Abstract
Stress, either physical or psychological, can have a dramatic impact on the immune system. Little progress, however, has been made in understanding stress-induced immune suppression. We report here that mice subjected to chronic 12-hour daily physical restraint for two days significantly increased the expression of Toll-like receptor 4 (TLR4). Interestingly, TLR4-deficient mice are resistant to stress-induced lymphocyte reduction. In addition, restraint stress caused dramatic decrease in T help 1 (Th1) cytokine IFN-γ and IL-2 levels but increase in Th2 cytokine IL-4 in wild type mice. Moreover, the restraint stress significantly inhibits changes of Th1 and Th2 cytokines in TLR4-deficient mice compared with the wild type mice. Therefore, stress modulates the immune system through a TLR4-dependent mechanism.
Keywords: Chronic stress, Mice, Microarray, TLR4, Th1 and Th2 cytokines
1. Introduction
Psychological and physical stressors influence cytokine responses (Stone and Bovbjerg, 1994; Wilder, 1995), cytolytic activity, lymphocyte proliferation (Gonzalez-Quijano et al., 1998), lymphocyte number (Yin et al., 2000; Pedersen and Nieman, 1998), and neuronal signal transmission (Marz et al., 1998). Many studies have revealed that exhausting physical activity and psychological stress have significant suppressive effects on the immune system (Ader and Cohen, 1993). Such suppression of the immune system has strong implications for disease susceptibility and progression (Dhabhar and McEwen, 1997), which is at least in part due to the reduction of lymphocytes (Zorrilla et al., 2001). Previous studies in both humans and animals have revealed that stress could promote autoimmunity (Shanks and Kusnecov, 1998) and infectious diseases by influencing the course and outcome of the pathological processes (Sheridan et al., 1998). Previous studies by Dhabhar and McEwen have shown that acute restraint stress could significantly enhance delayed-type hypersensitivity reaction (Dhabhar and McEwen, 1999), while chronic stress could decrease immune function and enhance susceptibility to diseases (Yin et al., 2000; Dhabhar and McEwen, 1997).
Apoptotic cell death is an active process mediated by various signaling pathways, which include cell death receptor Fas mediated pathway (Yin et al., 1999). Our previous studies utilizing the restraint stress model have shown that chronic restraint stress results in decreased splenic cellularity through Fas-mediated cell death pathway (Yin et al., 2000). We also have demonstrated that restraint stress could enhance the expression of pro-apoptotic genes (Yin et al., 2006). Though various alterations in the immune system have been revealed to be associated with stress, the exact mechanisms responsible for stress-modulated immune responses remain to be elucidated. To investigate which receptors and inflammatory cytokines might be involved in the effects of restraint stress, we compared gene expression patterns in the spleens of either stressed or non-stressed mice. Using oligo microarrays containing 113 genes of receptors and inflammatory cytokines, we found that 23 genes were up-regulated, including Toll-like receptor 4 (TLR4).
Toll-like receptors (TLRs) are an ancient and evolutionarily conserved receptor family that plays a critical role in the innate immune and inflammatory responses (Aderem and Ulevitch, 2000). TLRs recognize conserved pathogen-associated molecular patterns (PAMPs) shared by large groups of microorganisms and play an instructive role in induction of innate immune responses and also activation of adaptive immunity (Aderem and Ulevitch, 2000; Kaisho and Akira, 2002). The expression of TLRs has been detected in various types of cells including T cells (Caramalho et al., 2003), monocytes and macrophages (Guha and Mackman, 2001), and dendritic cells (Kaisho and Akira, 2003). TLR4 is essential for the recognition of lipopolysaccharide (LPS) from Gram-negative bacteria. TLR4 is expressed on dendritic cells and monocytes (Ahmad-Nejad et al., 2002), and T cells (Zanin-Zhorov et al., 2007; Xu et al., 2005; Caramalho et al., 2003), including CD4+ T cells (Xu et al., 2005; Caramalho et al., 2003) and CD8+ T cells (Xu et al., 2005). TLR4 can mediate cell death signaling through the interaction of the death domain of myeloid differentiation factor 88 (MyD88) with Fas associated death domain (FADD) (Haase et al., 2003). We show in this study that chronic stress modulates the immune function through a TLR4-dependent mechanism.
