Abstract
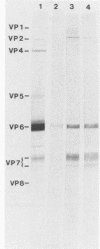
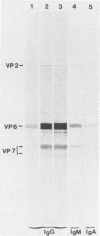
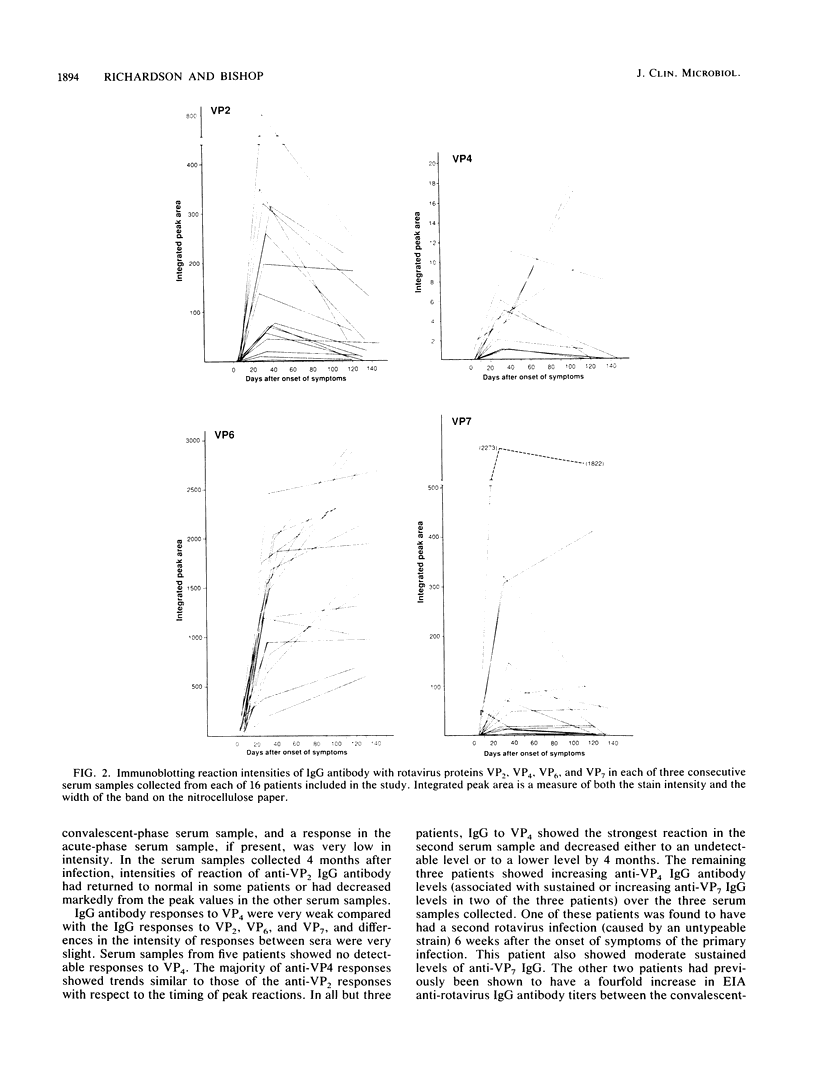
The class-specific antibody responses to serotype 1 rotavirus structural proteins were examined by immunoblotting with sera obtained from young children hospitalized with acute rotavirus diarrhea caused by serotype 1. All were believed to be primary infections. Three consecutive samples were obtained from 16 patients during the acute and convalescent phases of the disease and then approximately 4 months later. Immunoglobulin G (IgG)-class antibody responses to two inner capsid proteins (VP2 and VP6) and to the major homologous outer capsid protein (VP7) were detected in all patients. Antibody responses to VP6 were rapid, increased in intensity during 20 to 40 days after the onset of symptoms, and persisted for more than 4 months. Responses to VP2 and VP7 were more delayed, were maximal in convalescent-phase sera, and decreased markedly in intensity 4 months after the onset of symptoms in the majority of children. Two patients with evidence of mixed infection showed persisting high levels of antibody to VP7. Responses to the outer capsid protein VP4 were detected in 67% of patients, peaked at 20 to 40 days after the onset of symptoms, and were no longer detected at 4 months in the majority of patients. It is likely that the immunoblotting technique underestimated responses to VP4. Acute- and convalescent-phase sera (known to contain antirotavirus IgM or IgA measured by enzyme immunoassay) were also examined by immunoblotting. IgM- and IgA-class antibody responses to viral proteins VP2, VP4, and VP7 appeared to be qualitatively identical to those observed for IgG in the same serum samples.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Bishop R. F. Cultivation of human rotaviruses in cell culture. J Med Virol. 1984;13(4):377–383. doi: 10.1002/jmv.1890130409. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Borian F. E., Bell L. M., Modesto K., Gouvea V., Plotkin S. A. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988 Sep;158(3):570–587. doi: 10.1093/infdis/158.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Unicomb L. E., Pitson G. A., Bishop R. F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol. 1987 Mar;25(3):509–515. doi: 10.1128/jcm.25.3.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S. Variation in neutralization epitopes of human rotaviruses in relation to genomic RNA polymorphism. Virology. 1987 Aug;159(2):209–216. doi: 10.1016/0042-6822(87)90457-0. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y. Rotavirus antigens. Adv Exp Med Biol. 1985;185:201–214. doi: 10.1007/978-1-4684-7974-4_13. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Blanco M., Vilar M., Garcia D., Perez M., Daoud N., Midthun K., Kapikian A. Z. Reactions to and antigenicity of two human-rhesus rotavirus reassortant vaccine candidates of serotypes 1 and 2 in Venezuelan infants. J Clin Microbiol. 1989 Mar;27(3):512–518. doi: 10.1128/jcm.27.3.512-518.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Gonzalez M., Garcia D., Perez M., Daoud N., Cunto W., Chanock R. M., Kapikian A. Z. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet. 1987 Apr 18;1(8538):882–884. doi: 10.1016/s0140-6736(87)92858-3. