Abstract
Structural magnetic resonance imaging (MRI) studies of the human brain have reported evidence for sexual dimorphism. In addition to sex differences in overall cerebral volume, differences in the proportion of gray matter (GM) to white matter (WM) volume have been observed, particularly in the parietal lobe. To our knowledge there have been no studies examining the relationship between the sex differences in parietal lobe structure and function. The parietal lobe is thought to be involved in spatial ability, and particularly involved in mental rotation. The purpose of this study is to examine whether sex differences in parietal lobe structure are present, and if present to relate these differences to performance on the Mental Rotations Test (MRT). We found that women had proportionately greater gray matter volume in the parietal lobe compared to men, and this morphologic difference was disadvantageous for women in terms of performance on the MRT. In contrast, we found that men compared to women had proportionately greater parietal lobe surface area, and this morphologic difference was associated with a performance advantage for men on mental rotation. These findings support the possibility that the sexual dimorphism in the structure of the parietal lobe is a neurobiological substrate for the sex difference in performance on the Mental Rotations Test.
Keywords: sex differences, mental rotation, parietal lobe, spatial ability
Introduction
Significant sex differences in the morphology of the human brain have been reported in multiple studies. Males have larger brains even after eliminating differences in body size (Marshall, 1892; Nopoulos, Flaum, O’Leary, Andreasen, 2000; Raz, Gunning-Dixon, Head, Rodrigue, Williamson & Acker, 2004; Sowell, Peterson, Kan, Woods, Yoshii, Bansal, Xu, Zhu, Thompson, & Toga, 2006). In addition to the difference in size, there is evidence for sex differences in tissue proportions, with studies showing that women have proportionally greater gray matter (GM) volume than white matter (WM) volume compared to men (Gur, Turetsky, Matsui, Yan, Bilker, Hughett, & Gur, 1999; Goldstein, Eidman, Horton, Makris, Kennedy, Caviness, Faraone, & Tsuang, 2001; Luders, Narr, Thompson, Woods, Rex, Jancke, Steinmetz, & Toga, 2005). As a corollary to these studies evaluating volumes of tissue, imaging methods that map cortical thickness show increased cortical depth in women (Im, Lee, Lee, Shin, Kim, Kwon, & Kim, 2006; Luders, Narr, Thompson, Rex, Woods, DeLuca, Jancke, & Toga, 2006; Sowell, et al., 2006). A convergence of studies suggests that the proportional increase in gray matter in women is regionally specific to the parietal and posterior temporal lobes (Schlaepfer, Harris Tien, Peng, Lee, Pearlson, 1995; Nopoulos, et al., 2000; Im, et al., 2006; Luders, et al., 2006; Sowell, et al., 2006).
The parietal lobes are thought to play an important role in spatial processing in general, and mental rotation specifically (Save & Poucet, 2000;Culham & Kanwisher, 2000; Jagaroo, 2004). Mental rotation is the act of imagining an object turning in space (Corballis, 1997), be it two-dimensional images such as alphanumeric objects rotating around a central axis or three-dimensional objects being manipulated in terms of pitch, yaw, and roll. A variety of functional imaging studies using PET (e.g. Alivisatos & Petrides, 1997), EEG (e.g. Roberts & Bell, 2000), and fMRI (e.g.Seurinck, Vingerhoets, de Lange, 2004) have shown parietal lobe activation during mental rotation tasks. For example, as task demands are increased in a mental rotation task, activity in the superior parietal lobule increases (Tagaris, Kim, Strupp, Andersen, U urbil, & Georgopoulos, 1996). Lesion studies point to parietal involvement in spatial ability (see Corballis, 1997 for a review). Specifically, parietal lobe lesions result in deficits in mental rotation, which is in line with a broader role of the parietal lobe for spatial processing and neglect syndromes. It has been suggested that the right hemisphere may be dominant for mental rotation (e.g. Ditunno & Mann, 1990), however left parietal involvement may increase with mental rotation difficulty (e.g. Mehta, Newcombe, & Damasio, 1987).
In addition to possible hemispheric differences in mental rotation, gender differences on spatial tasks have been reported. In these reports, men excel relative to women, particularly on tasks involving mental rotation (Delgado & Prieto, 1996; Collins & Kimura, 1997). Meta-analyses have demonstrated consistent sex differences in mental rotation performance (Linn & Petersen, 1985, Voyer, Voyer, & Bryden 1995). Moreover, a growing body of fMRI studies has demonstrated sex differences in activation patterns between men and women on mental rotation tasks. For example, Jordan and colleagues (2002) observed bilateral activations in the parietal lobes, including superior and inferior parietal lobules and the intraparietal sulcus, inferior temporal gyrus and premotor areas in women whereas they observed right parieto-occipital sulcus, left intraparietal sulcus and left superior parietal lobule activation in men. Similarly, males have been observed to show predominantly parietal activation where females show additional inferior frontal activation when completing mental rotation tasks (Thomsen, Hugdahl, Ersland, Barndon, Lundevold, Smievoll, Roscher, & Sundberg, 2000). In line with the fMRI literature, ERP studies have also shown sex differences in neural activity during mental rotation tasks. In particular, parietal lobe activity has been reported to be more symmetrically organized in women than in men, where activity is biased to the right hemisphere (Johnson, McKenzie, & Hamm, 2002; Rescher & Rappelsberger, 1999).
To date, no study has evaluated the relationship of mental rotation performance to the sexually dimorphic structure of the human parietal lobe. Given that there are well-documented sex-related differences in parietal lobe morphology and mental rotation performance, and that the parietal lobe has been implicated in performing mental rotations in fMRI, PET, ERP, and lesion literature, the purpose of this study is to examine sex differences in parietal lobe structure and relate these differences to performance on a mental rotation task.
Methods
Subjects
The study sample consisted of 76 (n=38 female, n=38 male) healthy, normal adult volunteers recruited via newspaper advertising. The two groups were equivalent in age, verbal IQ (VIQ), performance IQ (PIQ), full-scale IQ (FSIQ), years of education, and parental socioeconomic status (SES). Demographics of the sample are listed in Table 1. Subjects were screened by an experienced research assistant with a structured interview to assess their medical and psychiatric histories using an abbreviated version of the Comprehensive Assessment of Symptoms and History (Andreasen, Flaum, & Arndt, 1992). Subjects were excluded if by self report they had significant (requiring medical intervention) medical, neurological, or psychiatric illness including alcohol and substance abuse. IQ measures were obtained using the Wechsler Adult Intelligence Scale-Revised (WAIS-R), which was given as part of a cognitive testing battery. Handedness was determined quantitatively by use of a handedness scale developed by Benton (1967). All subjects, except two (one left-handed male, one mix-handed male), were right-handed and all subjects were Caucasian. Neuropsychological testing and imaging were completed on the same day, in the morning and afternoon respectively. All subjects signed informed consent prior to participation. The protocol used was approved by the local institutional review board. The current sample overlaps substantially with our previous publication on sex differences in brain morphology Nopoulos, et al. (2000): n=32 females and n=28 males in are both samples.