2. Materials and Methods
2.1. Mice
TLR4 mutant mice, C.C3H-Tlr4lps-d, on a Balb/c background and wild type Balb/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained in the Division of Laboratory Animal Resources at East Tennessee State University (ETSU), a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All aspects of the animal care and experimental protocols were approved by the ETSU Committee on Animal Care.
2.2. Physical restraint stress
Six- to seven-week-old male mice were subjected to an established chronic physical restraint protocol used in our laboratory as well as others (Yin et al., 2000; Yin et al., 2006; Sheridan et al., 1998). Briefly, mice were placed in a 50-ml conical centrifuge tube with multiple punctures to allow ventilation. Mice were held horizontally in the tubes for 12 h followed by a 12-h rest. During the rest period food and water were provided ad libitum. Control littermates were kept in their original cage and food and water were provided only during the 12 h rest. At 2 days after physical restraint, mice were sacrificed by CO2 asphyxiation, and the spleens were harvested.
2.3. Isolation of RNA from spleen tissue
Total RNA was isolated from spleens by the VERSAGENE™ RNA Tissue Kit (Gentra SYSTEMS; Minnesota), as described previously (Yin et al., 2006). DNAse I treatment (Qiagen, Chatsworth, CA) was applied to eliminate genomic DNA interference. RNA quality was determined by running a sample with a RNA loading dye (Sigma–Aldrich) on a 1% agarose gel and inspecting for distinct 18S and 28S bands, indicating lack of degradation. Quantity was determined by A260 measurement (Yin et al., 1999; Yin et al., 2000). Samples were frozen at −80 °C until use.
2.4. Analysis of gene expression using oligo gene microarrays
The methodology for microarray analysis has been described previously (Yin et al., 2006). Specific gene expression oligo GEArray systems were purchased from SuperArray Bioscience Corporation (Frederick, MD). Oligo GEArray mouse inflammatory cytokines and receptors array (catalog number: OMM-011) consists of 113 genes known to be involved in inflammatory cytokines and receptors-mediated signaling. Using this array, we compared the gene expressions in spleen tissues from mice with or without physical restraint stress. Briefly, using the reagents provided, the RNA was enzymatically converted to biotinylated cRNA target with the TrueLabeling-AMP™ 2.0 Kit in a standard thermal cycler. The resulting biotinylated cRNA probe mixture was allowed to hybridize overnight to the GEArray microarrays in a HybTube in a standard hybridization oven at 60 °C. Loading was adjusted based on the intensity of hybridization signals to the housekeeping gene, and then gene expression was quantified by scanning densitometry. Data were acquired and analyzed with the web-based software package GEArrayR Expression Analysis Suite provided by SuperArray Bioscience Corporation (Frederick, MD).
2.5. Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR)
The real-time RT-PCR detection technique was performed as described previously (Yin et al., 2006). Briefly, first-strand cDNA was synthesized from 1 μg of total RNA in a final volume 50 μl using a Reaction Ready™ first strand cDNA synthesis kit (SuperArray Bioscience Corporation, Frederick, MD). Two μl of 1:2 diluted cDNA was subjected to real-time quantitative PCR using Bio-Rad iCycler iQ Multicolor Real-Time PCR Detection System (Bio-Rad Life Science Research, Hercules, CA). PCR was performed in a 50 μl volume using RT2 real-time™ SYBR Green Fluorescien PCR Master Mix (SuperArray Bioscience Corporation). All primers were purchased from SuperArray Bioscience Corporation. All PCR assays were performed in triplicate. The reaction conditions were: 95 °C for 12 min; followed by 40 cycles at 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-Actin were amplified from all samples on each plate as housekeeping genes to normalize expression levels of targets between different samples, and to monitor assay reproducibility. The reaction mixtures without template cDNA were used as negative controls.
