Abstract
Objective
To study the shape and characteristics of the vaginal high pressure zone (HPZ) by imaging a compliant fluid-filled bag placed in the vaginal HPZ with the 3-dimensional ultrasound (3D US) system.
Study Design
Nine nulliparous asymptomatic women underwent 3D US imaging and vaginal pressure measurements. A compliant bag was placed in the vagina and filled with various volumes of water. 3D US volumes of the pelvic floor were obtained at each bag volume while the subjects were at rest and during pelvic floor contraction.
Results
At low volumes, the bag was collapsed for a longitudinal extent of approximately 3.3 ± 0.2 cm (length of vaginal HPZ). With increasing bag volume, there was opening of the vaginal HPZ in the lateral dimension before the anterior-posterior (AP) dimension. Pelvic floor contraction produced a decrease in the AP dimension but not the lateral dimension of the bag in the region of the vaginal HPZ.
Conclusion
We propose that the shape and characteristics of the vaginal HPZ are consistent with the hypothesis that the puborectalis muscle is responsible for the genesis of the vaginal HPZ.
Keywords: Vaginal High Pressure Zone, 3-dimensional ultrasound, puborectalis muscle
Introduction
Recent studies from our laboratory suggest that the contraction of the puborectalis muscle (PRM) increases anal canal pressure1, 2. We questioned how can a “U” shaped PRM increase pressure in the anal canal because pressure in a circular tube, i.e., anal canal, is usually due to the circular sphincter muscles (internal and external anal sphincter)3–5. We hypothesize that the contraction of the PRM reduces the length of its two limbs thereby lifting the anal canal anteriorly, which compresses the structures located within the pelvic floor hiatus also known as the levator hiatus, against each other and also against the back of the symphysis pubis.
The anal canal and urethra have their own sphincter mechanisms, i.e., internal anal and external anal sphincter in the case of anal canal, and smooth and striated urethral sphincter complex in the case of urethra. The vagina, on the other hand, does not have any intrinsic sphincter mechanism; the vaginal high pressure zone (HPZ) is generally thought to be related to the pelvic floor muscle contraction6, 7. Using side-hole infusion manometery, we recently described the characteristics of the vaginal HPZ. The upper margin of the vaginal HPZ is located at its junction with the abdominal pressure and the lower margin at the junction with the atmospheric pressure. The vaginal HPZ is 3–4 cm in length with the peak pressure location, at approximately 2 cm above the hymen7.
The objective of this study was to determine the shape and characteristics of the vaginal high pressure zone (HPZ) by imaging a compliant, fluid-filled bag placed in the vagina with the 3-dimensional ultrasound (3D US) system. We wanted to determine if the anatomical features of the vaginal HPZ are consistent with the hypothesis that the PRM is responsible for the genesis of the vaginal HPZ. The Novel 3D US technique allowed us to study the anatomical location, configuration and dimensions of the vaginal HPZ.
Material and Methods
Nine nulliparous females participated in the study; none of them gave a history of trauma to the pelvic floor or prior pelvic floor surgery. The mean age of the subjects was 24±4 years (range 20 to 33). The University of California, San Diego Institutional Review Board approved the study protocol and all subjects gave permission and signed an informed consent prior to enrollment in the study. The study subjects received financial compensation for their participation. Each subject completed a medical history, a urinary and a fecal incontinence-scoring questionnaire to confirm the absence of incontinence symptoms. Prior to US imaging, each subject was instructed in the technique of pelvic floor muscle contraction with the investigator assessing if the maneuver was performed correctly by placing a finger in the vagina.
A polyethylene bag of 10 cm long, 35 mm maximal diameter and cylindrical in shape when fully distended, was used for these studies. A catheter with multiple side holes along its length was placed inside the bag for infusion of water into the bag. In-vitro studies were conducted prior to the placement of all bags in the vagina to determine the maximal volume of water to be used for the in-vivo studies. The bag was connected to the pressure transducer and gradually inflated with water using a syringe. The volume of water that caused the first increase in the bag pressure was called the maximal volume and all bag distensions for the in-vivo experiment were performed with less than the maximal volume. This testing assured that the bag itself did not contribute to the intra-bag pressure during the in-vivo experiments8. The bag was positioned lengthwise, carefully in the vagina such that approximately 2 cm of it was located below the hymen and the remainder was inside the vagina. The bag was inflated with water, with volumes ranging from 45 cc to 70 cc in 5 cc increments. At each bag volume, a 3-dimensional ultrasound (3D US) volume of the pelvic floor muscle was obtained using the 3D Philips HD11 US system (Phillips Medical Systems, Bothell, WA). With the subject in supine lithotomy position, the 3D US volume was obtained by placing a 3–9 MHz transvaginal US transducer on the perineum. The transducer head was directed in the cranial direction9, 10. The 3D US volumes were captured at rest and during sustained pelvic floor muscle contraction. The bag pressure was measured using a pressure transducer that was connected to the physiological recorder (Medtronic, Minneapolis St. Paul, MN).
