Abstract
Disruption of protein-protein interactions by small molecules is achievable but presents significant hurdles for effective compound design. In earlier work we identified a series of thiazolidinone inhibitors of the bacterial type III secretion system (T3SS) and demonstrated that this scaffold had the potential to be expanded into molecules with broad-spectrum anti-Gram negative activity. We now report on one series of thiazolidinone analogs in which the heterocycle is presented as a dimer at the termini of a series of linkers. Many of these dimers inhibited the T3SS-dependent secretion of a virulence protein at concentrations lower than that of the original monomeric compound identified in our screen.
A possible therapeutic solution to the problem of bacterial resistance to existing antibiotics is to discover drugs that will block pathogenic mechanisms rather than killing the infecting microbe. These pathogenic mechanisms include secretion systems such as the type III secretion system (T3SS) that deliver a variety of pathogen proteins using multicomponent oligomeric structures. Although many of the secreted virulence proteins are species-specific, the secretion systems are more conserved across species, indicating that disruption of such secretion systems is potentially a broad-spectrum therapeutic strategy. Because the T3SS is not required for bacterial growth per se, this strategy might spare commensals and limit bacterial resistance. In contrast, antibiotics that inhibit microbial growth exert a strong selection pressure for resistance.1 In recent years the T3SS machinery has become an aggregate target for drug discovery.2–4
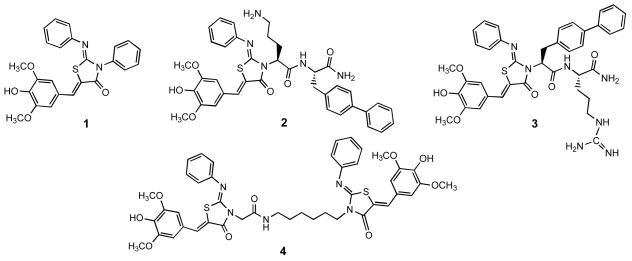
Previously our group identified a tris-aryl substituted 2-imino-5-arylidenethiazolidin-4-one, compound 1, as a broad spectrum inhibitor of Gram-negative bacterial secretion systems (Figure 1).5 Expansion of this chemotype enabled us to define the functional groups that could or could not be manipulated to synthetically evolve potent new analogs. Modifications at the heterocycle amido nitrogen were not only tolerated but gave rise to a series of novel dipeptide-modified congeners, for example 2 and 3, that showed enhanced potency and physiochemical properties.5, 6 We considered the functional architecture of the T3SS and speculated that these compounds might be fragments occupying only one of two inter-monomer binding sites. Prompted by this hypothesis we synthesized a bis-thiazolidinone dimer, 4.
Figure 1.