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Taniguchi K., Mackow E. R., Kapikian A. Z. Homotypic and heterotypic epitope-specific antibody responses in adult and infant rotavirus vaccinees: implications for vaccine development. J Infect Dis. 1990 Apr;161(4):667–679. doi: 10.1093/infdis/161.4.667. [DOI] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood K., Lund J. C., Coulson B. S., Hudson I. L., Bishop R. F., Barnes G. L. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol. 1988 Apr;26(4):732–738. doi: 10.1128/jcm.26.4.732-738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon P., Hanlon L., Marsh V., Byass P., Shenton F., Hassan-King M., Jobe O., Sillah H., Hayes R., M'Boge B. H. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987 Jun 13;1(8546):1342–1345. doi: 10.1016/s0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Greenberg H. B., Wyatt R. G., Kalica A. R., Banks C. E., James H. D., Jr, Flores J., Chanock R. M. Antigenic characterization of human and animal rotaviruses by immune adherence hemagglutination assay (IAHA): evidence for distinctness of IAHA and neutralization antigens. Infect Immun. 1981 Aug;33(2):415–425. doi: 10.1128/iai.33.2.415-425.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Midthun K., Hoshino Y., Green K. Y., Gorziglia M., Nishikawa K., Chanock R. M., Potash L., Perez-Schael I. Strategies for the development of a rotavirus vaccine against infantile diarrhea with an update on clinical trials of rotavirus vaccines. Adv Exp Med Biol. 1989;257:67–89. doi: 10.1007/978-1-4684-5712-4_9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Fong K. J., Losonsky G. A., Levine M. M., Maldonado Y., Yolken R., Flores J., Kapikian A. Z., Vo P. T., Greenberg H. B. Epitope-specific immune responses to rotavirus vaccination. Gastroenterology. 1987 Nov;93(5):941–950. doi: 10.1016/0016-5085(87)90555-5. [DOI] [PubMed] [Google Scholar]
- Svensson L., Sheshberadaran H., Vene S., Norrby E., Grandien M., Wadell G. Serum antibody responses to individual viral polypeptides in human rotavirus infections. J Gen Virol. 1987 Mar;68(Pt 3):643–651. doi: 10.1099/0022-1317-68-3-643. [DOI] [PubMed] [Google Scholar]
- Svensson L., Sheshberadaran H., Vesikari T., Norrby E., Wadell G. Immune response to rotavirus polypeptides after vaccination with heterologous rotavirus vaccines (RIT 4237, RRV-1). J Gen Virol. 1987 Jul;68(Pt 7):1993–1999. doi: 10.1099/0022-1317-68-7-1993. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Urasawa S. Reactivity patterns to human rotavirus strains of a monoclonal antibody against VP2, a component of the inner capsid of rotavirus. Brief report. Arch Virol. 1986;87(1-2):135–141. doi: 10.1007/BF01310550. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicomb L. E., Bishop R. F. Epidemiology of rotavirus strains infecting children throughout Australia during 1986-1987. A study of serotype and RNA electropherotype. Arch Virol. 1989;106(1-2):23–34. doi: 10.1007/BF01311035. [DOI] [PubMed] [Google Scholar]
- Ushijima H., Honma H., Ohnoda H., Mukoyama J., Oyanagi H., Araki K., Shinozaki T., Morikawa S., Kitamura T. Detection of anti-rotavirus IgG, IgM, and IgA antibodies in healthy subjects, rotavirus infections, and immunodeficiencies by immunoblotting. J Med Virol. 1989 Jan;27(1):13–18. doi: 10.1002/jmv.1890270104. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Schiff G. M., Hoshino Y., Greenberg H. B. Relative concentrations of serum neutralizing antibody to VP3 and VP7 proteins in adults infected with a human rotavirus. J Virol. 1988 May;62(5):1543–1549. doi: 10.1128/jvi.62.5.1543-1549.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]