Abstract
During pregnancy, VEGF (vascular endothelial growth factor) regulates in part endothelial angiogenesis and vasodilation. Herein we examine the relative roles of VEGFR (VEGF receptors) and associated signaling pathways mediating the effects of VEGF165 on eNOS (endothelial nitric oxide synthase) activation. Despite equal expression levels of VEGFR-1 and VEGFR-2 in UAEC (uterine artery endothelial cells) from NP (nonpregnant) vs P (pregnant) sheep, VEGF165 activates eNOS at a greater level in P- vs NP-UAEC, independently of Akt activation. The selective VEGFR-1 agonist PlGF-1 (placental growth factor) elicits only a modest activation of eNOS in P-UAEC compared to VEGF165, while the VEGFR-2 kinase inhibitor blocks VEGF165-stimulated eNOS activation, suggesting VEGF165 predominantly activates eNOS via VEGFR-2. Although VEGF165 also activates ERK-1/2 (extracellular-signal-regulated kinase), this is not necessary for eNOS activation since U0126 blocks ERK-1/2 phosphorylation but not eNOS activation, and VEGFR-2 kinase inhibitor inhibits eNOS activation but not ERK-1/2 phosphorylation. Furthermore, the inability of PlGF to activate ERK-1/2 and the ability of the VEGFR-2 selective agonist VEGF-E to activate ERK-1/2 and eNOS suggest again that both eNOS and ERK-1/2 activation occur predominately via VEGFR-2. The lack of VEGF165-stimulated Akt phosphorylation is consistent with a lack of robust phosphorylation of Ser-1179-eNOS. Although VEGF165-stimulated eNOS phosphorylation is observed at Ser-617 and Ser-635, pregnancy does not significantly alter this response. Our finding that VEGF165 activation of eNOS is completely inhibited by wortmannin but not LY294002 implies a downstream kinase, possibly a wortmannin-selective PI3-kinase, is acting between the VEGFR-2 and eNOS independently of Akt.
Keywords: VEGFR1, VEGFR2, ERK1/2, pregnancy, endothelium, nitric oxide synthase
INTRODUCTION
During pregnancy, blood flow to the uterus is increased dramatically to meet the rising demands of the growing fetus. Endocrine control of this phenomenon is complex but includes in part the action of many growth factors [1,2]. Vascular endothelial growth factor (VEGF) in particular is known to promote angiogenesis and also can promote activation of eNOS (endothelial nitric oxide synthase) in uterine artery endothelium. This phenomenon is observed in endothelial cells isolated from the uterine vasculature in the nonpregnant state, but is also substantially increased in a form of programmed adaptation during pregnancy [3].
Whereas pregnancy is characterized by dramatic increases in uterine blood flow, the state of preeclampsia is characterized by a noticeable lack or partial reversal of such adaptation, as well as a breakdown of the integrity of the vasculature at the maternal-fetal interface [4]. While the clinical presentation of fully developed preeclampsia is undoubtedly the result of a number of sequential endocrine and metabolic failures, VEGF excess has been reported to originate from the placenta itself and can clearly contribute to the negative symptoms of increased vascular permeability and hypercoagulation [5-7]. So the dilemma that arises is that VEGF may be beneficial to and a normal part of pregnancy adaptation underlying increased uterine blood flow, but an excess may be a part of the origin of preeclampsia, or at least contribute to the decline in the clinical situation. More recent studies have challenged this model. The role of VEGF itself has been challenged by the detection of sFlt-1 (soluble Fms related tyrosine kinase), the soluble form of VEGFR- (VEGF receptor) 1, and of abnormalities in circulating PlGF (placental growth factor) in diseased pregnancies [8]. Nonetheless, there are several technical issues regarding sample collection and assay of VEGF family peptides [9] that have not always been controlled for, and circulating sFlt-1 itself may affect both free-form levels of circulating VEGF family members and directly or indirectly affect expression of VEGFR isoforms [10,11]. This shift in focus away from VEGF may therefore be misplaced, and due consideration should be given to the possibility that the detrimental actions of sFlt-1 on the uterine endothelium may in fact be due to its combined potential to create an imbalance of locally produced members of the VEGF family and the receptors that respond to them.
To fully evaluate possible rescue of dysfunctional endothelium based on control of uteroplacental VEGF and the function of its locally expressed receptors, we must first understand the basis of normal VEGF action that is beneficial to pregnancy. More specifically, we must establish which receptors and associated signaling pathways are mediating the beneficial effects of VEGF165 and how they are enhanced in pregnancy. Such studies are relevant not only to our understanding of uterine blood flow, but also to the general field of VEGF-stimulated cell signaling mechanisms since, in contrast to findings in the widely studied HUVEC (human umbilical vein endothelial cell) [12,13] or bovine aortic endothelial cell [10,14] models, UAEC (uterine artery endothelial cells) achieve eNOS activation in response to ATP [15] and herein VEGF165, independently of Akt activation. Our focus on VEGF also contrasts the recently highly characterized response to ATP [15-20] in the same cells or vessels, which is mediated through a heterotrimeric G-protein coupled P2Y2 receptor, coupled in turn to PLC (phospholipase C) β3/Ca2+ signaling and the MEK/ERK [MAPK (mitogen activated protein kinase)/ERK (extracellular-signal-regulated) kinase] signaling pathway. The action of VEGF165, which signals in part through the intrinsic tyrosine kinase capability of its receptors, gives no consistent or substantial change in Ca2+ but is still able to mediate activation of the MEK/ERK-1/2 pathway [16,18]. In spite of these differences, both VEGF and ATP are capable of eNOS activation, which is further enhanced by pregnancy [16,18]. From a cell-signaling standpoint alone, a comparison of the effects of VEGF to that previously described for ATP in this unique endothelial cell model is of interest. From a pregnancy standpoint, an understanding of the integration of the control of eNOS activation is also essential before any therapy can be considered for endothelial dysfunction.
EXPERIMENTAL
Materials
VEGF165 and PlGF-1 were purchased from R&D Systems (Minneapolis, MN). Recombinant Orf virus VEGF-E was purchased through Angio-Proteomie (Boston, MA). Wortmannin and VEGFR tyrosine kinase inhibitor {4-[(4′-chloro-2′-fluoro)phenylamino]-6,7-dimethoxyquinazoline, a reasonably selective inhibitor of VEGFR-2 over VEGFR-1; IC50 = 100nM and 2μM, respectively} were purchased from EMD Chemicals (San Diego, CA). LY294002 was purchased from Cell Signaling Technology (Danvers, MA). U0126 was purchased from Promega Corp. (Madison, WI). All cell culture reagents were from Invitrogen (Carlsbad, CA), and all other chemicals were from Sigma (St. Louis, MO) unless indicated.
Cell culture
Uterine arteries were obtained from mixed Western breed nonpregnant sheep and pregnant ewes at 120–130 d of gestation during nonsurvival surgery, and UAEC were prepared by collagenase dispersion as described previously [16]. Cells were frozen at the end of passage 3 and stored in liquid nitrogen. Procedures for animal handling and protocols for experimental procedures were approved by the University of Wisconsin-Madison Research Animal Care Committees of both the School of Medicine and Public Health and the College of Agriculture and Life Sciences and followed the recommended American Veterinary Medicine Association guidelines for humane treatment and euthanasia of laboratory farm animals.
eNOS activation assay
The activity of eNOS was determined by incubation of cells with or without agonist (10 min) in the presence of [3H]arginine, followed by extraction and application of samples to AG 50W-X8 cation-exchange resin (Na+ form; Bio-Rad Laboratories, Hercules, CA) as described previously [15,21].