Table 1.
Demographics
| Women (N=38) | Men (N=38) | ||||
|---|---|---|---|---|---|
| Mean (S.D.) | Range | Mean (S.D.) | Range | P* | |
| Age | 26.1 (6.8) | 19–47 | 27.2 (7.5) | 18–47 | 0.512a |
| VIQ | 106.1 (9.1) | 86–126 | 109.1 (12.1) | 84–126 | 0.218a |
| PIQ | 115.6 (12.0) | 87–143 | 111.0 (11.5) | 87–136 | 0.092a |
| FSIQ | 111.4 (9.9) | 91–129 | 111.0 (11.8) | 86–130 | 0.892a |
| Education (years) | 14.9 (1.3)c | 12–17 | 14.3 (1.9) | 9–18 | 0.107b |
| Parental SES | 2.86 (0.5)c | 2–4 | 2.68 (0.5) | 2–4 | 0.145b |
| Height (cm) | 163.5 (8.0)c | 150–180 | 178.5 (5.9) | 168–193 | <0.0005b |
Mean values, standard deviations, and ranges for demographic variables of the sample separated by sex. P-values from independent samples t-tests to assess differences between men and women are reported.
d.f. = 74;
d.f. = 73;
N=37. Parental SES is measured on a scale from 1–4.
MRI acquisition
Images were obtained on a 1.5 Tesla GE Signa MR scanner. Three different sequences were acquired for each subject. T1 weighted images, using a spoiled grass sequence, were acquired with the following parameters: 1.5 mm coronal slices, 40° flip angle, TR = 24ms, TE = 5ms, NEX = 2, FOV = 26cm and a 256 × 192 matrix. The PD and T2 weighted images were acquired with the following parameters: 3.0 mm coronal slices, TE = 36ms (for PD) or TE = 96ms (for T2), TR = 3000ms, NEX = 1, FOV = 26cm, 256×192 matrix and an echo train length = 8. Processing of the images after acquisition was done using a locally developed family of software programs called BRAINS2 (acronym for Brain Research: Analysis of Images, Networks, and Systems). Details of the image analysis are published elsewhere (Magnotta, Harris, Andreasen, O’Leary, Yuh, & Heckel, 2002). Briefly, a three-dimensional data set is created, and the images are realigned, resampled, and the Talairach Atlas is warped onto the brain (Talairach & Tournoux, 1988).
Brain Volume Measures
Within the stereotactic space, boxes were assigned to specific brain regions. Intracranial volume was subdivided into brain tissue and cerebral spinal fluid. Brain tissue was subdivided into the cerebrum and cerebellum. Measures of cerebral tissue volume thus exclude cerebellar tissue. The cerebrum was then divided further into its four lobes. Volumes of tissue were obtained from each region in an automated fashion. Automated measures obtained using a stereotactically-based method have been reported by our lab and others to be efficient and accurate for cerebral lobe measures (Collins, Neelin, Peters, & Evans, 1994; Andreasen, Rajarethinam, Cizadlo, Arndt, Swayze, Flashman, O’Leary, Ehrhardt, Yuh, 1996).
Surface Anatomy
This method is described in detail in a manuscript by Magnotta, Andreasen, Schultz, Harris, Cizadlo, Heckel, Nopoulos, & Flaum (1999). To summarize here, the data were initially segmented using the method described above. The segmented image was then processed using our BRAINSURF program which extracts a triangle-based polygonal model of an iso-surface, representing the parametric center of the GM tissue class. Surface area is calculated as the sum total of the surface in a given region.
Mental Rotations Test
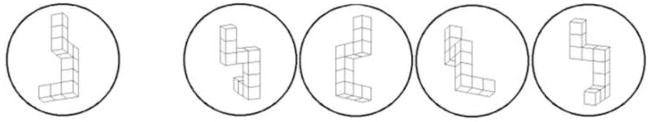
The Mental Rotations Test (MRT) developed by Vandenburg and Kuse (1978) was completed by participants as part of a neuropsychological battery. The MRT uses 2-dimensional renderings of 3-dimensional objects initially developed by Shepard and Metzler (1971). This test consists of 20 items, each item containing one target figure presented to the far left of each item and four choice figures presented to the right (see Figure 1). Participants were to identify the correct alternatives, which were identical to the target in form but displayed in a rotated position. Among the four choice figures, there are two correct alternatives and two incorrect alternatives. The MRT was scored with a possible total score of 40 (20 items, each with two correct responses). Participants were given 10 minutes to complete the task, yielding a measure of the total number of items individuals were able to complete within the time limit.
Fig. 1.
Example of a typical item on the Mental Rotations Test: The figure on the left is the target figure and subjects are asked to identify two of the four figures on the right that match the target figure.
Statistical Analysis
Analyses were completed using SPSS 16.0 for Windows. Brain tissue measures were analyzed using a one-way between-groups analysis of covariance (ANCOVA) procedure. Preliminary checks were performed to ensure that the assumptions of the ANCOVA procedure were not violated. Parietal tissue volumes (total volume, GM volume, and WM volume) were modeled using age and cerebral tissue volume as covariates. Parietal surface areas were modeled using age and cerebral surface area as covariates. Covariates differed for each of the morphological variables to control for differences between the sexes appropriate for the particular measure. For overall cerebral measures, differences in stature (i.e. height) may be responsible for some of the difference between men and women in brain size, thus height was used as a covariate for cerebral tissue volume and cerebral surface area. For parietal lobe measures, more precise covariates were used to control for sex differences in overall cerebral size. For parietal lobe tissue volumes, cerebral tissue volume was used as a covariate, whereas for parietal lobe surface areas, cerebral surface area was used as a covariate. In other words, volumetric covariates where used for volumetric variables to control for sex differences in overall brain size, and area measures were used as covariates for area variables.
Proportional tissue composition within the parietal lobe, i.e. gray matter-white matter ratio (GM-WM ratio), was calculated by dividing the parietal GM volume by the parietal WM volume. This gives a ratio such that larger relative amounts of GM yield a ratio greater than 1 and larger relative WM volumes give a ratio of less than 1. This method of calculating the ratio of GM to WM has been used in several other studies (e.g. Salat, Kaye, & Janowsky, 1999), particularly as a means of measuring the relative amounts of GM and WM and age-related change in tissue proportion. The GM-WM ratio was analyzed with an ANCOVA procedure, using age as a covariate, since this is a proportional measure covariates for cerebral size differences are unnecessary and thus excluded. All interaction effects were entered into the models, but were dropped if they were not significant, using an alpha value of 0.05 for all significance tests.
Analyses of MRT performance were calculated using independent samples t-tests, for both the total number of items completed and the percentage of items answered correctly.