The original HTS hit thiazolidinone 1, two potent N-3 dipeptide analogs 2 and 3, and the dimer 4.
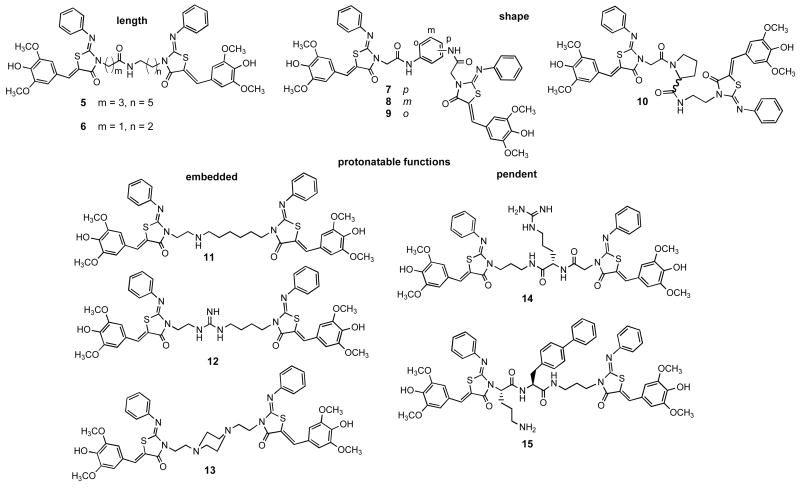
We analyzed dimer 4 for inhibition of the T3SS in S. typhimurium by monitoring secretion of a predominant substrate, SipA, into culture supernatants. Supernatant proteins were TCA precipitated, separated by SDS-PAGE and Western blotted with anti-SipA antibody. Evaluation of 4 showed a substantial increase in potency over 1, with IC50 values of 5μM versus 83 μM respectively, but the poor solubility of 4 precluded further biological characterization of this compound. The significant decrease in the IC50 prompted us to prepare a panel of dimers, with the goal of improving the solubility of this compound and exploring the optimal inter-thiazolidinone distance and juxtaposition. For this panel, tethers were constructed that varied in length, flexibility, charge, and pendent functional groups, providing divergent presentations of the terminal thiazolidinones (Figure 2). The linear analogs 5 and 6 expand and contract overall thiazolidinone-to-thiazolidinone distance and give different placements of the amide function. In contrast to the flexible amides, the para, meta, and ortho diamidophenyl central cores rigidly enforce three distinct shapes (7 – 9). Insertion of a proline (10) introduces two possible kinks in the tether depending on the populations of cis and trans conformations. The five analogs that are cationic at physiological pH (11 – 15) can be divided into the embedded and pendent classes. Monoamine 11 is highly flexible, whereas guanidine 12 will be somewhat more rigid, and piperazine 13 is likely to assume the shape determined by a di-equatorial chair conformation. The linker in compound 14 is flexible and projects the cationic function away from the axis of the dimer. Dipeptide 15 incorporates the beneficial sequence of the potent mono-thiazolidinone 25, 6 into the motif of 4.
Figure 2.
Dimeric analogs 5–15 use the tether to introduce spatial and functional group properties.
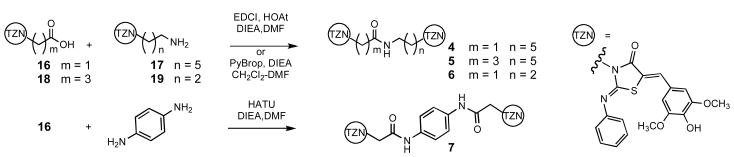
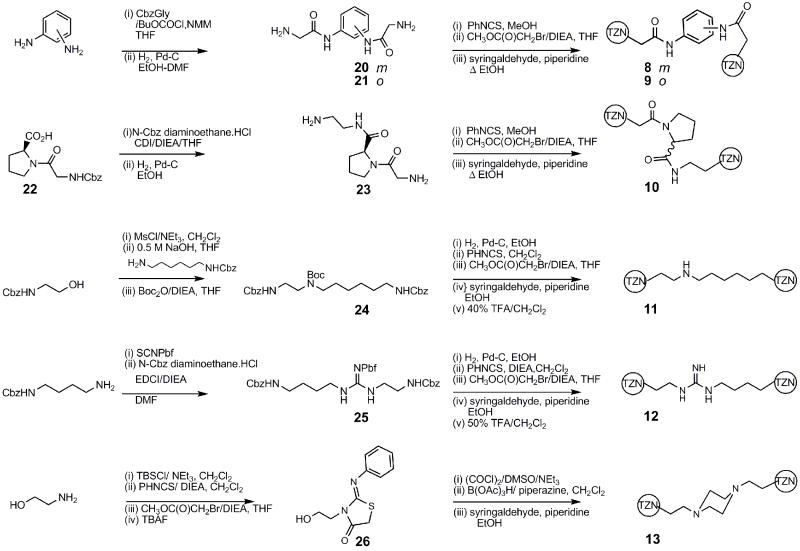
The syntheses of the dimers followed either a general end-to-end7 (Scheme 1) or a center-to-outside 8 (Scheme 2) strategy. In all the analogs, the substituted thiazolidinone ring was assembled by the method of Klika.9
Scheme 1.
Each completely substituted thiazolidinone terminates in either an amine or a carboxylic acid that reacts with the complementary function to form the dimer.
Scheme 2.