Western analyses
Treatment of cells on 60 mm dishes and subsequent Western blotting of extracted proteins were as described previously [15-17]. Analysis of VEGFR-1 and VEGFR-2 was on 6.5% polyacrylamide gels with 20 μg protein per lane; all other gels were 7.5% polyacrylamide with 15 μg protein per lane. VEGFR-1 was detected using anti-Flt-1 (1:100; #05-696, Millipore Corp., Billerica, MA) and rabbit anti-mouse HRP (horseradish peroxidase)-conjugated F(ab)2 (1:1250; #AQ160P, Millipore Corp.) VEGFR-2 was detected on separate membranes using Flk-1 (1:500; #sc-505, Santa Cruz Biotechnology Inc., Santa Cruz, CA) and goat anti-rabbit HRP-conjugated secondary antibody (1:2000; Cell Signaling Technology). Phosphorylation of proteins specified below was detected by use of phosphorylation-state specific antibodies; goat anti-rabbit HRP-conjugated secondary antibody (Cell Signaling Technology) was used for all. Phosphorylation of eNOS (Ser-1179, Thr-497, Thr-617, and Thr-635) was detected on 4 separate membranes using the following antibodies: eNOS Ser-1179 (Cell Signaling Technology) 1:750, 2° 1:3300; eNOS Thr-495 (Millipore Corp.) 1:3300, 2° 1:3300; eNOS Ser-617 (Millipore Corp.) 1:2000, 2° 1:4000; eNOS Ser-635 (Millipore Corp.) 1:10,000, 2° 1:4000. The blots were then frozen and blocked. Phosphorylation of ERK 1/2 and Akt were then measured using the following antibodies: ERK 1/2 Thr-202/Tyr-204 (Cell Signaling Technology) 1:2500, 2° 1:3000; Akt Ser-473 (Cell Signaling Technology) 1:750, 2° 1: 3000. To ensure consistent protein loading, all receptor and phosphorylation-state specific antibodies were normalized to levels of total Akt (Cell Signaling Technology; 1:1000, 2° 1:2000) or to Hsp 90 (ABR-Affinity BioReagents, Golden, CO; 1:5000, 2° 1:5000). For all proteins, specific binding was detected by enhanced chemiluminescence reagent detection system (Amersham Pharmacia). Hyperfilm was scanned using the HP Deskscan system (Hewlett-Packard, Palo Alto, CA) and bands quantified using Molecular Analyst (version 1.4, Bio-Rad Laboratories, Inc).
Statistical analyses
The data were analyzed by ANOVA or Student’s t test where appropriate. Results were considered significant at P < 0.05.
RESULTS
Previous immunocytochemistry studies demonstrated that both VEGFR-1 and VEGFR-2 are expressed by UAEC and VEGF can stimulate eNOS activation, ERK phosphorylation, and mitogenesis [16,18]. Western analysis shown herein further demonstrates that both VEGFR-1 and VEGFR-2 are expressed in equal amounts in NP- (nonpregnant) and P- (pregnant) UAEC (Figure 1). The ∼250 kDa bands detected here are similar to that reported previously for VEGFR-1 [22] and VEGFR-2 [23]. In recent studies of human placenta and ovine placental endothelial cells and tissues, the neuropilin-1 receptor was also identified by Western blot [24,25], but use of the same antisera on UAEC did not detect a similar protein (not shown).
Figure 1. Expression of VEGFR-1 and VEGFR-2 proteins in UAEC.
Untreated NP-UAEC (n = 8) and P-UAEC (n = 5), as well as HUVEC-CS (C) were grown to confluence in T75 flasks and then solubilized in lysis buffer as described; Western analysis with 20 μg of protein per sample on 6.5% polyacrylamide gels was performed as indicated in Materials and Methods. The lower VEGFR-1 and the VEGFR-2 bands were at 250 kDa. Representative Western blots for VEGFR-1, VEGFR-2, and total eNOS and Akt are shown. Graph represents mean ± SE with data normalized to total Akt protein and expressed as fold of the average P-UAEC level of expression.
Although there are several VEGF family peptides, we have previously only used the VEGF165 form, the predominant form expressed in ovine placenta [26], which activates both VEGFR-1 and VEGFR-2. Another agonist of interest is PlGF-1 because it is capable of activating VEGFR-1 but not VEGFR-2 (see [27]). Using VEGF165, we were able to extend our previous observations to show a time-dependent and pregnancy-enhanced activation of eNOS in UAEC (Figure 2A). In addition, whereas both NP- and P-UAEC show dose-dependent activation of eNOS by VEGF165, notably PlGF-1 was unable to reproduce this response in NP-UAEC and only stimulated a modest response starting at 10 ng/ml in P-UAEC (Figure 2B). The implication that VEGFR-2 predominantly mediates VEGF165 activation of eNOS is strengthened by the finding that the inhibitor of VEGFR-2 kinase was able to block the response with precisely the expected dose-dependency to near basal levels in P-UAEC (Figure 2C). Of interest, the inhibitor completely blocked eNOS activation in NP-UAEC, but with altered dose dependency (IC50 = 0.09 and 0.7 uM for P- and NP-UAEC, respectively).
Figure 2. Assessment of VEGF165-stimulated eNOS activity in UAEC.
A) Time course of VEGF165-stimulated eNOS activity. NP- and P-UAEC were treated with VEGF165 (10 ng/ml) for the times indicated, and activity was assessed as described in Materials and Methods. Data are expressed as fold of same-time unstimulated controls. Values shown are mean ± SE of n = 4–5 independent experiments (*, P < 0.05 relative to 0 control; #, P< 0.05 vs same NP-UAEC response). B) Dose dependency of eNOS activity after stimulation for 10 min with VEGF165 or PlGF-1 in ng/ml equivalent doses. Data are expressed as fold of unstimulated control. Values shown are mean ± SE of n = 4 independent experiments (*, P < 0.05 relative to control; #, P< 0.05 relative to same NP-UAEC response; ** indicates both P and NP at that dose of VEGF165 are different relative to control, P < 0.05). C) Effect of VEGFR-2 inhibitor on VEGF165-stimulated eNOS activation. NP-and P-UAEC were pretreated with VEGFR-2 inhibitor at doses given for 5 min, followed by treatment with VEGF165 (10 ng/ml) for 10 min. Data are expressed as percent of the VEGF response without inhibitor. Values shown are mean ± SE of n = 4–6 independent experiments (*, P < 0.05 relative to VEGF control without inhibitor; #, P< 0.05 relative to same P-UAEC response).