Correlations between brain measures and percent correct on the MRT were calculated using a partial correlation procedure. To reduce the number of tests that were run and thus reduce the likelihood of a Type I error, correlations between brain morphology and MRT percent correct were only analyzed for parietal surface area, and the parietal GM-WM ratio. In other words, correlations were only examined for the brain areas that were found to be significantly different between men and women. Age and cerebral surface area were used as control variables to account for overall brain size when examining parietal surface area. Age alone was used as a control when examining the parietal GM-WM ratio due to the proportional nature of this measure. A test for comparing independent correlations using Fisher’s r- to z- transformation was performed to examine differences in the correlations between men and women.
Correlations between total cerebral measures (tissue volume and surface area) and percent correct on the MRT (using age and height as covariates) were performed to test the assumption that mental rotation performance is specifically related to parietal lobe structure as opposed to a more global relationship to brain structure. Likewise, we explored correlations between VIQ and PIQ from the WAIS-R and MRT performance to examine whether our findings are specific to spatial ability, i.e. mental rotation, rather than cognitive ability in general.
Results
Brain Morphology
Table 2 presents the measures of cerebral and parietal lobe morphology, including tissue volumes (total, GM, and WM, both combined and in each hemisphere), surface area (both combined and in each hemisphere), and the parietal GM-WM ratio. There was a trend toward significant sex differences in cerebral tissue volume after controlling for age and height, with men having overall larger volumes of cerebral tissue [F(1,71)=3.375, p=0.070]. There was no significant sex difference in total cerebral surface area after co-varying age and height [F(1,71)=0.013, p=0.910].
Table 2.
Brain Morphology
| Women (N=38) | Men (N=38) | Sex Effect | |||
|---|---|---|---|---|---|
| Mean (S.D.) | Adjusted Mean (S.E.) | Mean (S.D.) | Adjusted Mean (S.E.) | F (P) | |
| Cerebral Tissue Volume (cc)a | 1083.7 (94.4) | 1120.8 (18.0) | 1207.6 (96.7) | 1175.1 (17.7) | 3.375 (0.070) |
| Cerebral Surface Area (mm2)a | 170809.4 (20471.0) | 176889.2 (3410.5) | 181540.6 (13416.2) | 176256.0 (3348.8) | 0.013 (0.910) |
| Parietal Lobe | |||||
| Tissue Volume (cc)b | 236.4 (22.6) | 249.6 (1.7) | 264.6 (23.0) | 251.4 (1.7) | 0.431 (0.514) |
| Gray Matter | 129.5 (11.5) | 135.6 (1.3) | 141.9 (13.7) | 135.7 (1.3) | 0.002 (0.968) |
| Right | 65.3 (6.0) | 68.7 (0.7) | 71.7 (7.6) | 68.3 (0.7) | 0.084 (0.772) |
| Left | 64.1 (6.1) | 67.0 (0.8) | 70.2 (6.5) | 67.4 (0.8) | 0.128 (0.721) |
| White Matter | 106.9 (12.8) | 114.0 (1.2) | 122.7 (12.2) | 115.6 (1.2) | 0.769 (0.383) |
| Right | 53.3 (6.6) | 56.9 (0.7) | 61.2 (6.6) | 57.5 (0.7) | 0.342 (0.561) |
| Left | 53.7 (6.5) | 57.0 (0.6) | 61.5 (5.8) | 58.1 (0.6) | 1.247 (0.268) |
| Surface Area (mm2)c | 40320.8 (4539.9) | 41334.6 (335.2) | 43436.8 (3289.4) | 42422.9 (335.2) | 5.003 (0.028)* |
| Right | 20492.2 (2481.5) | 21041.9 (199.1) | 22091.7 (1838.1) | 21542.0 (199.1) | 2.996 (0.088) |
| Left | 19828.5 (2119.4) | 20292.7 (164.3) | 21345.1 (1576.7) | 20880.9 (164.3) | 6.081 (0.016)* |
| Gray/White Matter Ratiod | 1.22 (0.11) | 1.22 (0.02) | 1.16 (0.11) | 1.16 (0.02) | 5.167 (0.026)* |
| Right | 1.24 (0.11) | 1.23 (0.02) | 1.18 (0.12) | 1.18 (0.02) | 4.176 (0.045)* |
| Left | 1.20 (0.12) | 1.20 (0.02) | 1.14 (0.10) | 1.15 (0.02) | 4.994 (0.028)* |
Mean values and standard deviations for both men and women, for brain morphology measures, including overall cerebral volume and surface area and target region, parietal lobe, measures. Adjusted means, standard errors, and sex effects are the result of ANCOVA:
using age and height as covariates (d.f. 1, 71);
using age and cerebral tissue volume as covariates (d.f. 1, 72);
using age and cerebral surface area as covariates (d.f. 1, 72);
using age as a covariate (d.f. 1, 73);
p<0.05. Briefly, it was found that men had greater parietal lobe surface area predominately in the left hemisphere and women had proportionally more gray matter in the parietal lobe more strongly in the left hemisphere.
Parietal Tissue Volume
After controlling for age and cerebral tissue volume, there was no observed main effect of sex on total parietal tissue volume [F(1,72)=0.431, p=0.514].
Parietal Gray Matter Volume
There were no observed sex differences in parietal GM volume after controlling for age and cerebral tissue volume [F(1,72)=0.002, p=0.968]. Likewise, there were no sex differences in the left hemisphere [F(1,72)=0.084, p=0.772] or the right hemisphere [F(1,72)=0.128, p=0.721] parietal GM volume. Thus, the volume of parietal lobe cortex, with respect to the rest of the cerebrum, is not sexually dimorphic.
Parietal White Matter Volume
There were no observed differences in parietal WM volume after controlling for age and cerebral tissue volume [F(1,72)=0.769, p=0.383]. This was true of both hemispheres individually as well [right: F(1,72)=0.342, p=0.561; left: F(1,71)=1.247, p=0.268].
Parietal Surface Area
After controlling for age and cerebral surface area, significant sex differences were observed for total parietal surface area [F(1,72)=5.003, p=0.028], particularly in the left hemisphere [F(1,72)=6.081, p=0.016] and a weak trend in the right hemisphere [F(1,72)=2.996, p=0.088]. Thus, even after controlling for overall differences in brain size, by co-varying for cerebral surface area, compared to women, men have larger surface areas of the parietal lobe (mean difference=1088.3 mm2), particularly left parietal (mean difference=588.2 mm2), with a consistent pattern in the right parietal (mean difference=500.1mm2). One female outlier was identified in terms of parietal lobe surface area, however removal of this data point had no effect on the pattern of results.
Parietal Gray Matter-White Matter Ratio
Sex differences in tissue composition were observed in the parietal lobe [F(1,73)=5.167, p=0.026] with women having a higher ratio of GM to WM than men. This increase in the GM-WM ratio in women compared to men was observed bilaterally [right: F(1,73)=4.176, p=0.045; left: F(1,73)=4.994, p=0.028]. It is important to contrast this finding with the GM and WM volume findings above. In the previous analysis, parietal gray and white matter volumes, in proportion to the entire cerebrum, were not different between the sexes. However, the ratio analysis looks at proportion of GM and WM within the parietal lobe, in which women have proportionately larger GM volumes.