Each tether terminates in two free amino groups that are simultaneously assembled into the substituted thiazolidinone.
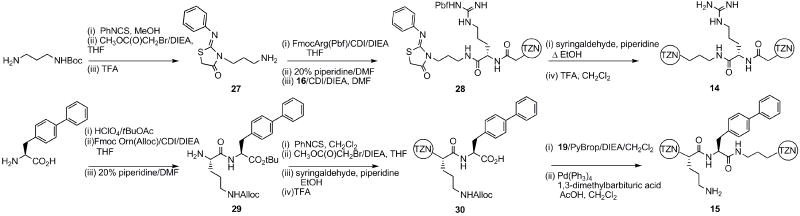
The analogs presenting pendent amino acids, 14 and 15, were prepared by essentially linear routes (Scheme 3).
Scheme 3.
The synthesis of 14 and 15.
We evaluated these dimeric thiazolidinones for inhibition of the T3SS in S. typhimurium by again analyzing secretion of the SipA protein into culture supernatants. All of the dimeric compounds, with the exception of 7, which was too insoluble to evaluate, were comparable to or slightly more potent than the original hit compound 1 (Table 1). These data suggest that these compounds may bind as 4-substituted thiazolidinone monomers, with the additional ring and intervening tether being innocuous but not overwhelmingly beneficial. Amide 4 is more potent than the corresponding amine 11 or guanidine 12. This may indicate a role for the carbonyl in a critical hydrogen bond and/or result from a deleterious effect of cationic charge along the tether. The greater potency of 5 compared with 6 would argue against the carbonyl’s position as a critical feature. Indeed, it is the longest linear amide 5 and the most rigidly kinked ortho diaminobenzene amide 9, two uncharged analogs, that distinguish themselves among the new compounds by having potency significantly greater than 1. Overall, compound 4 remains the most potent of the dimers, and may represent an optimum of shape, flexibility, and carbonyl placement for the cognate binding site. Alternative binding sites along the inter-protein interface are also possible and would be in agreement with the lack of a single comprehensive structure-activity trend among the dimers. The specific contribution of each individual thiazolidinone ring to the activity of the dimer remains undetermined, and we have no evidence that these rings are acting in tandem. While a definitive identification of the thiazolidinone binding site(s) will be best determined by structural biology, our results are consistent with these compounds inhibiting protein-protein interactions along a large oligomeric interface.
Table 1.
Inhibition of SipA secretion by the dimeric analogs of 1.
| compound | IC50 (μM) |
|---|---|
| 1 | 83 |
| 4 | 5 |
| 5 | 17 |
| 6 | 48 |
| 7 | n.d. |
| 8 | 80 |
| 9 | 22 |
| 10 | 55 |
| 11 | 65 |
| 12 | 47 |
| 13 | 44 |
| 14 | 48 |
| 15 | 49 |
Supplementary Material
Experimental data including analytical characterization of all compounds can be found in Supplementary data accessible online.