We have previously described in the UAEC model the activation of ERK-1/2 kinase signaling in response to ATP and VEGF, and further that eNOS is phosphorylated at multiple sites in response to ATP in a manner further enhanced by pregnancy [15,28]. Like ATP, VEGF165 is capable of stimulating a time- and dose-dependent phosphorylation of ERK-1 and ERK-2 in a manner consistent with eNOS activation (Figure 3A and B). Of note however, a causal link between ERK-1/2 phosphorylation and eNOS activation is immediately challenged by the finding that the VEGFR-2 inhibitor, which blocks eNOS activation (Figure 2C, above), does not block ERK-1/2 phosphorylation in NP- or P-UAEC when used over the same dose range (see Supplementary Figure S1). Furthermore, although the MEK inhibitor U0126 can dose-dependently block the activation of ERK-1/2 (Figure 4A), it is not an inhibitor of VEGF-stimulated eNOS activation (Figure 4B), and indeed to some degree mildly enhances the response, as was previously observed for ATP [15]. To examine the possible direct coupling of VEGFR-2 and VEGFR-1 to both eNOS activation and the MEK/ERK-1/2 pathway, we further examined the effects of VEGF-E and PlGF-1. VEGF-E, an isoform that selectively activates VEGFR-2 [29,30], was capable of ERK-1/2 activation with expected dose dependency [29-31] and with greater effect in P- vs NP-UAEC (Figure 5A). The magnitude of ERK-1/2 activation achieved at 100 ng/ml equivalents of VEGF-E was equal to that for VEGF165 at 10 ng/ml. In contrast, PlGF alone achieved no activation of ERK-1/2 and, combined with VEGF-E at 10 ng/ml equivalents, showed no synergy over that achieved by 10ng/ml equivalent VEGF-E alone.
Figure 3. Phosphorylation of ERK 1 and ERK 2 in response to VEGF165 stimulation in UAEC.
A) Time course of VEGF-stimulated phosphorylation of ERK 1/2. NP- and P-UAEC were treated with 10 ng/ml VEGF165 for the times given, and phospho-specific Western analysis of ERK 1/2 was performed; representative blots of VEGF-stimulated samples are shown (no difference in same-time controls was determined; blots not shown). Data were normalized to total Akt protein and expressed as fold of same-time unstimulated control. Values shown are mean ± SE of n = 4 independent experiments (*, P < 0.05 relative to 0 control). B) Dose-dependent response of ERK 1/2 phosphorylation to VEGF stimulation. NP- and P-UAEC were treated for 10 min with VEGF165 at doses indicated and phospho-specific Western analysis of ERK 1/2 was performed; representative blots are shown. Data were normalized to total Akt protein and expressed as fold of unstimulated control. Values shown are mean ± SE of n = 4–6 independent experiments (*, P < 0.05 relative to control; #, P < 0.05 relative to same P-UAEC response).
Figure 4. Effect of U0126 on VEGF165 responses in UAEC.
A) Dose-dependent response of VEGF165-stimulated ERK 1/2 phosphorylation to U0126. P-UAEC were treated with U0126 at doses given for 20 min followed by VEGF165 treatment (10 ng/ml) for 10 min, and phospho-specific Western analysis of ERK 1/2 was performed; representative blots are shown. Data were normalized to total Akt protein and expressed as percent of the VEGF response without inhibitor. Values shown are mean ± SE of n = 4 independent experiments (*, P < 0.05 relative to VEGF control without inhibitor). B) Effect of U0126 on VEGF165-stimulated eNOS activation. NP-and P-UAEC were pretreated with U0126 (10 uM) for 20 min followed by VEGF165 treatment (10 ng/ml) for 10 min. Data are expressed as percent of the VEGF response without inhibitor. Values shown are mean ± SE of n = 4 independent experiments (*, P < 0.05 relative to VEGF control without inhibitor).
Figure 5. Dose-dependent effects of VEGF-E in UAEC.
NP- and P-UAEC were treated for 10 min with VEGF-E at doses indicated as well as with 10 ng/ml VEGF165, 10 ng/ml PlGF, or 10 ng/ml VEGF-E with 10 ng/ml PlGF (E+PlGF; for VEGF-E and PlGF, ng/ml equivalents were used). Values shown are mean ± SE of n = 4–6 independent experiments (*, P < 0.05 relative to control; #, P < 0.05 relative to same NP-UAEC response; ‡, P < 0.05 for E+PlGF vs PlGF alone). A) Dose-dependent response of ERK 1/2 phosphorylation to VEGF-E stimulation. Phospho-specific Western analysis of ERK 1/2 was performed, and representative blots are shown. Data were normalized to total Hsp90 protein and expressed as fold of unstimulated control. B) Dose-dependent response of eNOS activation to VEGF-E stimulation. Data are expressed as fold of unstimulated control.
Regarding eNOS activation (Figure 5B), VEGF-E activated eNOS in both NP- and P-UAEC at a magnitude at 100ng/ml equivalents that was comparable to VEGF165 at 10 ng/ml. In contrast, PlGF alone did not stimulate a significant rise in eNOS activity in NP-UAEC but gave rise to a small response in P-UAEC. The combination of VEGF-E and PlGF as above showed a small but significant increase in P-UAEC alone above that by PlGF alone, which was not significantly different from VEGF-E alone at the same dose. A comparison of the dose dependency of VEGF-E on ERK-1/2 and eNOS activation showed some discrepancy, which again suggests that ERK-1/2 activation is not the basis for eNOS activation and further that while both responses may be VEGFR-2 mediated, the VEGFR-2 signaling events are not the same in each case.
Another pathway often implicated in eNOS activation in a number of endothelial cell studies is the phosphatidylinositol 3 (PI3)-kinase/Akt pathway. We have previously shown that ATP does not couple to this pathway in UAEC [15,17] and that the PI3 kinase inhibitor LY294002 does not block ATP-stimulated activation of eNOS [15]. Herein we also report that VEGF165 has no time- or dose-dependent effect on phosphorylation of Akt (see Supplementary Figure S2), and this pathway is unaffected by the VEGFR-2 kinase inhibitor (not shown). Although LY294002 was able to reduce the level of phosphorylated Akt below basal (see Supplementary Figure S3A), the effect of LY294002 on eNOS activity was slight and only achieved significance at 1 and 10 uM LY294002 in NP- and P-UAEC, respectively (see Supplementary Figure S3B). This small degree of inhibition was, however, not nearly as effective as the VEGFR-2 kinase inhibitor (Figure 2C).