Mental Rotations Test
Independent samples t-tests revealed significant sex differences in performance on the MRT (see Table 3). Women completed significantly fewer items than men (t(74)=3.465, p=0.001). Men had significantly higher percent correct compared to women (t(74)=2.965, p=0.004).
Table 3.
Mental Rotations Test
| Women (N=38) | Men (N=38) | t(p)* | |
|---|---|---|---|
| Mean (S.D.) | Mean (S.D.) | ||
| # of Items Completed | 27.1 (7.9) | 33.1 (7.0) | 3.465 (0.001)** |
| Percent Correct | 53.0 (18.9) | 66.0 (19.2) | 2.965 (0.004)** |
Means and standard deviations by sex for results on the Mental Rotations Test (Vandenburg & Kuse, 1978), including the total number of items out of 40 that were completed and the percent of the total that were answered correctly. Independent samples t-tests reveal that men completed significantly more items and answered more items correctly.
d.f. 74;
p<0.005.
Correlations
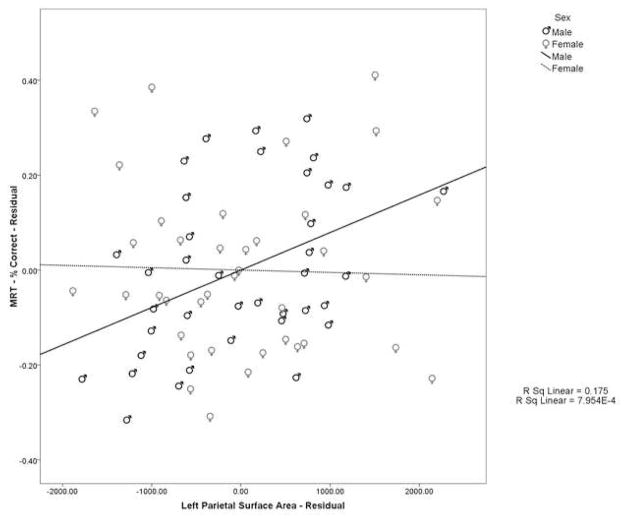
Table 4 displays the correlations between MRT performance and brain structure for each group. In addition, the Fisher’s tests comparing the male and female correlations are shown. Men and women show a different pattern of correlations between parietal lobe measures and performance on the MRT (see Table 4). For men, the correlations between the percent of items answered correctly and brain measures were all positive in direction. They reached significance for total parietal surface area (r=0.423, p=0.010), right parietal surface area (r=0.331, p=0.048), and left parietal surface area (r=0.425, p=0.010) (see Fig. 2). For women, most of the correlations were in the negative direction, reaching significance for the left parietal GM-WM ratio (r= −0.469, p=0.003) (see Fig. 3) and total parietal GM-WM ratio (r= −0.361, p=0.028).
Table 4.
Correlations – Brain Measures and MRT Percent Correct
| Women (N=38) | Men (N=38) | Zobs (ptwo-tailed) | |
|---|---|---|---|
| r (ptwo-tailed) | r (ptwo-tailed) | ||
| Cerebral Tissue Volume (cc) | 0.161 (0.356) | 0.173 (0.312) | -- |
| Parietal Lobe | |||
| Surface Area (mm2)a | 0.119 (0.490) | 0.423 (0.010)** | −1.39 (0.165) |
| Right | 0.223 (0.191) | 0.331 (0.048)* | −0.49 (0.624) |
| Left | −0.026 (0.882) | 0.425 (0.010)** | −2.01 (0.045)* |
| Gray/White Matter Ratiob | −0.361 (0.028)* | 0.025 (0.881) | −1.69 (0.092) |
| Right | −0.198 (0.239) | 0.010 (0.951) | −0.89 (0.378) |
| Left | −0.469 (0.003)** | 0.043 (0.802) | −2.31 (0.021)* |
Partial correlations between sexually dimorphisms in the parietal lobe and percent of items answered correctly on the Mental Rotations Test.
using age and cerebral surface area as control variables (d.f. 34);
using age as a control variable (d.f. 35);
p < 0.05;
p ≤ 0.01. Relationships are specific for each sex, in women a significant negative relation was observed between the left parietal GM-WM ratio and MRT performance, whereas in men positive relationships were observed between parietal surface area, predominately left hemisphere, and MRT performance. Results of Fisher’s r-to-z transformation show that the correlations were significantly different between men and women for left hemisphere measures.
Fig. 2.
Scatterplot of the relationship between left parietal lobe surface area and mental rotation performance. Values represent the residuals after factoring out the effects of age and cerebral surface area, including positive relationship between these variables in men (solid line) and the lack of a relationship in women (dashed line).
Fig. 3.
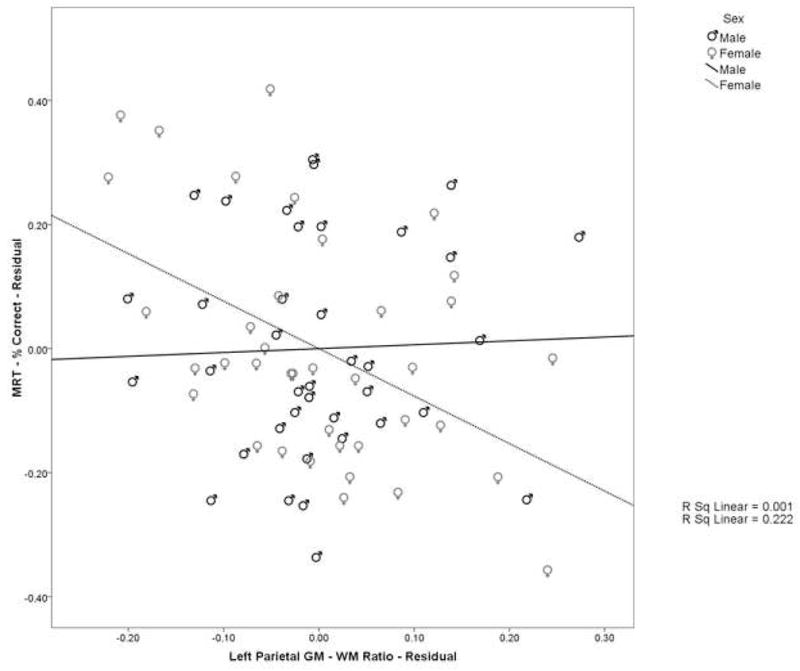
Scatterplot of the relationship between the left parietal GM-WM ratio and mental rotation performance. Values represent the residuals after factoring out the effect of age, including the negative relationship between these variables in women (dashed line) and the lack of a relationship in men (solid line)
Significant results were found when evaluating the correlations between percent correct and parietal lobe measures in women compared to the same correlations in men. The correlations were significantly different between men and women for left parietal surface area (Zobs=−2.01, ptwo-tailed=0.045), and left parietal GM-WM ratio (Zobs=−2.31, ptwo-tailed=0.021). These correlations were significantly negative or at least negative in direction in women and significantly positive or at least positive in direction in men.