Threshold cycle numbers (CT) were determined with Bio-Rad iCycler iQ Multicolor Real-Time PCR Detection System (version 1.1 software) and transformed using the ΔCT comparative method. Gene-specific expression values were normalized to expression values of GAPDH and/or β-Actin (endogenous control) within each sample. The amount of target, normalized to an endogenous reference and relative to a calibrator, was determined by the comparative Ct method (ΔΔCT). In brief, the ΔCT value was determined by subtracting the average GAPDH CT and/or β-Actin value from the average target gene value in the same sample. The calculation of ΔΔCT involves subtraction of the ΔCT calibrator value.
2.6. Enzyme linked immunosorbent assay (ELISA) for cytokines
Splenic lymphocytes from TLR4-deficient mice and wild type mice were adjusted to a final concentration of 5 × 105 cells/ml in 96-well plates. Lymphocytes were treated with Con A (5 μg/ml). The supernatants were harvested after 24 hr (IL-2 and IFN-γ detection) or 48 hr (IL-4) of cultivation. The presence of cytokines in the supernatants was determined using cytokine-specific sandwich Enzyme linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacture’s instructions.
2.7. Statistical analysis
The results were presented as mean ± S.D. The data were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni tests to determine where differences among groups existed. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Microarray analysis of chronic stress-induced gene expression of inflammatory cytokines and receptors
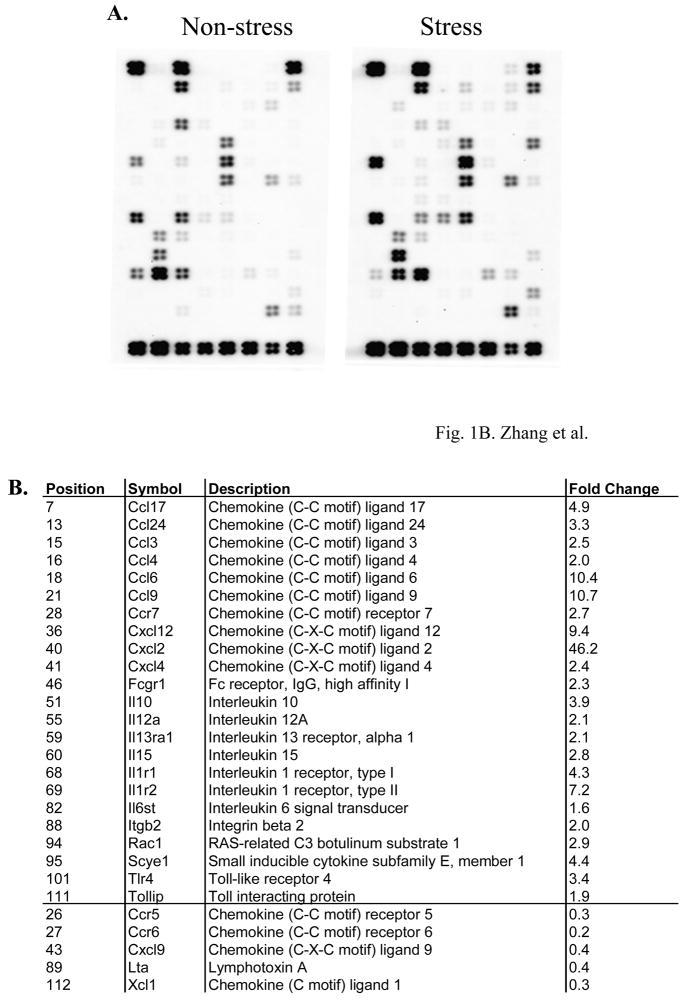
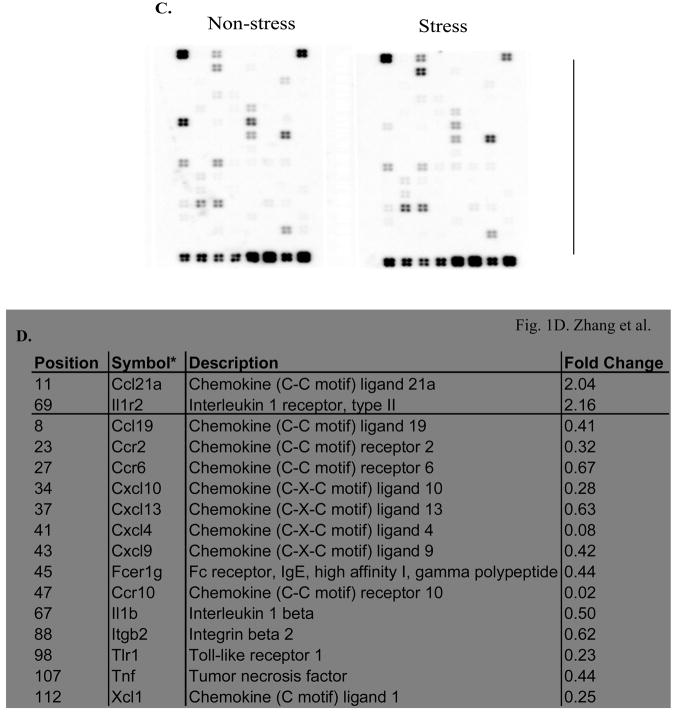