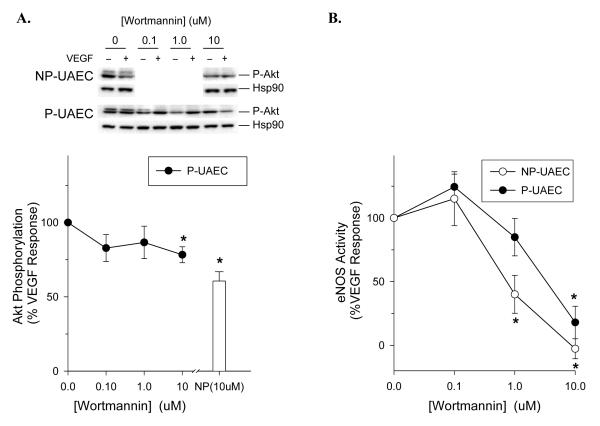
Although it is clear Akt does not play a major role in the activation of eNOS by VEGF or ATP, the question of a possible role for PI3-kinase remains. LY294002 has been shown both here and previously to be at best a modest inhibitor of eNOS activation in UAEC, but another reported PI3-kinase inhibitor, wortmannin, has been shown to be an effective inhibitor of ATP-stimulated eNOS activation in both NP-and P-UAEC [15]. The effect of wortmannin on Akt phosphorylation (Figure 6A) was similar in magnitude to that of LY294002 (Supplementary Figure S3A), but challenge of VEGF165-stimulated eNOS activation with wortmannin resulted in significant inhibition in both NP and P-UAEC (Figure 6B). Once again there was a slight difference in the dose dependency between NP- and P-UEAC, but effective inhibition was seen in both cases. To establish if this relates to altered eNOS protein phosphorylation, we first examined VEGF165-mediated changes in eNOS phosphorylation at positions Ser-1179, Thr-497, Ser-617, and Ser-635. VEGF promoted a slow and small fold change in Ser-1179 phosphorylation, no detectable change in Thr-497 phosphorylation, and more rapid (within 10 min) changes in phosphorylation above basal at positions Ser-617 and Ser-635 (see Supplementary Figure S4). Of note, PlGF failed to stimulate phosphorylation of any of these sites in NP- and P-UAEC (data not shown). Although we previously showed the ATP-stimulated phosphorylation levels at positions Ser-617, Ser-635, and Ser-1179 were higher in P-UAEC [15], no significant difference was observed between NP- and P-UAEC in response to VEGF165. When the challenge with VEGF165 was then repeated with wortmannin present, phosphorylation at Ser-1179 in P-UAEC was reduced at 10 uM wortmannin, and phosphorylation at Ser-617 was reduced up to 50% in a dose-dependent manner (Figure 7). In NP-UAEC, significant inhibition of phosphorylation was also observed at both Ser-1179 and Ser-617 to 50% of control. Nonetheless, it should be noted that the effect of VEGF alone on eNOS phosphorylation is at best modest, and the effect of wortmannin co-treatment on phosphorylation levels in the presence of VEGF was similar to the effect of the same dose of wortmannin alone (data not shown). Thus an apparent inhibitory effect of even 50% magnitude, although significant, may be of little physiological relevance.
Figure 6. Effect of wortmannin on VEGF165 responses in UAEC.
A) Dose-dependent effect of wortmannin on Akt phosphorylation with VEGF. NP- and P-UAEC were treated with wortmannin at doses given for 20 min followed by VEGF165 treatment (10 ng/ml) for 10 min, and phospho-specific Western analysis of Akt was performed; representative blots are shown. Data were normalized to total Hsp90 protein and expressed as percent of the VEGF response without inhibitor. Values shown are mean ± SE of n = 4 independent experiments (*, P < 0.05 relative to VEGF control without inhibitor). B) Dose-dependent effect of wortmannin on VEGF-stimulated eNOS activation. NP-and P-UAEC were pretreated with wortmannin at the doses shown for 20 min followed by VEGF165 treatment (10 ng/ml) for 10 min. Data are expressed as percent of the VEGF response without inhibitor. Values shown are mean ± SE of n = 4 independent experiments (*, P < 0.05 relative to VEGF control without inhibitor).
Figure 7. Dose-dependent effect of wortmannin on eNOS phosphorylation.
NP- and P-UAEC were pretreated with wortmannin at doses given for 20 min followed by VEGF165 (10 ng/ml) treatment for 10 min, and phospho-specific Western analyses of eNOS at sites A) Ser-1179, B) Thr-497, C) Ser-617, and D) Ser-635 were performed; representative blots are shown. Data were normalized to total Hsp90 protein and expressed as percent of the VEGF165 response without inhibitor. Values shown are mean ± SE of n = 4 independent experiments (*, P < 0.05 relative to VEGF165 control without inhibitor; #, p < 0.05 relative to P-UAEC at same dose).
DISCUSSION
From a pregnancy-specific standpoint, VEGF is known to be a product of both the uterus [32] and the placenta [24] and is critical for uteroplacental angiogenesis [2]. VEGF165 production in particular is also known to increase during critical periods of pregnancy in humans and sheep [33,34]. VEGF165 gene expression in the placenta may in part be driven by local hypoxia [7]. Of interest, pregnancy is also associated with higher levels of estrogen [35], which may also regulate expression of VEGFR isoforms in uterine tissues including vascular endothelium [36] and may act on UAEC to stimulate VEGF production and capillary tube-like formation (R. R. Magness, personal communication). Thus, VEGF may act both alone and as a partial mediator of estrogen action to control blood flow through the uteroplacental unit. The need for a greater understanding of the role of VEGF in control of vascular function in normal pregnancy and diseases of pregnancy is clear, yet reports of alterations in levels or isoforms of VEGF, PlGF-1, or sFlt-1 in preeclamptic pregnancy show major quantitative differences which may be due to methodologic considerations [9]. Bearing that in mind, studies such as those of Polliotti et al. [37] and Levine et al. [8] have attempted to confirm that circulating VEGF peptide family member levels are altered in pregnancy, or that isoforms of VEGF or PlGF-1 are altered in preeclamptic pregnancy. Nonetheless, it is absolutely clear that the hypoxia common to many pregnancy-related disorders (including preeclampsia and intrauterine growth retardation) leads to an increase in placental production of VEGF family peptides and probably sFlt-1 [7,38]. Thus while methodologic difficulties and interaction with sFlt-1 continue to make it difficult to reliably determine if the levels of bioactive VEGF family proteins actually rise in the circulation, local levels of these peptide hormones are elevated at the uteroplacental interface and, more importantly, are altered relative to each other. The increases in sFlt-1 may also alter relative VEGFR subtype expression in endothelium, changing the relative level of VEGFR-2 vs VEGFR-1 and/or neuropilin-1 [10,11]. Either way such studies suggest normal cell function requires a balance of VEGF family peptides relative to VEGFR proteins which, if disturbed, will likely lead to inappropriate signaling inside the cell and altered physiologic control. Despite the ongoing debate on the possible changing levels of VEGF family peptides, a fundamental understanding of the positive vs negative effects of VEGF and its associated receptors in the uterine circulation requires us to first know what the ‘normal’ response to VEGF is and how pregnancy normally augments that response at the receptor and post-receptor level. It is therefore vital that we clarify the specific points of cell signaling that discriminate eNOS activation at the level of uterine artery endothelium itself and understand how it is potentiated in pregnancy. The UAEC model gives us that opportunity.
In several previous studies we have shown that one important mechanism by which the uterine artery acquires altered function is through the reprogramming of cell signaling at the level of the endothelium in order to achieve a potentiated vasodilator response to a number of different agonists. Of note, responses to several G-protein coupled receptors and growth factor receptors in UAEC are altered [16-18], suggesting changes have occurred at the post-receptor level. Responses to ATP are perhaps the best characterized in UAEC to date. We have previously shown that responses to ATP appear to couple to eNOS activation in a manner predominantly mediated by a P2Y2 receptor mediated Ca2+-sensitive mechanism [16-20] and to a lesser extent by a Ca2+-independent, wortmannin-sensitive but LY294002-insensitive mechanism [15]. ATP can also stimulate the MEK/ERK-1/2 pathway in a manner potentiated by pregnancy, but this is not necessary for eNOS activation and may even exert a mildly inhibitory influence [15], perhaps through direct phosphorylation of an ERK consensus sequence on eNOS at position Thr-97 [39]. Another surprise, and a unique feature of UAEC compared to other endothelial models [10,12-14], is that Akt is not a mediator of eNOS activation. Even expression of constitutively active Akt failed to stimulate eNOS activity in UAEC and did not overcome the inhibitory effect of wortmannin, either alone or in the presence of ATP [15].