To examine the specificity of these findings, we explored possible correlations between VIQ and PIQ from the WAIS-R and MRT performance. Neither VIQ (men: r=0.052, p=0.754; women: r=−0.042, p=0.802) nor PIQ (men: r=0.205, p=0.218; women: r=0.219; p=0.186) were significantly related to the percentage of items answered correctly on the MRT. This suggests that the relationship to sex differences in parietal lobe morphology is specific to mental rotation rather than to cognitive function in general. Similarly, we did not observe a relationship between mental rotation performance and overall cerebral tissue volume (men: r=0.173, p=0.312; women: r=0.161, p=0.356) or overall cerebral surface area (men: r=0.171, p=0.318; women: r=0.272, p=0.114). This suggests that the relationship to mental rotation is specific to the parietal lobe rather than to brain morphology in general.
Discussion
Consistent with previous research, this study has shown regional morphological differences between male and female brains. In regard to parietal lobe morphology, men have significantly larger volumes of parietal lobe GM, WM, and cortical surface area compare to women. After controlling for cerebral volume, there were no sex differences in parietal lobe GM or WM. However, after controlling for cerebral surface area, men had a greater parietal lobe surface area compared to women. This finding compliments a previous neuropathologic study that also found increased cortical surface area of the inferior occipital lobe in the human male (Amunts, et al., 2007). In addition, women were found to have proportionally larger GM-WM ratios in the parietal lobe compared to men. This sexual dimorphism in tissue distribution is consistent with several other studies (Schlaepfer, et al., 1995; Nopoulos, et al., 2000; Im, et al., 2006; Luders, et al., 2006; Sowell, et al., 2006).
In regard to the structure – function relationship, an interesting pattern emerges. Women had proportionately more GM volume in the parietal lobe compared to men, and this morphologic difference was disadvantageous for women in terms of performance on the MRT. In contrast, we found that men compared to women had proportionately greater parietal lobe surface area, and this morphologic difference was associated with a performance advantage for men on mental rotation. Moreover, both of these relationships are unique and specific to each sex. That is, the negative correlation between proportional GM and performance is present only in the women and the positive correlation between surface area and MRT performance is seen only in the men. These findings support the possibility that the structural differences in the brain underlie the differing structure – function relationships between the sexes.
The pattern of relationships between parietal lobe measures and MRT performance and the lack of a significant relation between MRT performance and WAIS-R measures suggests that the structure –function relationships are specific to mental rotation and not to cognitive processes in general. Men did not show a generalized enhancement of cognitive processing due to increased parietal lobe surface area nor did women show a generalized diminishment of cognitive processing due to proportionately greater parietal GM. Likewise, the lack of a relationship between MRT performance and overall cerebral volume and surface area suggests that the relationship is specifically related to morphological differences in the parietal lobe not overall differences across the whole brain. Together, these findings suggest that the sexual dimorphisms in the parietal lobe represent a specific adaption for spatial abilities, i.e. mental rotation, in men and potentially a specialization for other cognitive processes in women rather than general cognitive enhancement or deficit.
Gur, et al. (1999) suggested that the proportionately larger GM volume found in women increased the amount of tissue available for computational processing, because the somatodendritic tissue is where processing occurs. In other words, increased proportions of GM are posited to compensate for smaller overall tissue volumes and surface area. Results from the current study, particularly the negative correlations between proportional GM and performance on MRT, do not support this hypothesis in regard to this region and this task, though it may be true elsewhere in the brain and/or for different tasks. Rather than being a compensatory mechanism for differences in computational power due to large-scale differences in brain morphology, our results suggest that differences in local brain morphology, i.e. proportionally increased GM or surface area in the parietal lobe, relate to specific specializations that result in different, adaptive skill sets in different individuals.
Our demonstration that an increase in proportional GM in the parietal interferes with performance on the MRT may seem counterintuitive. One possible explanation is that the left parietal lobe in women is not specialized for tasks that involve the same mental rotation abilities as are found in men. Rather, the higher proportion of GM observed in women may be related to specialization in a different sort of spatial task. The specialization for another spatial task may require a very different type of processing that may necessarily come at the expense of mental rotation performance and relate to differences in the underlying cytoarchitecture leading to the morphological differences observed between men and women. There are certain spatial tasks in which women have superior performance to that of men, particularly tasks that require spatial memory. For example, on an Object Location Memory Task, women perform better than men (Eals & Silverman, 1994). In that task, participants examine an array of objects and are asked to determine which objects are in different spatial locations in a later array. Of particular interest is the empirical evidence that supports the role of the parietal lobe for both spatial memory (Naghavi & Nyberg, 2005; Wagner, Shannon, Kahn, & Buckner, 2005; Klingberg, 2006) and visuomotor control (Battaglia-Mayer, Archambault, & Caminiti, 2006). The results from the current study suggest that specific morphological characteristics, such as increased surface area or increased proportional GM volume, are related to specialization in specific areas of expertise. Where parietal lobe surface area may represent an adaptation that drives superior mental rotation performance, we predict that increased proportional GM volume will relate specifically to superior spatial memory. It seems possible that there are spatial tasks that are complementary, in this case mental rotation ability may be complementary to spatial memory. One can be an ‘expert’ at one of these but this may interfere with becoming an ‘expert’ in the complementary spatial domain, due to the different structural requirements of the substrate needed to process these disparate tasks. Intuitively, it appears that increased neuropil, with an increased number and complexity of synaptic connections would benefit memory encoding and storage, and that increased surface area, increasing the number of functional columns, may benefit active manipulation of information. However, this is highly speculative and requires further research to resolve exactly how the fine-scale, structural make up of brain maps to cognitive processes.
Cerebral cytoarchitecure may also relate to strategy use in completing the mental rotation task. Previous research has suggested that when one object must be rotated to match another, as in the MRT, the best performance arises when the two hemispheres work in cooperation, with the right hemisphere holding the target shape and the left hemisphere rotating the other to match (Cook, Fruh, Mehr, Regard, & Landis, 1994). Perhaps, when one has a high proportion of GM, there is not enough underlying WM to facilitate efficient and appropriate communication between brain regions, which need to work together to accomplish the mental rotation and the comparison. Perhaps the greater proportion of WM to GM in men facilitates performance by allowing parallel processing of the target object and simultaneous rotation of the other object to match. In contrast, women may use a different strategy, which does not allow for high-speed parallel processing, perhaps a serial type of processing in which the target object is processed then the other object is rotated and then a comparison is made. The postulated increased processing power due to higher proportions of GM in women may be required to overcome a lack of specialization. Whereas in men, the right parietal may be specialized to manipulate the target object and the left parietal may be specialized to manipulate the comparison object, in women there may not be as much specialization requiring increased processing power in one side as each side must manipulate both the target object and the comparison object. However, the contributions of each hemisphere to mental rotation are not entirely clear, and have been shown to be modulated by numerous factors, including task difficulty and solution strategy.