Acknowledgments
The support of NIAID (U54 A105714) is gratefully acknowledged. The investigators are members of the Northwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Keyser P, Elofsson M, Rosell S, Wolf-Watz H. J Intern Med. 2008;264:17. doi: 10.1111/j.1365-2796.2008.01941.x. [DOI] [PubMed] [Google Scholar]
- 2.Kauppi AM, Andersson CD, Norberg HA, Sundin C, Linusson A, Elofsson M. Bioorg Med Chem. 2007;15:6994. doi: 10.1016/j.bmc.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 3.Kauppi AM, Nordfelth R, Uvell H, Wolf-Watz H, Elofsson M. Chem Biol. 2003;10:241. doi: 10.1016/s1074-5521(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 4.Negrea A, Bjur E, Ygberg SE, Elofsson M, Wolf-Watz H, Rhen M. Antimicrob Agents Chemother. 2007;51:2867. doi: 10.1128/AAC.00223-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felise HB, Nguyen HV, Pfuetzner RA, Barry KC, Jackson SR, Blanc MP, Bronstein PA, Kline T, Miller SI. Cell Host Microbe. 2008;4:325. doi: 10.1016/j.chom.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kline T, Felise HB, Barry KC, Jackson SR, Nguyen HV, Miller SI. J Med Chem. 2008;51:7065. doi: 10.1021/jm8004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.4 2-((2Z,5Z)-5-(4-hydroxy-3,5-dimethoxybenzylidene)-4-oxo-2-(phenylimino)thiazolidin-3-yl)-N-(6-((2Z,5Z)-5-(4-hydroxy-3,5-dimethoxybenzylidene)-4-oxo-2-(phenylimino)thiazolidin-3-yl)hexyl)acetamide (4). To a solution of 176 (24 mg, 0.053 mmol) in 0.5 mL DMF at 0 °C was added 166 (25 mg, 0.061 mmol), DIEA (9.2 μL, 0.053 mmol), HOAt (7 mg, 0.053 mmol), and after 5 min, EDCI (16 mg, 0.053 mmol). The reaction mixture was allowed to warm to room temperature and stirred overnight. The crude reaction mixture was suspended in CHCl3 and washed with H2O, 1 mM citric acid, and NaHCO3. The organic layer was dried over Na2SO4 and concentrated in vacuo. The crude solid was purified via silica gel chromatography using a gradient from 0 to 10% MeOH in CHCl3 to give 4 (5.3 mg, 0.006 mmol). 1H NMR (300 MHz, DMSO-d6, δ): 1.28–1.40 (m, 4H), 1.40–1.53 (m, 2H), 1.60–1.76 (m, 2H), 3.06–3.19 (m, 2H), 3.74 (s, 12H), 3.87 (t, J = 7.5 Hz, 2H), 4.47 (s, 2H), 6.83 (s, 2H), 6.84 (s, 2H), 6.98 (d, J = 7.5 Hz, 2H), 7.03 (d, J = 7.5 Hz, 2H), 7.11–7.23 (m, 2H), 7.32–7.46 (m, 4H), 7.68 (s, 1H), 7.70 (s, 1H), 8.20 (t, J = 5.3 Hz, 1H), 9.25 (s, 1H), 9.27 (s, 1H). 13C NMR (500 MHz, DMSO-d6, δ): 30.36, 30.48, 31.26, 33.44, 43.04, 47.03, 49.43, 60.62, 112.61, 121.91, 121.99, 125.51, 125.57, 128.07, 128.12, 129.28, 133.87, 135.72, 135.93, 142.90, 142.97, 151.90, 152.27, 152.64, 154.14, 154.25, 169.76, 170.27, 170.37. MS m/z 852 [M + H]+, 874 [M + Na]+. HRMS (m/z): [M + Na]+ calcd for C44H45N5O9NaS2, 874.2551; found 874.2551.
- 8.1-(4-(4-oxo-2-(phenylimino)thiazolidin-3-yl)butyl)-3-(2-(4-oxo-2-(phenylimino)thiazolidin-3-yl)ethyl) 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl guanidine (12). Benzyl 4-aminobutylcarbamate (89 mg, 0.40 mmol) was dissolved in CH2Cl2 (2 mL), 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl isothiocyanate (125 mg, 0.40 mmol) was added, and the solution was stirred for 16 h. The reaction mixture was partitioned between EtOAc (10 mL), and water (5 mL). The organic phase was washed with 5 mL of, successively, NaHCO3 and NaCl, dried, and concentrated in vacuo to the crude