The 3D US volumes were stored on the ultrasound machine hard drive and on a compact disk for off-line data analysis. The 3D volumes were viewed with the Q-lab 4.2 software program (Phillips Medical Systems, Bothell, WA). The US volumes were displayed in a standard, symmetrical orientation to study the 2D images in the axial, coronal and sagittal planes. The observers recognized the anatomical structures and with marker dot positioning was able to identify the structures in the exact same location in all three orthogonal planes simultaneously.
The determination of the anatomical location and measurements were performed on the 2D mid sagittal and transverse plane images. The mid sagittal plane image allowed measurements of the vertical extent of the vaginal HPZ and its relationship to the other anatomical structures of the perineum (Figure 1). The transverse or axial images of the levator hiatus were obtained in the axis of the PRM muscle (Figure 2). The PRM axis was defined as a line connecting the lower end of the pubic symphsis to the apex of the anorectal angle11, 12.
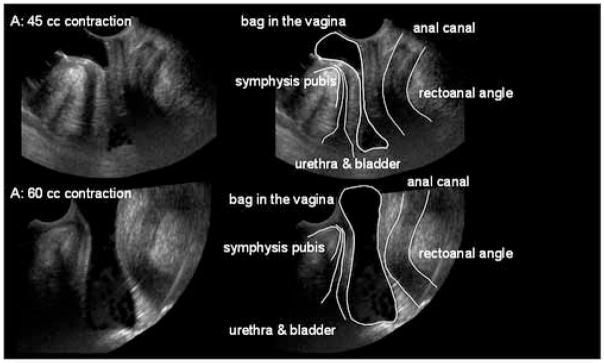
Figure 1. Vaginal high pressure zone in the Sagittal plane.
A collapsible bag was placed partly outside and partly inside the vagina and distended with 45 cc (A) and 60cc (B) water. A 3D US volume was obtained and 2D images in the sagittal plane were extracted from the 3D US volume. The vaginal HPZ is seen as an impression on the US images. Note the relationship between vaginal HPZ with the perineal body and the other pelvic floor structures. At the distension volumes of 45 cc, the bag is distended at its two ends but totally collapsed in the center. Between the totally distended and totally collapsed segments there is gradually tapering of the bag at both ends. Note that at distension volume of 60 cc, the shape of the bag changes to an hourglass.
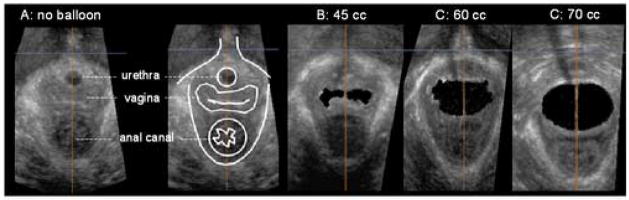
Figure 2. Vaginal high-pressure zone in the transverse (axial) plane.
These 2D US images in the transverse plane (in the plane of the puborectalis muscle; a line connecting the lower end of the pubic symphysis with the ano-rectal angle) were extracted from the 3D US volumes. These 3D US volumes were obtained with no bag in the vagina and the bag distended with 45cc, 60cc and 70 cc of water. Note that the bag dimensions are significantly greater in the lateral as compared to the AP direction. With the increase in the bag volumes the lateral dimensions of the bag do not change but there is a gradual increase in the AP dimension of the vaginal HPZ.
The lower end of the pubic symphysis was best visualized in the coronal plane image and the apex of the anorectal angle was best imaged in the sagital plane using the standard rotation technique described by our group recently13. Measurements were made from the transverse image in the axis of the PRM. The AP length of the levator hiatus was the straight-line distance from the symphysis pubis to the outer border of the anal canal or the inner edge of the PRM. The AP and lateral lengths of the vaginal bag, in the PRM axis were also determined.