Our studies herein focused upon VEGF action and extend our previous finding that VEGFR-1 and VEGFR-2 are both expressed in UAEC, and further determine that the neuropilin-1 receptor is undetectable in UAEC. Furthermore, UAEC derived from pregnant ewes do not show altered levels of VEGFR-1 and VEGFR-2 receptor protein expression relative to those from nonpregnant ewes. Given also that UAEC from NP and P ewes utilized in these studies at passage 4 have similar levels of eNOS protein [16], we must conclude that enhanced cell responses in P-UAEC in response to VEGF165 compared to NP-UAEC are due to altered post-receptor signaling rather than receptor levels, and that at least some of the signaling pathways that are enhanced in P-UAEC are similar to those enhanced in response to ATP [15]. Comparing the effects of VEGF165 with that of VEGFR-1-selective PlGF-1 show clearly that PlGF can elicit a small eNOS activation in P-UAEC alone, but VEGF165 is by far the most effective activator of eNOS in NP- and P-UAEC. This combined with the inhibitory action of the VEGFR-2 kinase inhibitor on eNOS activity in both NP- and P-UAEC suggests that VEGFR-2 has a predominant role in the eNOS activation process in response to VEGF165. Further examination of the effects of the VEGFR-2-selective isoform VEGF-E confirms that activation of VEGFR-2 alone is capable of leading to eNOS activation; and the lack of synergy with the combination of a submaximal dose of VEGF-E with PlGF suggests co-activation of VEGFR-1 is not necessary for the maximal response to the VEGFR-2 selective agonist. Nonetheless, VEGF165 is not VEGFR-2 selective in vivo, and our other data suggests that a role for VEGFR-1 in response to VEGF165 cannot be ignored. The fact we did see some small degree of eNOS activation in response to PlGF in P-UAEC confirms PlGF does activate the VEGFR-1 receptor and can contribute to eNOS activation, and the fact this is not seen in NP-UAEC confirms VEGFR-1 function/coupling may change in pregnancy. Indeed other subtle differences in the dose-dependency of inhibition of VEGF165-stimulated activation by the kinase inhibitor allude to a subtle change between the NP and P state. This dose-dependency of the inhibitor in the P-UAEC is consistent with the literature for this compound, whereas that in NP-UAEC is somewhat shifted to the right. It is interesting to note that at doses of VEGFR inhibitor of 0.1 uM and greater, the response of P-UAEC, expressed as fold of control, was similar to NP-UAEC. A change in VEGFR-2 receptor dimerization or interaction with other proteins including VEGFR-1 is a possible explanation for this response difference that will require further study.
It is clear from studies of other endothelial cell models that VEGFR-2 is capable of coupling to the MEK/ERK-1/2 pathway [27] which, given our implication of VEGFR-2 in eNOS activation in UAEC, begs the question of whether ERK-1/2 activation mediates eNOS activation in UAEC. While the ability of both VEGF165 and VEGF-E (but not PlGF) to robustly activate both eNOS and ERK-1/2 in NP-and P-UAEC may imply that activation of eNOS by VEGF165 requires the ERK-1/2 pathway, further studies clearly refute that. Although the VEGFR-2 kinase inhibitor blocks eNOS activation by VEGF165 in both NP- and P-UAEC, it has no effect on ERK-1/2 in either NP- or P-UAEC. In addition, the agent U0126, which blocks ERK-1/2 phosphorylation, fails to inhibit eNOS activation and indeed mildly potentiates it to similar levels in both NP- and P-UAEC. Combined, these data suggest that although ERK-1/2 and eNOS activation are mediated by the same receptor, the VEGFR-2 signaling events leading to ERK-1/2 activation and to eNOS activation are distinct. While the exact nature of these distinct receptor-mediated events requires further study, it is clear that activation of ERK-1/2 by VEGFR-2 is not sufficient to explain activation of eNOS, so further signaling pathways responsible for eNOS activation need to be considered, along with their potential activation in pregnancy. Interestingly, studies using site-directed mutagenesis of VEGFR-2 have already suggested the autophosphorylation site Tyr-801 is necessary for eNOS activation in bovine aortic endothelial cells [14], yet it is not the same as the autophosphorylation sites commonly associated with ERK-1/2 activation or indeed PLC-γ recruitment [40]. Such studies would have predicted, since both sites are autophosphorylation sites on VEGFR-2, that we should have seen equal inhibition of eNOS and ERK-1/2 responses by the VEGFR-2 kinase inhibitor. Certainly the dose dependency for the inhibition of eNOS in P-UAEC was exactly as expected and in NP-UAEC was only slightly shifted, so it is reasonable to assume the compound was indeed inhibiting autophosphorylation of VEGFR-2. The fact we did not see similar inhibition of VEGFR-2 mediated ERK-1/2 activation suggests that while ERK-1/2 activation is coupled to VEGR-2, it may not be through the ‘traditional’ recruitment of PLC-γ and/or other adaptor proteins. Clearly such possibilities warrant further consideration in this most unusual cell model.
Others [13] have suggested VEGFR-1 couples to eNOS activation in HUVEC via the PI3 kinase/Akt pathway, yet our studies herein suggest no such coupling of the VEGF165 response to the Akt pathway in UAEC, and this is consistent with our former report for no clear role for Akt in response to ATP [15].We also see no clear requirement for Ser-1179 phosphorylation in the activation of eNOS in response to VEGF, consistent again with the response to ATP [15]; the lack of these responses reinforce the uniqueness of the UAEC model. The PI3-kinase inhibitor LY294002 is ineffective in substantially blocking eNOS activation in response to VEGF, and we confirmed that the inhibitor did indeed lower basal phosphorylated Akt levels in the cell, with no parallel effect seen on the ERK-1/2 pathway until used at much higher doses (ERK-1 inhibited at less specific dose of 100 uM; data not shown). The intriguing finding, as previously observed for responses to ATP [15], was that another widely used PI3-kinase inhibitor, wortmannin, was an effective inhibitor of eNOS activation and actually could fully inhibit the response to VEGF165 in both NP- and P-UAEC. A discrepancy in action between LY294002 and wortmannin on various cell responses has previously been reported in many other cell systems [41-44]. The extent of inhibition by wortmannin in UAEC was greater for the response to VEGF165 than was seen previously for ATP. The question that immediately arises is what is the true target for wortmannin? Clearly it is not acting by preventing Akt phosphorylation and associated activation. Also, wortmannin did not affect VEGF-stimulated ERK-1/2 activity (data not shown). Although both wortmannin and LY294002 are both known reported inhibitors of PI3-kinase (phosphatidylinositol 3-kinase), we must ask if this kinase is the real target in UAEC. In view of the dose dependency of the wortmannin effects observed, an unidentified target of wortmannin is possible, as wortmannin has been reported to alter several pathways that are PI3-kinase-independent [42,43,45,46]. Alternatively, since the known mechanisms of action of these two drugs to inhibit PI3-kinase are different, it also follows that an alternate, wortmannin-sensitive, LY294002-insensitive isoform of PI-3 kinase may be involved in eNOS activation and may mediate this effect independently of the Akt pathway. Indeed, the class II PI-3 kinase β isoform, which demonstrates sensitivity to wortmannin and resistance to LY294002 [47,48], is a downstream target of activated growth factor receptors [49]. Our data herein suggests whatever the target, it is clearly important in UAEC to eNOS activation and its function may be considerably enhanced by pregnancy. It is interesting to note that the above-mentioned autophosphorylated residue Tyr-801 on VEGFR-2 necessary for eNOS activation in bovine aortic endothelial cells has been reported to signal through the PI3-kinase pathway independently of PLCγ. [14]. Further studies will be necessary to determine if this residue is in fact playing a pregnancy-enhanced role in UAEC to directly or indirectly recruit specific wortmannin-sensitive isoforms of the PI3-kinase family that signal independently of Akt.