Psychological and physical stress influences cytokine responses (Stone and Bovbjerg, 1994; Wilder, 1995). We have reported previously that chronic stress induces lymphocyte cell death in the spleen through pro-apoptotic genes (Yin et al., 2000; Yin et al., 2006). To investigate whether inflammatory cytokines and receptors are involved in restraint stress-induced lymphocyte cell death we subjected Balb/c mice to a 12-h physical restraint daily for 2 days (Yin et al., 2000; Shi et al., 2003; Yin et al., 2006) and total RNA was isolated from the spleens of stressed and non-stressed mice and subsequent microarray analysis (Yin et al., 2006). Genes with more than a 2-fold increase or decrease in their expression were considered significant in the selection criteria. Microarray experiments were repeated three times with representative images shown in Figure 1A. The gene expression pattern was normalized for β-actin and GAPDH. Cyclophilin A (PPIA) expression was used as a control. Twenty three of the 113 genes were increased and 5 were decreased in physical restraint stress compared with non-stressed mice (Fig. 1B). Moreover, the expression of fold changes of some of these genes was verified by quantitative real time RT-PCR (see below). TLR4 is not only important in the modulation of innate and adaptive immune responses (Aderem and Ulevitch, 2000; Medzhitov et al., 1997; Gan and Li, 2006), but also participated in noninfectious inflammatory diseases (Jiang et al., 2005; Seki et al., 2007). To determine the response of inflammatory cytokines and receptors to restraint stress in TLR4-deficient mice, 6-8-week-old TLR4-deficient mice were subjected to a 12-h daily physical restraint daily for 2 days (Yin et al., 2000; Shi et al., 2003; Yin et al., 2006) and the spleens were harvested for total RNA isolation and subsequent microarray analysis. Microarray experiments with representative images shown in Figure 1C. Fourteen of 113 genes were decreased and 2 were increased in restraint stress of TLR4-deficient mice compared with non-stressed mice (Fig. 1D).
Fig. 1.