Statistical Analysis
The measured values (mean ± standard error of mean, SEM) at rest and with pelvic floor contraction were compared using the paired t-test. Statistical significance was defined as p < 0.05. A Spearman’s correlation coefficient was used to determine the correlation between the balloon size and the intra-balloon pressure, AP length of the levator hiatus, as well as the AP and lateral lengths of the bag inside the vagina (SPSS 11.5, SPSS Inc., Chicago, IL, USA).
Results
The Effect of Bag Volume on the Bag Pressure
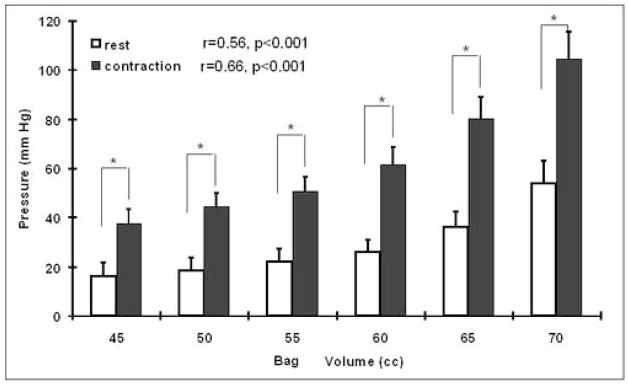
The graded increase in the bag volume resulted in a gradual increase in the bag pressure. At the bag volumes used (45 –70cc), the bag pressure increased with increase in the bag volume (figure 3). The pelvic floor contraction at each bag volume resulted in a statistically significant (p<0.001) increase in the bag pressure when compared to rest. A direct relationship between bag pressure and bag volume was observed both at rest as well as during the pelvic floor contraction, r- value 0.56 at rest (p<0.001) and 0.66 for contraction (p<0.001). At a bag volume of 70 cc, the bag pressures were 54±9 and 105±11 mmHg, at rest and pelvic floor contraction respectively.
Figure 3. The effect of bag volume on the bag pressure.
Note an increase in the bag pressure with the increase in the bag volume. At each bag volume the pelvic floor contraction is associated with an increase in the bag pressure. The difference between the rest and pelvic floor muscle contraction pressure was statistically significant at each bag volume (*, p<0.001).
Vaginal HPZ in the Sagittal plane: Effect of Bag Volume
The vaginal HPZ was assessed by its constricting impression on the fluid filled bag. The sagittal plane images show the vertical extent of the vaginal HPZ and its relationship with the perineal and other pelvic floor structures (figure 1). At bag volumes of 45 cc, the bag is fully distended at its two ends with a totally collapsed segment in the middle. Between the totally distended and totally collapsed vaginal segments there is a gradually tapering of the bag at both the cranial and caudal ends. The vertical extent of the totally collapsed zone is 1.8 ± 0.1 cm. However, if one measures the vertical extent from the onset of the narrowing of the bag at the two ends, the constriction extends for about 3.3 ± 0.1 cm. The lower end of the collapsed segment is located at the cranial margin of the perineal body and the upper end is adjacent to the bladder neck. The cranio-caudal length of the totally collapsed segment decreases with the increase in the bag volume and bag pressure. The anal canal is located posterior, and the urethra anterior to the vaginal HPZ with a relatively thin space intervening between the edges of the bag and the outer walls of the anal canal and the urethra. At the cranial end, the bag is deflected in the posterior direction above the vaginal HPZ suggesting that the upper vagina is located in a different coronal plane than the vaginal HPZ.
Vaginal HPZ in the Transverse plane: Effect of Bag Volume
The vaginal bag in the transverse or axial plane is completely collapsed at the low bag volumes and opens gradually as the volume and bag pressure increases with a characteristic shape. The vaginal HPZ, in the absence of the bag, is shaped like an “H”. The bag conforms to the intraluminal shape of the vagina and at low bag volumes it is also shaped like an “H”. At the 45 cc bag volume, the dimensions of the vagina are significantly greater in the lateral as compared to the anterior-posterior direction, 2.2 ± 0.1 and 0.4 ± 0.1 mm respectively. As the bag is gradually distended, the lateral dimensions of the vagina and the bag do not change but there is a gradual increase in the AP dimensions (figure 2). The AP length of the pelvic floor hiatus also increases with the increase in the bag volume and bag pressure (figure 4).