A final ongoing question in the area of regulation of eNOS activity in general and pregnancy enhancement of this response in UAEC in particular is the phosphorylation not only of position Ser-1179, but of other sites on eNOS and their relationship to NOS activity. A number of former studies in UAEC have agreed that in response to ATP, eNOS phosphorylation occurs at positions Ser-1179, Ser-617, and Ser-635, and these events are further enhanced in P-UAEC [15,28], In contrast, phosphorylation of position Thr-497 is largely unaffected by ATP and not enhanced in P-UAEC. We have also found in UAEC, as well as in the ovine eNOS cDNA as studied by site-directed mutagenesis, that there is no clear relationship between any one or any pair of sites and eNOS activation [15,28]. It is possible, and even highly likely, that the influence of such sites on activity is simply indirect through altering the surface charge in general, or by targeting the protein to different locations and/or coactivators and so making activation possible. In the case of ATP stimulation, this lack of a key role for phosphorylation is understandable in view of the additional substantial and pregnancy-enhanced elevation of intracellular Ca2+, which is another known activator of eNOS [15,17-20]. However, VEGF does not bring about such a generalized and substantial elevation of Ca2+ [16-18] and, consistent with this, wortmannin is able to only partially inhibit the eNOS activation response to ATP, but more completely inhibit the response to VEGF. In view of the potential differences, we have examined the effects of VEGF on eNOS phosphorylation. While PlGF has no effect on phosphorylation of these sites, we find that time-dependent changes in phosphorylation of Ser-1179, Ser-617, and Ser-635 are indeed observed. Nonetheless, there is not so clear a difference between the effects of VEGF165 on eNOS phosphorylation in NP- vs P-UAEC in contrast to that previously observed in response to ATP [15,28]. Most interesting of all, however, is the finding that wortmannin, which so effectively inhibits VEGF-stimulated eNOS activity in a dose-dependent manner, only shows comparable dose-dependent inhibition of the Ser-617 site in P-UAEC. A causal link between phosphorylation and activity is therefore possible, but is not clearly established and will require further confirmation.
In summary, we have investigated in detail the potential role for VEGFR-1 and VEGFR-2 in mediating activation of eNOS in UAEC and its enhancement by pregnancy. The relative weakness of PlGF-1 in eliciting an eNOS response in P-UAEC, combined with the effectiveness of the VEGFR-2 kinase inhibitor to block VEGF165 stimulated eNOS activation in both NP-and P-UAEC, suggests any positive effect of VEGF165 on activation is largely mediated via the VEGFR-2 subclass, which is further confirmed by the stimulatory effect of VEGF-E. While VEGFR-2 may play the dominant role in eNOS activation, a less critical modulatory role of VEGFR-1 may occur in pregnancy and certainly cannot be ruled out by the current studies. There is no evidence that VEGF165 couples to Akt phosphorylation as a means of controlling eNOS activity, and this is consistent with a lack of any strong evidence for activation via robust phosphorylation at position Ser-1179 in NP- or P-UAEC. Although VEGFR-2 appears to couple to the MEK/ERK-1/2 signaling pathway, MEK/ERK-1/2 is not the mediator of eNOS activation, and the independent mechanism by which VEGFR-2 activates the MEK/ERK-1/2 pathway requires further study. Our finding herein that VEGF165 activation of eNOS can also be completely inhibited by wortmannin but not LY294002 implies a downstream kinase, possibly a wortmannin-selective PI3-kinase, is acting between the VEGFR-2 kinase and eNOS itself, but functions independently of Akt. A link between eNOS activation and eNOS phosphorylation at position 617 is possible, but by no means proven.
In conclusion, we have now substantially clarified the nature of the VEGF receptors that are present in UAEC and how they couple VEGF165 stimulation to eNOS activation. Clearly both VEGFR-1 and VEGFR-2 are present, yet VEGFR-2 plays the major role in activation of eNOS in a manner that is distinct from activation of ERK-1/2. While the involvement of VEGFR-2 in eNOS activation is not unique to UAEC, the possible pregnancy-induced role of VEGFR-1 in modulating VEGFR-2 action, and the distinctly different signaling events that mediate eNOS activation in UAEC continue to contrast the findings of studies in other endothelial cell models. Furthermore, our findings for VEGF stimulation of eNOS activity agree with our own recent reports for ATP stimulation, that eNOS activation is not mediated solely through the phosphorylation of a single or pair of eNOS phosphorylation sites [15,28]. While the former response to ATP may be in large part due to P2Y2 receptor mediated Ca2+ mobilization secondary to activation of PLC-β3, this is certainly not the case for responses to VEGF acting through its distinctly different class of receptors. In addition, the effects of wortmannin vs LY294002 on responses to VEGF and ATP suggest an as yet unknown regulatory mechanism plays a major role in controlling eNOS, and changes in this signaling pathway may be the basis of pregnancy-enhanced eNOS activation in UAEC. An understanding of this mechanism may well provide an ability to restore normal vascular function necessary for optimal fetal growth and development that is otherwise impaired in conditions such as preeclampsia. Knowledge of such a mechanism may also have direct relevance to the broader area of vascular function and provide novel therapeutic targets to resist the effects of aging on cardiovascular function in both men and women.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Terrance Phernetton for assistance in animal preparation. This work was supported by Grants HL64601, HL079020, HL49210, and HD38843. This paper reports part of the studies of J.S. towards a PhD in the University of Wisconsin Endocrinology Reproductive Physiology Training Program.
This work was undertaken using funding from the NIH and as such is required to be made publicly available within 12 months of publication. This must either be achieved by the journal or by uploading of the accepted manuscript to PubMed Central by the authors.