A further explanation concerning sex differences on mental rotation tasks is the fact that mental rotation tests are often timed tests, and that the sex differences arise from men completing the rotation faster and thus completing more of them in the allotted time (e.g. Goldstein, Haldane, & Mitchell, 1990; Voyer, 1997). However, the results of time effects on mental rotation performance are mixed. Other studies have found that performance was independent of time to complete the task, i.e. men maintain a performance advantage even under conditions were time is unlimited, and that an emphasis on speed decreased performance for both sexes (Delgado & Prieto, 1996; Peters, 2005). The current study does not resolve the issue of performance factors on mental rotation ability, however we suggest that the neural substrates underlying mental rotation ability differ between the sexes and that these differences translate into a performance advantage for men and a performance disadvantage for women. It is important to note however that performance in real-time in real-life poses time constraints not dissimilar from those on standardized tests. The presence of a speed-accuracy trade-off in women and the lack thereof in men is suggestive of a real difference in mental rotation ability and, as is shown in this study, may translate to morphological differences in the neural substrates underlying this ability.
Previous research had not identified sex differences in surface area (Nopoulos, et al., 2000; Barta & Dazzan, 2003) however, these studies had examined total cerebral surface area, not regional differences. It is possible that the smaller surface area observed in women is due to larger proportional surface areas of other brain regions (i.e. occipital, temporal, or frontal lobes) and the larger parietal lobes observed in men are at the expense of smaller regions elsewhere in the brain.
The number of neurons in the cortex has been reported to be proportional to its surface area and not its volume (Rockel, Hiorns, & Powell, 1974, 1980). However, Rockell, Hiorns, and Powell (1980) utilized only male human subjects and compared across species rather than across sexes. The functional columnar organization of many areas of the cortex is preserved across species (the exception being the occipital lobe, which is expanded in primates, particularly humans) as is the basic number of neurons per column, in other words the packing density in a given functional column appears to remain constant across species (Rockel, et al., 1980). The greater surface area in male parietal lobes may allow for more neurons and thus more functional columns than in females, which may lead to male superiority on the mental rotation task used in this study. In contrast, it has been observed that neuronal density is greater in women in posterior temporal cortex (Witelson, Glezer, & Kigar, 1995), however, it has been observed that male brains on average have more neocortical neurons than female brains, 23 billion to 19 billion (Pakkenberg & Gunderson, 1997). Assuming that the number of neurons per functional column is constant across sexes, men would thus have more functional columns. On the other hand, increased packing density may mean that the above assumption does not hold across the sexes and women may have more functional columns per unit area. In a similar vein, the increased thickness of cortex in women may allow for greater arborization of dendrites and more elaborate connections between functional columns, which may not lead to a performance advantage for mental rotation as does increased surface area, though it may lead to performance advantages on different spatial tasks. In other words, the ability to mentally rotate objects may require a large number of functional columns rather than fewer, but better connected columns. This may also be reflected by the different strategies that men and women have been reported to use for mental rotation tasks, with men using a holistic strategy of rotating the whole object, which may be dependent on the processing of increasing numbers of cell columns, and women using an atomistic strategy of matching object parts, which may better utilize fewer highly connected cell columns. For example, Pezaris and Casey (1991) found that in general boys relied on a spatial strategy for mental rotation whereas girls relied on a more verbal strategy for mental rotation.
Many plausible evolutionary scenarios have been put forth to explain why sex differences in spatial ability might have evolved (see Jones, Braithwaite, & Healy, 2003; Ecuyer-Dab & Robert, 2004 for comparisons of these theories), including: a division of labor between the sexes, i.e. the hunter-gatherer theory, in which each has evolved specialized cognitive skills to support respective behavioral niches, men hunting and women gathering (Silverman & Eals, 1992). Male proficiency at mental rotation and female proficiency at object location memory consistently across cultures lends some support to this theory (Silverman, Choi, & Peters, 2007). Another theory posits that sexual selection of spatial skills is dependent on the mating system of a given species. Polygynous species, in which there is an unequal maximum reproductive rate between the sexes, the sex with the higher maximum will have a larger territorial range and enhanced spatial skills to navigate and locate more mates (Gaulin, 1992). In the genus Microtus (voles), polygynous species exhibit sex differences in range and spatial ability, favoring males, while the monogamous species of Microtus do not show differences in range or spatial ability (Gaulin, 1992). Further evolutionary scenarios posit that selection occurs in females, where large ranges and energetic costs conflict with reproduction and maternal survival, thus the apparent, enhanced male performance in spatial ability may in part be due to a simultaneous selective decrease in female spatial ability necessary for long-range navigation to avoid the risks associated with a large territorial range, such as predation, and a selective increase in female spatial ability related to more detailed processing of immediate environments to maximize local resources (Ecuyer-Dab & Robert, 2004; Panter-Brick, 2002; Sherry & Hampson, 1997). Other theories have suggested that these sex differences may be due to male-male warfare (Buss & Shackelford, 1997), female-choice (Sherry & Hampson, 1997), or as by-products of selective pressures on the optimal rate of fetal development (Wynn, Tierson, & Palmer, 1996).
In isolation, each of these theories has intuitive appeal as well as potential logical and empirical limitations. Taken together it appears there are myriad benefits for enhanced spatial ability in men and strict costs and risks unequally affecting women. The pattern of differential spatial ability may be an adaptive means of maximizing resource use and reproductive potential. Under the assumption that there are some if not many selective factors favoring sex differences in spatial ability, one would expect the brain structures involved in processing spatial information to be sexual dimorphic such that the brain ‘optimally’ supports the performance dependent on the sex of the individual. Indeed this is observed in Microtus pennsylvanicus, or meadow vole, in which males display superior performance in the Morris water maze as well as significantly increased dendritic arborization in frontal and parietal areas compared to females (Kavaliers, Ossenkorp, Galea, & Kolb, 1998). Whatever the evolutionary mechanism, our results support the notion that there has been disruptive selection1 for sex differences in spatial ability at some point during human evolution such that both sexes are cognitively and neurologically equipped to behave adaptively. However, the possibility that sex differences in human spatial ability may be due to socialization and experience remains open for debate.