Figure 4. Anterior-posterior length of the levator hiatus with bag distension.
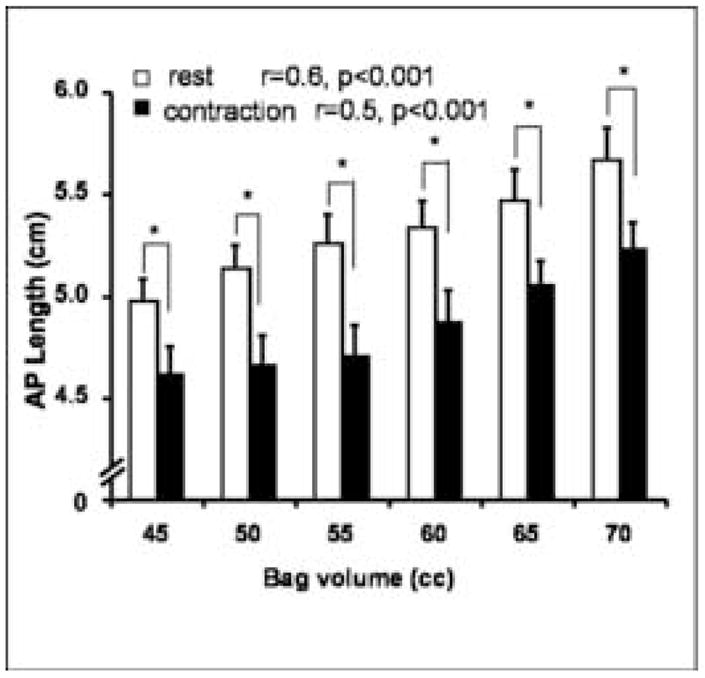
The AP length of the pelvic floor hiatus increase with increase in the bag volume. Also note a decrease in the AP length with the pelvic floor contraction in the presence of the bag.
Effect of Pelvic Floor Contraction on the Pelvic Floor Hiatus and the Vaginal Bag
The AP length of the pelvic floor hiatus, which is largely related to the length of the PRM, decreases significantly with the pelvic floor contraction, both in the absence and the presence of the bag. In the absence of the vaginal bag, the rest and contraction AP lengths of the levator hiatus are 5.0 and 4.6 cm respectively. The AP length of the pelvic floor hiatus increases in a linear fashion with the increase in the bag volume, both at rest and during the pelvic floor contraction, correlation coefficient of 0.57 (p<0.001) and 0.50 (p=0.001) respectively. At the bag volume of 70 cc, the rest and contraction lengths of the pelvic floor hiatus were 5.7 and 5.2 cm, respectively (figure 4). The pelvic floor contraction resulted in characteristic changes in the dimensions of the vaginal bag (figure 5). There is a consistent decrease in the AP but not the lateral length of the vaginal bag with the pelvic floor contraction (figure 6). At 60 cc bag volume, the AP length decreased from 1.2 ± 0.1 to 1.0 ± 0.1 but there was no change in the lateral lengths 2.9 ± 0.1 and 2.9 ± 0.1 cm. With bag distension there is an increase in the vaginal bag dimension and the AP length of the pelvic floor hiatus but the former is greater than the latter (at 60 cc the two values are 1.2 and 0.2 cm respectively). The latter suggests a displacement (retro pubic fat & other structures) or compression of the tissue from the axial plane of the pelvic floor hiatus during vaginal distension.
Fig. 5. Effect of pelvic floor contraction on AP and lateral dimension of the vaginal HPZ.