Grants supporting this work: HL64601, HL079020, HL49210 and HD38843
Abbreviations used
- ERK
extracellular-signal-regulated kinase
- eNOS
endothelial nitric oxide synthase
- HRP
horseradish peroxidase
- HUVEC
human umbilical vein endothelial cell
- MEK
MAPK (mitogen activated protein kinase)/ERK kinase
- NP
nonpregnant
- P
pregnant
- PI3-kinase
phosphatidylinositol 3-kinase
- PLC
phospholipase C
- PlGF
placental growth factor
- sFlt
soluble Fms related tyrosine kinase
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- UAEC
uterine artery endothelial cells
REFERENCES
- 1.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. doi:10.1038/35025215 [doi] [DOI] [PubMed] [Google Scholar]
- 2.Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol. Reprod. 2001;64:1033–1040. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- 3.Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R245–58. doi: 10.1152/ajpregu.00108.2002. doi:10.1152/ajpregu.00108.2002 [doi]; 284/2/R245 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Brown MA. The physiology of pre-eclampsia. Clin. Exp. Pharmacol. Physiol. 1995;22:781–791. doi: 10.1111/j.1440-1681.1995.tb01937.x. [DOI] [PubMed] [Google Scholar]
- 5.Hayman R, Brockelsby J, Kenny L, Baker P. Preeclampsia: The endothelium, circulating factor(s) and vascular endothelial growth factor. J. Soc. Gynecol. Investig. 1999;6:3–10. doi:S1071-5576(98)00044-6 [pii] [PubMed] [Google Scholar]
- 6.Murakami Y, Kobayashi T, Omatsu K, Suzuki M, Ohashi R, Matsuura T, Sugimura M, Kanayama N. Exogenous vascular endothelial growth factor can induce preeclampsia-like symptoms in pregnant mice. Semin. Thromb. Hemost. 2005;31:307–313. doi: 10.1055/s-2005-872437. doi:10.1055/s-2005-872437 [doi] [DOI] [PubMed] [Google Scholar]
- 7.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J. Clin. Endocrinol. Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. doi:jc.2005-0078 [pii]; 10.1210/jc.2005-0078 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. doi:10.1056/NEJMoa031884 [doi]; NEJMoa031884 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Jelkmann W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin. Chem. 2001;47:617–623. [PubMed] [Google Scholar]
- 10.Kou R, SenBanerjee S, Jain MK, Michel T. Differential regulation of vascular endothelial growth factor receptors (VEGFR) revealed by RNA interference: Interactions of VEGFR-1 and VEGFR-2 in endothelial cell signaling. Biochemistry. 2005;44:15064–15073. doi: 10.1021/bi0509898. doi:10.1021/bi0509898 [doi] [DOI] [PubMed] [Google Scholar]
- 11.Krysiak O, Bretschneider A, Zhong E, Webb J, Hopp H, Verlohren S, Fuhr N, Lanowska M, Nonnenmacher A, Vetter R, Jankowski J, Paul M, Schonfelder G. Soluble vascular endothelial growth factor receptor-1 (sFLT-1) mediates downregulation of FLT-1 and prevents activated neutrophils from women with preeclampsia from additional migration by VEGF. Circ. Res. 2005;97:1253–1261. doi: 10.1161/01.RES.0000194324.29363.82. doi:01.RES.0000194324.29363.82 [pii]; 10.1161/01.RES.0000194324.29363.82 [doi] [DOI] [PubMed] [Google Scholar]
- 12.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. doi:10.1038/21224 [doi] [DOI] [PubMed] [Google Scholar]
- 13.Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, le Noble F, Wang L, Mukhopadhyay D, Ahmed A. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ. Res. 2006;99:715–722. doi: 10.1161/01.RES.0000243989.46006.b9. doi:10.1161/01.RES.0000243989.46006.b9. [DOI] [PubMed] [Google Scholar]
- 14.Blanes MG, Oubaha M, Rautureau Y, Gratton JP. Phosphorylation of tyrosine 801 of vascular endothelial growth factor receptor-2 is necessary for akt-dependent endothelial nitric-oxide synthase activation and nitric oxide release from endothelial cells. J. Biol. Chem. 2007;282:10660–10669. doi: 10.1074/jbc.M609048200. doi:10.1074/jbc.M609048200. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan JA, Grummer MA, Yi FX, Bird IM. Pregnancy-enhanced endothelial nitric oxide synthase (eNOS) activation in uterine artery endothelial cells shows altered sensitivity to Ca2+, U0126, and wortmannin but not LY294002--evidence that pregnancy adaptation of eNOS activation occurs at multiple levels of cell signaling. Endocrinology. 2006;147:2442–2457. doi: 10.1210/en.2005-0399. doi:en.2005-0399 [pii]; 10.1210/en.2005-0399 [doi] [DOI] [PubMed] [Google Scholar]
- 16.Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J, Magness RR. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology. 2000;141:1107–1117. doi: 10.1210/endo.141.3.7367. [DOI] [PubMed] [Google Scholar]
- 17.Di T, Sullivan JA, Magness RR, Zhang L, Bird IM. Pregnancy-specific enhancement of agonist-stimulated ERK-1/2 signaling in uterine artery endothelial cells increases ca(2+) sensitivity of endothelial nitric oxide synthase as well as cytosolic phospholipase A(2) Endocrinology. 2001;142:3014–3026. doi: 10.1210/endo.142.7.8278. [DOI] [PubMed] [Google Scholar]
- 18.Gifford SM, Cale JM, Tsoi S, Magness RR, Bird IM. Pregnancy-specific changes in uterine artery endothelial cell signaling in vivo are both programmed and retained in primary culture. Endocrinology. 2003;144:3639–3650. doi: 10.1210/en.2002-0006. [DOI] [PubMed] [Google Scholar]
- 19.Gifford SM, Yi FX, Bird IM. Pregnancy-enhanced store-operated Ca2+ channel function in uterine artery endothelial cells is associated with enhanced agonist-specific transient receptor potential channel 3-inositol 1,4,5-trisphosphate receptor 2 interaction. J. Endocrinol. 2006;190:385–395. doi: 10.1677/joe.1.06773. doi:190/2/385 [pii]; 10.1677/joe.1.06773 [doi] [DOI] [PubMed] [Google Scholar]
- 20.Gifford SM, Yi FX, Bird IM. Pregnancy-enhanced Ca2+ responses to ATP in uterine artery endothelial cells is due to greater capacitative Ca2+ entry rather than altered receptor coupling. J. Endocrinol. 2006;190:373–384. doi: 10.1677/joe.1.06635. doi:190/2/373 [pii]; 10.1677/joe.1.06635 [doi] [DOI] [PubMed] [Google Scholar]
- 21.Cale JM, Tsoi SC, Toppe M, Grummer MA, Ochiai M, Magness RR, Bird IM. Molecular cloning of ovine endothelial nitric oxide synthase and expression in COS-7 cells. J. Soc. Gynecol. Investig. 2005;12:156–168. doi: 10.1016/j.jsgi.2004.11.006. doi:S1071-5576(04)00321-1 [pii]; 10.1016/j.jsgi.2004.11.006 [doi] [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Shibuya M. The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 1997;14:2079–2089. doi: 10.1038/sj.onc.1201047. doi:10.1038/sj.onc.1201047. [DOI] [PubMed] [Google Scholar]
- 23.Nadeau S, Baribeau J, Janvier A, Perreault T. Changes in expression of vascular endothelial growth factor and its receptors in neonatal hypoxia-induced pulmonary hypertension. Pediatr. Res. 2005;58:199–205. doi: 10.1203/01.PDR.0000169969.18669.D2. doi:10.1203/01.PDR.0000169969.18669.D2. [DOI] [PubMed] [Google Scholar]