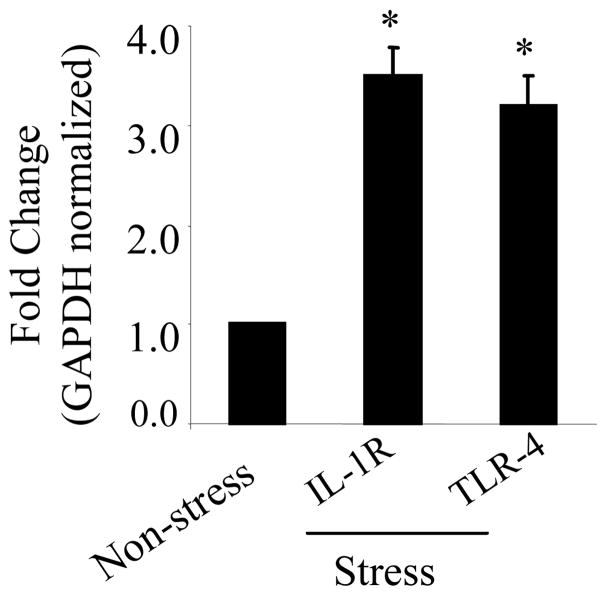
3.2. Verification of differentially expressed genes with quantitative real-time RT-PCR
To validate the gene expression data obtained from microarray analysis, we analyzed the mRNA by quantitative RT-PCR for 2 genes that were up-regulated in the spleens of restraint stress mice compared to the non-stressed control mice. Quantitative real time RT-PCR demonstrated a significant increase in expression of TLR4 and interleukin 1 receptor (IL-1R) in the spleen from the restraint stressed mice (Fig. 2). Gene expression of TLR4 and IL-1R from the spleens of non-stressed mice was used as experimental control and GAPDH as the housekeeping gene for normalizing expression.
Fig. 2.
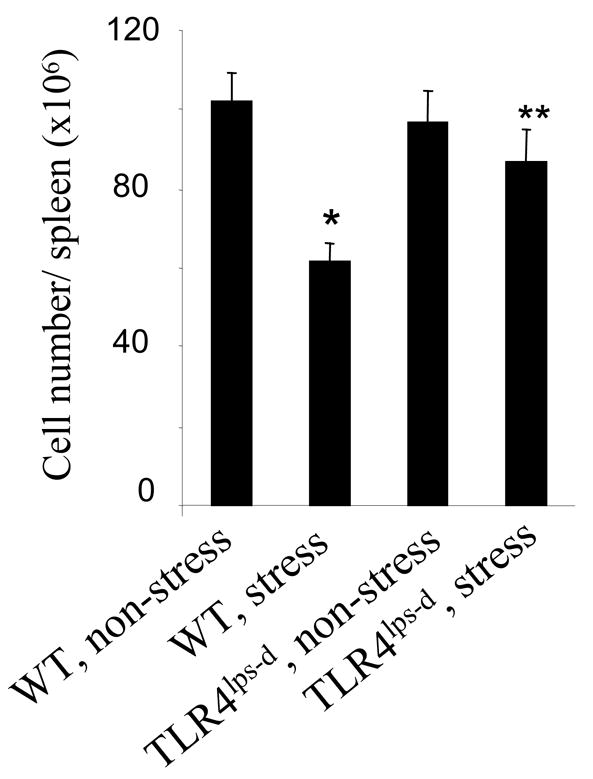
3.3. TLR4 deficient mice are resistant to restraint stress induced lymphocyte reduction
It has been reported that overexpression of TLR4 sensitizes cells to serum deprivation-induced apoptosis (Fan et al., 2005). Since restraint stress dramatically increased TLR4 expression (Figs. 1 and 2), its consequence on the TLR4 function was next examined in restraint stress. We subjected mice with loss of function mutations in TLR4 (C.C3H-TLR4lps-d), and their appropriate control Balb/c (wild type), to physical restraint for 12 hours daily. At 2 days after restraint stress, a reduction in the number of splenocytes was only observed in wild type Balb/c mice, but not in C.C3H-TLR4lps-d mice (Fig. 3). Therefore, mice without functional TLR4 lose their sensitivity to restraint stress-induced reduction in the number of splenocytes, supporting the conspicuous role of TLR4 in stress response.
Fig. 3.