These specializations by sex are not necessarily innate (i.e. biologically driven by chromosomal differences). Instead, they may be due to environmental influences and circumstances that affect socialization and that thereby have downstream effects on brain structure and cognitive performance. These may change slowly over time, as changes occur in role expectations. Men and women have different yet complementary social and reproductive roles in society, which may shape not only in which tasks each sex will become ‘experts,’ but also alter the underlying neural architecture that supports specialization. As role expectations change (e.g. as more and more women become well-educated and enter male-dominated fields – a very recent secular trend), neural structure and cognitive performance may change as well. Future work could compare women who excel in historically male-dominated fields such as physics and mathematics, with women who perform more traditional social roles. This may help to determine whether or not the sex differences reported in the present study are modulated by learning and experience. Previous research has suggested that mental rotation abilities may be modulated by learning, where female performance can be increased to the level of male performance, through training and feedback with an appropriate instructional strategy (Kass, Ahlers, & Dugger, 1998) and in boys and girls that respond equally to training in mental rotation (Sanz de Acedo Lizarraga & García Ganuza, 2003). However, these studies employ mental rotation tasks that differ substantially from the Vandenburg and Kuse (1978) version of the task so conclusions are limited in relation to this study.
The extent to which biology and environment can affect mental rotation performance is an important question. Gross brain morphology is largely determined throughout development and likely becomes relatively stable during adulthood. However, experience may modify neuronal circuits, through changing synaptic efficacy and local connections. Unfortunately, we may never know about environmental effects on mental abilities because human males and females are not reared in gender-neutral environments. So it is possible that the findings could be due to nurture rather than nature. Perhaps training can modify neural circuitry to substantially improve spatial abilities. Moreover, a biological propensity to develop superior spatial abilities may never be realized without the proper experience to activate a biological predisposition. The most likely relationship between biological and environmental influences on spatial abilities is likely one in which a biological propensity to excel at, to easily learn, and to be motivated to acquire superior spatial abilities may be present in human males that leads to seeking and developing male spatial skills. On the other hand, human females with different biological propensities may allow them to excel at and be motivated to acquire a different, but complementary skill set due to a different set of selective pressures throughout human evolution that become manifest in modern society.
In summary, the present study has shown that sex differences in the structure of the parietal lobe are related to performance on a particular spatial task, mental rotation. Unique and specific relationships between parietal lobe structure and task performance were observed: men were found to have greater parietal surface area, and this was associated with enhanced mental rotation performance; women were found to have increased proportional GM, and this was found to be disadvantageous to MRT performance. The structure – function relationships between parietal lobe structure and mental rotation performance are an excellent example of the importance of studying sex differences in the field of neuroscience.
Acknowledgments
This research was supported in part by NIMH Grants MH31593, MH40856 and MH43271
Footnotes
Disruptive selection refers to a process of differential selective pressure on members of a given species such that a divergence of phenotype between members of the species is favored.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alivisatos B, Petrides M. Functional activation of the human brain during mental rotation. Neuropsychologia. 1997;35(2):111–118. doi: 10.1016/s0028-3932(96)00083-8. [DOI] [PubMed] [Google Scholar]
- Amunts K, Armstrong E, Malikovic A, Hömke L, Mohlberg H, Schleicher A, Zilles K. Gender-specific left-right asymmetries in human visual cortex. The Journal of Neuroscience. 2007;27:1356–1364. doi: 10.1523/JNEUROSCI.4753-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH): An instrument for assessing psychopathology and diagnosis. Archives of General Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW, II, Flashman LA, O’Leary DS, Ehrhardt JC, Yuh WTC. Automatic atlas-based volume estimation of human brain regions from MR images. Journal of Computer Assisted Tomography. 1996;20(1):98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Barta P, Dazzan P. Hemispheric surface area: Sex, laterality and age effects. Cerebral Cortex. 2003;13:364–370. doi: 10.1093/cercor/13.4.364. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Archambault PS, Caminiti R. The cortical network for eye-hand coordination and its relevance to understanding motor disorders of parietal patients. Neuropsychologia. 2006;44:2607–2620. doi: 10.1016/j.neuropsychologia.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Buss DM, Shackelford TK. Human aggression in evolutionary psychological perspective. Clinicial Psychology Review. 1997;17:605–619. doi: 10.1016/s0272-7358(97)00037-8. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18:192–205. [PubMed] [Google Scholar]
- Collins DW, Kimura D. A large sex difference on a two-dimensional mental rotation task. Behavioral Neuroscience. 1997;111(4):845–849. doi: 10.1037//0735-7044.111.4.845. [DOI] [PubMed] [Google Scholar]
- Cook ND, Fruh H, Mehr A, Regard M, Landis T. Hemispheric cooperation in visuospatial rotations: Evidence for manipulative role for the left hemisphere and a reference role for the right hemisphere. Brain and Cognition. 1994;25:240–249. doi: 10.1006/brcg.1994.1034. [DOI] [PubMed] [Google Scholar]
- Corballis MC. Mental rotation and the right hemisphere. Brain and Language. 1997;57:100–121. doi: 10.1006/brln.1997.1835. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functionsin human parietal cortex. Current Opinion in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Delgado AR, Prieto G. Sex differences in visuospatial ability: Do performance factors play such an important role? Memory & Cognition. 1996;24(4):504–510. doi: 10.3758/bf03200938. [DOI] [PubMed] [Google Scholar]
- Ditunno PL, Mann VA. Right hemisphere specialization for mental rotation in normals and brain damaged subjects. Cortex. 1990;26(2):177–188. doi: 10.1016/s0010-9452(13)80349-8. [DOI] [PubMed] [Google Scholar]
- Eals M, Silverman I. The hunter-gatherer theory of spatial sex differences: Proximate factors mediating the female advantage of recall of object arrays. Ethology and Sociobiology. 1994;15:95–105. [Google Scholar]
- Ecuyer-Dab I, Robert M. Have sex differences in spatial ability evolved from male competition for mating and female concern for survival? Cognition. 2004;91:221–257. doi: 10.1016/j.cognition.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Gaulin SJC. Evolution of sex differences in spatial ability. Yearbook of Physical Anthropology. 1992;35:125–151. [Google Scholar]
- Goldstein JM, Eidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism in adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goldstein D, Haldane D, Mitchell C. Sex differences in visual spatial ability: The role of performance factors. Memory & Cognition. 1990;18:546–550. doi: 10.3758/bf03198487. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RC. Sex differences in brain gray and white matter in healthy young adults: Correlations with cognitive performance. Journal of Neuroscience. 1999;19(10):4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Lee J, Shin YW, Kim IY, Kwon JS, Kim SI. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. NeuroImage. 2006;31:31–38. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Jagaroo V. Mental rotation and the parietal question in functional neuroimaging: A discussion of two views. European Journal of Cognitive Psychology. 2004;16(5):717–728. [Google Scholar]