- 24.Chung JY, Song Y, Wang Y, Magness RR, Zheng J. Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2004;89:2484–2490. doi: 10.1210/jc.2003-031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsoi SC, Wen Y, Chung JY, Chen D, Magness RR, Zheng J. Co-expression of vascular endothelial growth factor and neuropilin-1 in ovine feto-placental artery endothelial cells. Mol. Cell. Endocrinol. 2002;196:95–106. doi: 10.1016/s0303-7207(02)00190-9. doi:S0303720702001909 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Cheung CY, Singh M, Ebaugh MJ, Brace RA. Vascular endothelial growth factor gene expression in ovine placenta and fetal membranes. Am. J. Obstet. Gynecol. 1995;173:753–759. doi: 10.1016/0002-9378(95)90335-6. doi:0002-9378(95)90335-6 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Zachary I. VEGF signalling: Integration and multi-tasking in endothelial cell biology. Biochem. Soc. Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. doi:10.1042/ [doi] [DOI] [PubMed] [Google Scholar]
- 28.Cale JM, Bird IM. Dissociation of endothelial nitric oxide synthase phosphorylation and activity in uterine artery endothelial cells. Am J Physiol Heart Circ Physiol. 2006;290:H1433–H1445. doi: 10.1152/ajpheart.00942.2005. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa S, Oku A, Sawano A, Yamaguchi S, Yazaki Y, Shibuya M. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J. Biol. Chem. 1998;273:31273–31282. doi: 10.1074/jbc.273.47.31273. [DOI] [PubMed] [Google Scholar]
- 30.Meyer M, Clauss M, Lepple-Wienhues A, Waltenberger J, Augustin HG, Ziche M, Lanz C, Buttner M, Rziha HJ, Dehio C. A novel vascular endothelial growth factor encoded by orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (flt-1) receptor tyrosine kinases. EMBO J. 1999;18:363–374. doi: 10.1093/emboj/18.2.363. doi:10.1093/emboj/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cudmore M, Ahmad S, Al-Ani B, Hewett P, Ahmed S, Ahmed A. VEGF-E activates endothelial nitric oxide synthase to induce angiogenesis via cGMP and PKG-independent pathways. Biochem. Biophys. Res. Commun. 2006;345:1275–1282. doi: 10.1016/j.bbrc.2006.04.031. doi:10.1016/j.bbrc.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 32.Bausero P, Cavaille F, Meduri G, Freitas S, Perrot-Applanat M. Paracrine action of vascular endothelial growth factor in the human endometrium: Production and target sites, and hormonal regulation. Angiogenesis. 1998;2:167–182. doi: 10.1023/a:1009292506879. [DOI] [PubMed] [Google Scholar]
- 33.Wheeler T, Evans PW, Anthony FW, Godfrey KM, Howe DT, Osmond C. Relationship between maternal serum vascular endothelial growth factor concentration in early pregnancy and fetal and placental growth. Hum. Reprod. 1999;14:1619–1623. doi: 10.1093/humrep/14.6.1619. [DOI] [PubMed] [Google Scholar]
- 34.Vonnahme KA, Wilson ME, Li Y, Rupnow HL, Phernetton TM, Ford SP, Magness RR. Circulating levels of nitric oxide and vascular endothelial growth factor throughout ovine pregnancy. J. Physiol. 2005;565:101–109. doi: 10.1113/jphysiol.2004.082321. doi:jphysiol.2004.082321 [pii]; 10.1113/jphysiol.2004.082321 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magness RR. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy. In: Bazer F, editor. Endocrinology of Pregnancy. Humana Press; Totowa, NJ: 1998. pp. 507–539. [Google Scholar]
- 36.Sugino N, Kashida S, Karube-Harada A, Takiguchi S, Kato H. Expression of vascular endothelial growth factor (VEGF) and its receptors in human endometrium throughout the menstrual cycle and in early pregnancy. Reproduction. 2002;123:379–387. doi: 10.1530/rep.0.1230379. [DOI] [PubMed] [Google Scholar]
- 37.Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstet. Gynecol. 2003;101:1266–1274. doi: 10.1016/s0029-7844(03)00338-7. doi:S0029784403003387 [pii] [DOI] [PubMed] [Google Scholar]
- 38.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, Caniggia I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R1085–93. doi: 10.1152/ajpregu.00794.2005. doi:00794.2005 [pii]; 10.1152/ajpregu.00794.2005 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cale JM, Bird IM. Inhibition of MEK/ERK1/2 signalling alters endothelial nitric oxide synthase activity in an agonist-dependent manner. Biochem. J. 2006;398:279–288. doi: 10.1042/BJ20060371. doi:BJ20060371 [pii]; 10.1042/BJ20060371 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahimi N. Vascular endothelial growth factor receptors: Molecular mechanisms of activation and therapeutic potentials. Exp. Eye Res. 2006;83:1005–1016. doi: 10.1016/j.exer.2006.03.019. doi:10.1016/j.exer.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosado JA, Sage SO. Phosphoinositides are required for store-mediated calcium entry in human platelets. J. Biol. Chem. 2000;275:9110–9113. doi: 10.1074/jbc.275.13.9110. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Wang Z. Regulation of epidermal growth factor receptor endocytosis by wortmannin through activation of Rab5 rather than inhibition of phosphatidylinositol 3-kinase. EMBO Rep. 2001;2:842–849. doi: 10.1093/embo-reports/kve179. doi:10.1093/embo-reports/kve179 [doi]; 2/9/842 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warashina A. Mechanism by which wortmannin and LY294002 inhibit catecholamine secretion in the rat adrenal medullary cells. Cell Calcium. 2001;29:239–247. doi: 10.1054/ceca.2000.0187. doi:10.1054/ceca.2000.0187 [doi]; S0143-4160(00)90187-8 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Jandu N, Shen S, Wickham ME, Prajapati R, Finlay BB, Karmali MA, Sherman PM. Multiple seropathotypes of verotoxin-producing escherichia coli (VTEC) disrupt interferon-gamma-induced tyrosine phosphorylation of signal transducer and activator of transcription (stat)-1. Microb. Pathog. 2007;42:62–71. doi: 10.1016/j.micpath.2006.10.005. doi:S0882-4010(06)00143-4 [pii]; 10.1016/j.micpath.2006.10.005 [doi] [DOI] [PubMed] [Google Scholar]
- 45.Jenner S, Farndale RW, Sage SO. Wortmannin inhibits store-mediated calcium entry and protein tyrosine phosphorylation in human platelets. FEBS Lett. 1996;381:249–251. doi: 10.1016/0014-5793(96)00130-5. doi:0014-5793(96)00130-5 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Cross MJ, Stewart A, Hodgkin MN, Kerr DJ, Wakelam MJ. Wortmannin and its structural analogue demethoxyviridin inhibit stimulated phospholipase A2 activity in swiss 3T3 cells. wortmannin is not a specific inhibitor of phosphatidylinositol 3-kinase. J. Biol. Chem. 1995;270:25352–25355. doi: 10.1074/jbc.270.43.25352. [DOI] [PubMed] [Google Scholar]
- 47.Arcaro A, Volinia S, Zvelebil MJ, Stein R, Watton SJ, Layton MJ, Gout I, Ahmadi K, Downward J, Waterfield MD. Human phosphoinositide 3-kinase C2beta, the role of calcium and the C2 domain in enzyme activity. J. Biol. Chem. 1998;273:33082–33090. doi: 10.1074/jbc.273.49.33082. [DOI] [PubMed] [Google Scholar]
- 48.Maffucci T, Cooke FT, Foster FM, Traer CJ, Fry MJ, Falasca M. Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J. Cell Biol. 2005;169:789–799. doi: 10.1083/jcb.200408005. doi:10.1083/jcb.200408005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arcaro A, Zvelebil MJ, Wallasch C, Ullrich A, Waterfield MD, Domin J. Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors. Mol. Cell. Biol. 2000;20:3817–3830. doi: 10.1128/mcb.20.11.3817-3830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.