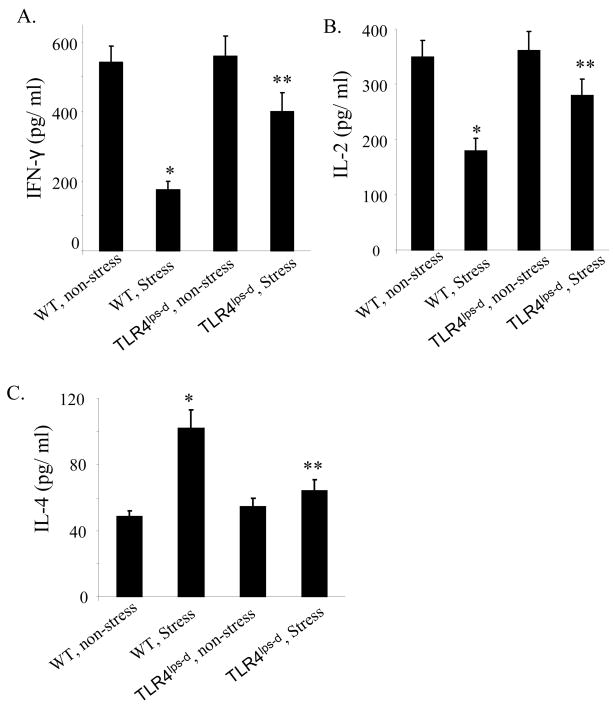
3.5. Effect of TLR4 on the balance between Th1 and Th2 cytokine levels in restraint stress
Since TLRs play a critical role in regulating inflammatory response and innate immunity (Baeuerle and Baltimore, 1996; Wang et al., 2006), we next determined the effects of restraint stress on the T helper 1 (Th1) and Th2 cytokine production in TLR4-deficient mice and wild type mice. At 2 days after restraint stress of mice, culture supernatants from Con A-stimulated lymphocytes were analyzed for the levels of Th1 cytokines, IFN-γ and IL-2, and Th2 cytokine IL-4 by ELISA. We found that splenocytes from stressed wild type mice produced dramatically less IFN-γ (Fig. 4A) and IL-2 (Fig. 4B) and significantly more IL-4 production (Fig. 4C) than splenocytes from non-stressed wild type mice. Although restraint stress of mice significantly alters the levels of Th1 and Th2 cytokines in TLR4-deficient mice compared with non-stressed mice, interestingly, the restraint stress significantly inhibits changes of Th1 and Th2 cytokines in TLR4-deficient mice compared with the wild type mice (Fig. 4). Therefore, the inhibition of Th1 cytokines and the induction of Th2 cytokines by restraint stress are mediated through a TLR4-dependent mechanism.
Fig. 4.
4. Discussion
Various studies with different model systems have revealed that stress could either enhance or reduce immune function (Ader and Cohen, 1993). It is generally believed that acute and moderate stress could enhance immune function, while chronic stress often results in reduction of immune function and increase in the susceptibility to diseases (Dhabhar and McEwen, 1997; Shi et al., 2003). However, the mechanisms by which stress affects immune function remain to be elucidated. We have reported previously that restraint stress of mice decreased the number of lymphocytes through cell death receptor Fas (Yin et al., 2000; Yin et al., 2006).