- Johnson BW, McKenzie KJ, Hamm JP. Cerebral asymmetry for mental rotation: Effects of response hand, handedness and gender. NeuroReport. 2002;13(15):1929–1932. doi: 10.1097/00001756-200210280-00020. [DOI] [PubMed] [Google Scholar]
- Jones CM, Braithwaite VA, Healy SD. The evolution of sex differences in spatial ability. Behavioral Neurosci ence. 2003;117(3):403–411. doi: 10.1037/0735-7044.117.3.403. [DOI] [PubMed] [Google Scholar]
- Jordan K, Wüstenberg T, Heinze HJ, Peters M, Jäncke L. Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia. 2002;40:2397–2408. doi: 10.1016/s0028-3932(02)00076-3. [DOI] [PubMed] [Google Scholar]
- Kass SJ, Ahlers RH, Dugger M. Eliminating gender differences through practice in an applied visual spatial task. Human Performance. 1998;11(4):337–349. [Google Scholar]
- Kavaliers M, Ossenkorp KP, Galea LAM, Kolb B. Sex differences in spatial learning and prefrontal and parietal cortical dendritic morphology in the meadow vole. Brain Research. 1998;810:41–47. doi: 10.1016/s0006-8993(98)00868-3. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Linn MC, Petersen AC. Emergence and characterization of sex differences in spatial ability: A meta-analysis. Child Development. 1985;56(6):1479–1498. [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Woods RP, Rex DE, Jancke L, Steinmetz H, Toga AW. Mapping cortical gray matter in the young adult brain: Effects of gender. NeuroImage. 2005;26:493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, DeLuca H, Jancke L, Toga AW. Gender differences on cortical thickness and the influence of scaling. Human Brain Mapping. 2006;27:314–324. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotta VA, Andreasen NC, Schultz SK, Harris G, Cizadlo T, Heckel D, Nopoulos P, Flaum M. Quantitative in vivo measurement of gyrification in the human brain: Changes associated with aging. Cerebral Cortex. 1999;9:151–160. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- Magnotta V, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Computerized Medical Imaging and Graphics. 2002;26(4):251–64. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Marshall J. On the relation between the weight of the brain and its parts, and the stature and mass of the body in man. Journal of Anatomy and Physiology. 1892;26:445–500. [PMC free article] [PubMed] [Google Scholar]
- Mehta Z, Newcombe F, Damasio H. A left hemisphere contribution to visuospatial processing. Cortex. 1987;23:447–461. doi: 10.1016/s0010-9452(87)80006-0. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: Shared demands on integration? Consciousness and Cognition. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: Evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Research: Neuroimaging. 2000;98:1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJG. Neocortical number in humans: Effects of sex and age. The Journal of Comparative Neurology. 1997;384:312–320. [PubMed] [Google Scholar]
- Panter-Brick C. Sexual division of labor: Energetic and evolutionary scenarios. American Journal of Human Biology. 2002;14:627–640. doi: 10.1002/ajhb.10074. [DOI] [PubMed] [Google Scholar]
- Peters M. Sex differences and the factor of time in solving Vandenberg and Kuse mental rotation problems. Brain and Cognition. 2005;57:176–184. doi: 10.1016/j.bandc.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Pezaris E, Casey MB. Girls who use “masculine problem-solving strategies on a spatial task: Predisposed genetic and environmental factors. Brain and Cognition. 1991;17(1):1–22. doi: 10.1016/0278-2626(91)90062-d. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiology of Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Rescher B, Rappelsberger P. Gender dependent EEG-changes during a mental rotation task. International Journal of Psychophysiology. 1999;33:209–222. doi: 10.1016/s0167-8760(99)00063-x. [DOI] [PubMed] [Google Scholar]
- Roberts NE, Bell MA. Sex differences on a mental rotation task: Variations in electroencephalogram hemispheric activation between children and college students. Developmental Neuropsychology. 2000;17(2):199–223. doi: 10.1207/S15326942DN1702_04. [DOI] [PubMed] [Google Scholar]
- Rockel AJ, Hiorns RW, Powell TPS. Numbers of neurons through the full depth of the neocortex. Journal of Anatomy. 1974;118:371. [PubMed] [Google Scholar]
- Rockel AJ, Hiorns RW, Powell TPS. The basic uniformity in structure of the neocortex. Brain. 1980;103:221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Archives of Neurology. 1999;56:338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Sans de Acedo Lizarraga ML, García Ganuza JM. Improvement of mental rotation in girls and boys. Sex Roles. 2003;49(56):277–286. [Google Scholar]
- Save E, Poucet B. Hippocampal-parietal cortical interactions in spatial cognition. Hippocampus. 2000;10:491–499. doi: 10.1002/1098-1063(2000)10:4<491::AID-HIPO16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, Peng L, Lee S, Pearlson GD. Structural differences in the cerebral cortex of healthy female and male subjects: A magnetic resonance imaging study. Psychiatry Research: Neuroimaging. 1995;61:129–135. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- Seurnick R, Vingerhoets G, de Lange FP, Achten E. Does egocentric mental rotation elicit sex differences? NeuroImage. 2004;23:1440–1449. doi: 10.1016/j.neuroimage.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 171:701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Sherry DF, Hampson E. Evolution and hormonal control of sexually-dimorphic spatial abilities in humans. Trends in Cognitive Sciences. 1997;1(2):50–56. doi: 10.1016/S1364-6613(97)01015-2. [DOI] [PubMed] [Google Scholar]
- Silverman I, Choi J, Peters M. The hunter-gatherer theory of sex differences in spatial abilities: Data from 40 countries. Archives of Sexual Be havior. 2007;36:261–268. doi: 10.1007/s10508-006-9168-6. [DOI] [PubMed] [Google Scholar]
- Silverman I, Eals M. Sex differences in spatial abilities: evolutionary theory and data. In: Barkow J, Cosmides L, Tooby J, editors. The Adapted Mind: Evolutionary Psychology and the Generation of Culture. New York: Oxford University Press; 1992. pp. 487–503. [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex. 2006;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaris GA, Kim SG, Strupp JP, Andersen P, U urbil K, Georgopoulos AP. Quantitiative relations between parietal activation and performance in mental rotation. NeuroReport. 1996;7:773–776. doi: 10.1097/00001756-199602290-00022. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- Thomsen T, Hugdahl K, Ersland L, Barndon R, Lundevold A, Smievoll AI, Roscher BE, Sundberg H. Functional magnetic resonance imaging (fMRI) study of sex differences in a mental rotation task. Medical Science Monitor. 2000;6(6):1186–1196. [PubMed] [Google Scholar]
- Vandenburg SG, Kuse AR. Mental rotations, a group test of three-dimensional spatial visualizations. Perceptual and Motor Skills. 1978;47:599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: A meta-anlysis and consideration of crucial variables. Psychological Bulletin. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Voyer D. Scoring procedure, performance factors, and magnitude of sex differences in spatial performance. American Journal of Psychology. 1997;110(2):259–277. [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. The Journal of Neuroscience. 1995;15(5):3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TG, Tierson FD, Palmer CT. Evolution of sex differences in spatial cognition. Yearbook of physical anthropology. 1996;39:11–42. [Google Scholar]