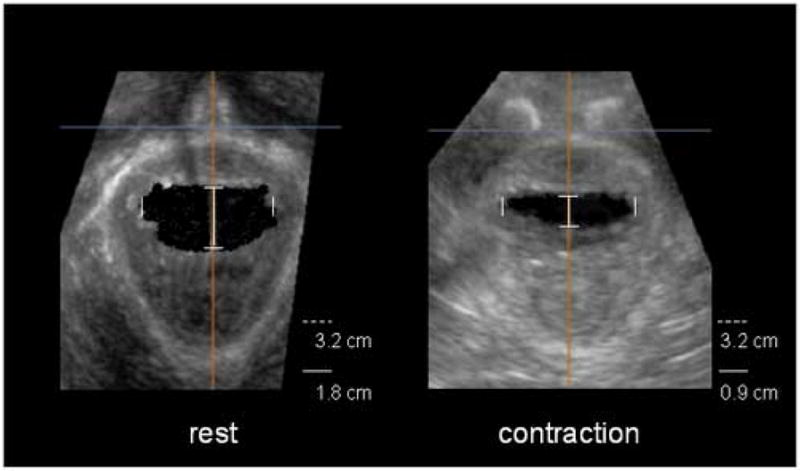
These transverse images (in the plane of the puborectalis muscle) of the pelvic floor hiatus were obtained at bag volume of 60 cc. Note that the pelvic floor contraction resulted in characteristics changes in the dimension of the vaginal bag. The AP length of the bag decreased from 1.8 cm at the baseline to 0.9 cm during pelvic floor contraction. The lateral dimension of the bag is not affected by the pelvic floor contraction; 3.2 cm at both rest and with pelvic floor muscle contraction.
Figure 6. Effect of bag volume and pelvic floor contraction on the vaginal HPZ.
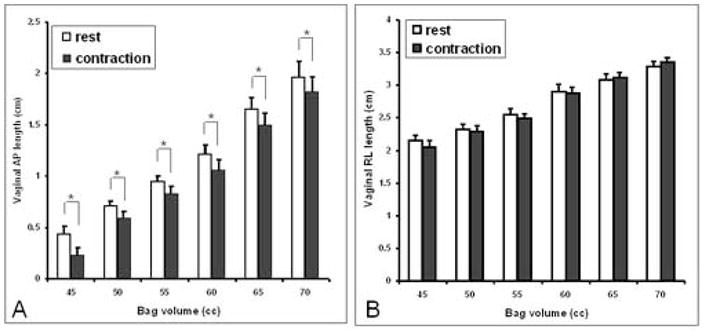
note a reduction in the AP but not the lateral dimension of the bag with pelvic floor contraction with each bag volume. The bag volumes that resulted in the opening of the vaginal HPZ revealed a consistent decrease in the AP length of the vaginal bag with each contraction but there was no effect on the lateral dimension of the vaginal bag (*, p<0.05).
Comment
In summary, our data show the following 1; the vaginal HPZ extends for a distance of 3.3 ± 0.1 cm above the perineal body, with the maximal site of constriction at its center, 2; the distension of a bag inside the vagina shows expansion in the lateral direction before the AP direction, 3; the bag distension in the vagina causes an increase in the AP length of the pelvic floor hiatus and puborectalis muscle, 4; pelvic floor contraction reduces the AP length of the pelvic floor hiatus, 5; In the setting of a distended vaginal bag, the pelvic floor contraction results in the reduction of its AP but not the lateral dimension.
We assessed the vaginal HPZ using side-hole infusion manometry in a previous study and our data revealed that it is approximately 3–4 cm in length, which is fairly close to the measurement obtained in the present study. The pressures obtained by infusion manometry with the infusion port in a specific direction demonstrate that the pressures are greater in the AP direction as compared to the right-left lateral direction7. The effect of probe size on the vaginal HPZ was investigated in a separate study, and it revealed that the pressure increases with the increase in the probe size14. The latter study also detected greater pressures when the infusion port was in the anterior-posterior direction rather than the right and left lateral direction. Furthermore, the pressure when the infusion port was directed anteriorly was found to be significantly (3–4 times) higher than the pressure when the infusion port was posterior, the precise reason for which is not known. Similar to the manometric study of the vaginal HPZ, we observed a gradual increase in the bag pressure with the increase in the bag volume in the current study, which is consistent with the studies of Morin et al.15 and Morgan et al.16. These authors described vaginal speculums, which allow increase in the anterior-posterior dimension of the vaginal canal and at the same time measures the force exerted by the pelvic floor muscle. Their data also show a linear increase in the pelvic floor force with the increase in the anterior-posterior length of the vaginal canal. In our study, increase in the bag volume also resulted in an increase in the anterior-posterior length of the PRM. The peak vaginal HPZ pressure measured with infusion manometry is significantly higher (anterior vaginal pressure ranging from 220–800 mmHg)7, 14 than the one found in the current study. The bag pressure measured in the current study does not have any directionality; it represents average pressure around the circumference, which we believe is the reason for the pressures to be lower in the current study as compared to the previous manometric study.