In the present study, we assessed the involvement of inflammatory cytokine levels and receptor gene expression profile during the restraint stress of wild type mice using microarray analysis. Among 113 genes analyzed, 28 genes showed dramatic differences between stressed and non-stressed mice. The differentially expressed genes for the TLR4 and IL-1 receptor were confirmed by real-time quantitative RT-PCR (Fig. 2). We also examined the responses of inflammatory cytokines and receptor gene expression profile in restraint stress of TLR4-deficient mice using microarray analysis. We found that in restraint stress of TLR4-deficient mice the levels of 14 genes decreased, including pro-inflammatory genes, such as tumor necrosis factor (TNF-alpha), interleukin 1 beta (IL-1 beta) (Figs. 1C and 1D). Our data support that TLR4 plays an important role in inflammatory responses (Jiang et al., 2005; Seki et al., 2007). Our results are consistent with the findings from other laboratories (Shahrara et al., 2006; Loos et al., 2006), who have reported that activation of TLR4 signaling increases pro-inflammatory cytokine levels. Specifically, we investigated the role of TLR4 in stress-induced immune suppression. TLR-mediated signaling mainly modulates intracellular signaling pathways, such as nuclear factor-kappaB (NF-κB), which plays a fundamental role in regulating innate immunity and inflammation as well as cell proliferation, and cell apoptosis (Baeuerle and Baltimore, 1996; Wang et al., 2006). It is known that different TLRs have distinct patterns of cellular expression (Ahmad-Nejad et al., 2002). TLR4 is expressed on dendritic cells and monocytes (Ahmad-Nejad et al., 2002), CD4+ T cells (Xu et al., 2005; Caramalho et al., 2003), and CD8+ T cells (Xu et al., 2005). The most significant finding reported herein is the observation that TLR4-deficient mice showed a significant inhibition of restraint stress-induced reduction in lymphocyte numbers compared with age-matched wild type control mice, indicating that TLR4 is a novel receptor contributing to the lymphocyte reduction induced by chronic stress.
We observed that restraint stressed mice developed dramatic decreases in Th1 cytokine IFN-γ and IL-2 levels. We also found that the Th2 cytokine IL-4 was significantly increased by restraint stress (Fig. 4). These data demonstrated that restraint stress induces an imbalance the Th1 and Th2 responses. Interestingly, we showed that restraint stress of TLR4-deficient mice significantly inhibits changes of Th1 and Th2 cytokines compared with restraint stress of the wild type mice (Fig. 4). These results indicate that TLR4 plays a critical role in chronic restraint stress-induced imbalance of Th1/Th2 cytokines. Thus, restraint stress-induced immune suppression is mediated through a TLR4-dependent mechanism. In our previous publication (Yin et al., 2000), we performed restraint stress in adrenalectomized mice. We found that adrenalectomy did not significantly affect the stress-induced splenocyte reduction (Yin et al., 2000). Therefore, the hypothalamo-pituitary-adrenal (HPA) axis is unlikely to be involved in mediating the reduction of splenocytes in our established restraint stress model, which is an important finding. Our finding of the absence of an effect of adrenalectomy on restraint stress–induced reduction in splenocytes is in contrast with the demonstration of the role of the adrenal gland in acute stress–induced enhancement of delayed-type hypersensitivity response (Dhabhar and McEwen, 1996). Indeed, McEwen et al. (McEwen et al., 1997) have indicated that the spleen is a relatively privileged site and is inaccessible to endogenously produced corticosteroids. Therefore, we did not further determine the effect of the HPA axis in the same restraint stress animal model.
In summary, to our knowledge this study is the first to demonstrate that the TLR4 is involved in restraint stress-mediated immune alterations. Our data indicate that inhibition of TLR4 activation pathway may be an effective approach for prevention and/or treatment of stress-induced immune suppression.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant DA020120 and East Tennessee State University Research Development Committee (ETSU RDC) 0048 to D. Yin. This work was also supported in part by ETSU RDC grant 07-026M to G. Hanley and National Natural Science Foundation of China (30470404) to J. Miao.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ader R, Cohen N. Psychoneuroimmunology: conditioning and stress. Annu Rev Psychol. 1993;44:53–85. doi: 10.1146/annurev.ps.44.020193.000413. [DOI] [PubMed] [Google Scholar]