thiourea. This was dissolved in DMF (3 mL) and diisopropylethylamine (35 μL, 0.20 mmol), benzyl 2-aminoethylcarbamate HCl (28 mg, 0.14 mmol), and EDCI (35 mg, 0.12 mmol) were added. After stirring 48 h the solution was concentrated in vacuo, dissolved in EtOAc (10 mL), and water (10 mL) was added. The organic layer was washed with water (2×10 mL) and NaCl solution (10 mL), dried, and concentrated in vacuo. The residue was purified via silica gel chromatography using a gradient from 0 to 80 % EtOAc in CHCl3 to give 25 (65 mg, 0.09 mmol). 1H NMR (500 MHz, CDCl3, δ): 1.43–1.48 (m, 10H), 2.03 (s, 3H), 2.50, (s, 3H), 2.57 (s, 3H), 2.91 (s, 2H), 3.14–3.27 (m, 4H), 5.05 (s, 4H), 7.30 (s, 10H). MS m/z 694.4 [M + H]+. Biscarbamate 25 (65 mg, 0.09 mmol) was dissolved in EtOH (5 mL), Pd-C (80 mg) was added, and hydrogen gas was bubbled through the slurry for 2 h. The resulting suspension was filtered through celite, rinsed with MeOH (5 mL), and concentrated in vacuo. The residue was dissolved in CH2Cl2 (4 mL), and phenyl isothiocyanate (28 μL, 0.23 mmol) and diisopropylethylamine (100 μL, 0.57 mmol) were added. After 16 h, the solution was concentrated in vacuo and redissolved in THF (4 mL). Diisopropylethylamine (80 μL, 0.46 mmol) and methyl bromoacetate (25 μL, 0.27 mmol) were added, and the solution was stirred for 16 h. EtOAc (5 mL) and NaHCO3 solution (5 mL) were added, the organic layer was washed with NaCl (5 mL), dried and concentrated in vacuo. The residue was purified via silica gel chromatography using a gradient from 0 to 80 % EtOAc in CHCl3 to give the thiazolidinone (50 mg, 0.07 mmol). 1H NMR (300 MHz, CDCl3, δ): 1.44 (s, 6H), 1.50–1.65 (m, 4H), 2.07 (s, 3H), 2.52 (s, 3H), 2.59 (s, 3H), 2.92 (s, 2H), 2.95 (br, 2H), 3.55 (br, 2H), 3.71–3.82 (m, 6H), 3.98 (br, 2H), 6.92 (t, J = 7.0 Hz, 4H), 7.14 (t, J = 7.3 Hz, 2H), 7.30–7.37 (m, 4H). MS m/z 776.3 [M + H]+. To a solution of the thiazolidinone (11 mg, .014 mmol) and piperidine (25 μL, .253 mmol) in EtOH (3 mL) syringaldehyde (10 mg, .055 mmol) was added and the solution was heated to 90 °C for 48 h. The solution was concentrated in vacuo, and the residue was dissolved in CH2Cl2 (0.5 mL) and trifluoroacetic acid (0.5 mL), and stirred for 1 h. After removing the volatiles in vacuo, the residue was purified via preparative HPLC using a gradient of 10 to 40% B in 5 min, then 40 to 95% B in 20 min to give 12 (4.3 mg, .005 mmol). 1H NMR (500 MHz, CDCl3, δ): 1.68–1.86 (m, 4H), 3.65 (t, J = 5.5 Hz, 2H), 3.72 (s, 12H), 3.96 (t, J = 6.8 Hz, 2H), 4.16 (t, J = 5.5 Hz, 2H), 6.62 (s, 4H), 7.00 (d, J = 7.5 Hz, 2H), 7.06 (d, J = 7.5 Hz, 2H), 7.17–7.20 (m, 4H), 7.55 (s, 1H), 7.59 (s, 1H). 13C NMR (500 MHz, CD3OD, δ): 25.82, 25.84, 27.15, 42.51, 43.41, 56.75, 56.78, 108.98, 109.02, 118.98, 122.39, 122.49, 125.55, 125.66, 126.15, 126.35, 130.48, 130.53, 133.00, 133.64, 139.79, 149.18, 149.48, 149.53, 152.27, 152.35, 157.77, 168.60. HRMS (m/z): [M + H]+ calcd for C43H46N7O8S2, 852.2844; found 852.2840.
- 9.Klika KD, Valtamo P, Janovec L, Suchar G, Kristian P, Imrich J, Kivela H, Alfoldi J, Pihlaja K. Rapid Commun Mass Spectrom. 2004;18:87. doi: 10.1002/rcm.1290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental data including analytical characterization of all compounds can be found in Supplementary data accessible online.