. Olfactory memory traces in Drosophila. Prog. Brain Res. 2008;169:293–304. doi: 10.1016/S0079-6123(07)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr. Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 28.Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 29.Yu D, Akalal DB, Davis RL. Drosophila α/β mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–855. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burrows M. The Neurobiology of an Insect Brain. Oxford Univ. Press; New York, USA: 1996. [Google Scholar]
- 31.Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- 33.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 34.Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.