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Stress-induced enhancement of antigen-specific cell-mediated immunity. J Immunol. 1996;156:2608–2615. [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Ha T, Li Y, Ozment-Skelton T, Williams DL, Kelley J, Browder IW, Li C. Overexpression of TLR2 and TLR4 susceptibility to serum deprivation-induced apoptosis in CHO cells. Biochem Biophys Res Commun. 2005;337:840–848. doi: 10.1016/j.bbrc.2005.09.123. [DOI] [PubMed] [Google Scholar]
- Gan L, Li L. Regulations and roles of the interleukin-1 receptor associated kinases (IRAKs) in innate and adaptive immunity. Immunol Res. 2006;35:295–302. doi: 10.1385/IR:35:3:295. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quijano MI, Martin M, Millan S, Lopez-Calderon A. Lymphocyte response to mitogens: influence of life events and personality. Neuropsychobiology. 1998;38:90–96. doi: 10.1159/000026523. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Haase R, Kirschning CJ, Sing A, Schrottner P, Fukase K, Kusumoto S, Wagner H, Heesemann J, Ruckdeschel K. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J Immunol. 2003;171:4294–4303. doi: 10.4049/jimmunol.171.8.4294. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr Mol Med. 2003;3:373–385. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- Loos T, Dekeyzer L, Struyf S, Schutyser E, Gijsbers K, Gouwy M, Fraeyman A, Put W, Ronsse I, Grillet B, Opdenakker G, Van Damme J, Proost P. TLR ligands and cytokines induce CXCR3 ligands in endothelial cells: enhanced CXCL9 in autoimmune arthritis. Lab Invest. 2006;86:902–916. doi: 10.1038/labinvest.3700453. [DOI] [PubMed] [Google Scholar]
- Marz P, Cheng JG, Gadient RA, Patterson PH, Stoyan T, Otten U, Rose-John S. Sympathetic neurons can produce and respond to interleukin 6. Proc Natl Acad Sci U S A. 1998;95:3251–3256. doi: 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Nieman DC. Exercise immunology: integration and regulation. Immunol Today. 1998;19:204–206. doi: 10.1016/s0167-5699(98)01255-9. [DOI] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- Shahrara S, Park CC, Temkin V, Jarvis JW, Volin MV, Pope RM. RANTES modulates TLR4-induced cytokine secretion in human peripheral blood monocytes. J Immunol. 2006;177:5077–5087. doi: 10.4049/jimmunol.177.8.5077. [DOI] [PubMed] [Google Scholar]
- Shanks N, Kusnecov AW. Differential immune reactivity to stress in BALB/cByJ and C57BL/6J mice: in vivo dependence on macrophages. Physiol Behav. 1998;65:95–103. doi: 10.1016/s0031-9384(98)00149-8. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D, Glaser R. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann N Y Acad Sci. 1998;840:803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Devadas S, Greeneltch KM, Yin D, Allan MR, Zhou JN. Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology. Brain Behav Immun . 2003;17(Suppl 1):S18–S26. doi: 10.1016/s0889-1591(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Stone AA, Bovbjerg DH. Stress and humoral immunity: a review of the human studies. Adv Neuroimmunol. 1994;4:49–56. doi: 10.1016/s0960-5428(06)80189-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- Wilder RL. Neuroendocrine-immune system interactions and autoimmunity. Annu Rev Immunol. 1995;13:307–338. doi: 10.1146/annurev.iy.13.040195.001515. [DOI] [PubMed] [Google Scholar]
- Xu D, Komai-Koma M, Liew FY. Expression and function of Toll-like receptor on T cells. Cell Immunol. 2005;233:85–89. doi: 10.1016/j.cellimm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Yin D, Mufson RA, Wang R, Shi Y. Fas-mediated cell death promoted by opioids. Nature. 1999;397:218. doi: 10.1038/16612. [DOI] [PubMed] [Google Scholar]
- Yin D, Tuthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J Exp Med. 2000;191:1423–1428. doi: 10.1084/jem.191.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D, Zhang Y, Stuart C, Miao J, Zhang Y, Li C, Zeng X, Hanley G, Moorman J, Yao Z, Woodruff M. Chronic restraint stress modulates expression of genes in murine spleen. J Neuroimmunol. 2006;177:11–17. doi: 10.1016/j.jneuroim.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, Cohen-Sfady M, Pevsner-Fischer M, Lider O, Cohen IR. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J Immunol. 2007;179:41–44. doi: 10.4049/jimmunol.179.1.41. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]