The vagina does not have an intrinsic sphincter mechanism of its own and the vaginal HPZ is related to the pelvic floor muscle contraction6, 10, 15, 16. The vagina is a relatively compliant organ and as a result its shape is influenced by the direction of forces exerted by the pelvic floor muscle and the adjacent structures. The bag placed in the vagina conforms to the shape of the vagina and therefore the impression on the bag reveals the direction of forces being exerted on the vagina. It is interesting that Kerremans used impressions on the compressible material (moulds) to identify the nature of forces responsible for the genesis of the HPZ in the anal canal17, the impression on the collapsible bag, too a certain extent exemplifies the same principles. The 3D US imaging technique provide additional details of the vaginal high pressure zone. Sagittal images reveal the vertical extent of the vagina HPZ and the site of maximal force development, i.e., the site of maximal constriction is the levator hiatus. Equally interesting is our observation regarding the shape of the vaginal HPZ in the transverse plane. The bag assumes the shape of the vagina and when gradually distended it expands in the lateral direction with greater ease than in the anterior and posterior direction. Expansion of the bag is expected to be greater in the area of lower pressure as compared to the higher pressure. The increase in the lateral dimension prior to the AP dimension of the vaginal bag proves that the force responsible for the genesis of vaginal HPZ is preferentially directed in the AP direction. Easier expansion of the bag in the lateral as compared to the AP direction is consistent with the lower pressures in the lateral directions observed in our previous study, where we measured the vaginal HPZ using infusion manometry7. With the increase in the bag volume and bag pressure, and following full expansion in the lateral directions, the bag starts to expand in the AP direction, suggesting higher pressure in anterior-posterior direction.
From the analysis of the transverse US images of the vaginal HPZ, it is clear that the bag shape is related to the indentations of the vagina by the anal canal and the urethra in the posterior and anterior directions respectively. Although it was difficult to quantitate, ultrasound images revealed that the expansion of the vaginal HPZ occurs more easily in the posterior as compared to the anterior direction. The latter finding is consistent with our findings from infusion manometry 7, 14 where we observed greater pressures in the anterior as compared to the posterior direction. The most revealing finding of our study is the changes in the dimensions of the bag with the pelvic floor contraction in the setting of a moderately distended bag. It showed that with the pelvic floor contraction there is a decrease in the anterior-posterior but not the lateral dimension of the bag. The latter observation proves that the major force responsible for the genesis of the vaginal HPZ is directed in the anterior-posterior plane. A reduction in the anterior posterior length of the pelvic floor hiatus proves that with pelvic floor contraction the anal canal is lifted in the anterior direction, thus compressing the vagina between the anal canal and the urethra, the two relatively non-compliant structures. We propose that the vaginal compression between the anal canal and urethra results from the anterior lift of the levator hiatus and the latter is responsible for the genesis of the vaginal HPZ.
The levator hiatus is formed by the levator ani or pelvic floor muscle. The human pelvic floor is made up of several distinct muscles (pubococcygeus, iliococcygeus, and puborectalis); pubococcygeus and puborectalis collectively have also been referred to as the “pubovisceral” muscle18. The puborectalis muscle (PRM) located caudal (inferior) to the pubococcygeus is a relatively thick, U- shaped muscle. The two anterior ends of PRM insert on the inferior pubic rami and the arcus tendineus of the levator ani muscle that traverses the fascia of the obturator internus muscle, while the posterior ends join with each other posterior to the anorectal junction. Recent advances in imaging of the pelvic floor by 3 D ultrasound9 and MRI19 demonstrate a “U-shaped” PRM that originates from the pubis and creates a sling around the vagina, and anorectum. Since the only muscle that originates at the pubis and slings behind the ano-rectum is the PRM, we believe that measures of the levator hiatus are actually measures of the puborectalis muscle. Our measurements of the levator hiatus are quite reproducible13 and similar to the ones made from the MR images20. We propose that the vaginal HPZ is largely related to the contraction of the PRM. Does the pubococcygeus muscle contribute to the vaginal HPZ? Even though we can not be sure, we believe it is unlikely because unlike puborectalis muscle, the pubococcygeus does not form a sling posterior to the anal canal and therefore may not result in the anterior lift of the anal canal. However, a definite proof of our hypothesis requires one to determine the effect of PRM myotomy on the vaginal pressure, which requires studies in an animal model.
What is the physiologic and clinical relevance of our observations? Our studies suggest that the puborectalis muscle is likely to be important in the constrictor function of the pelvic floor. If our hypothesis is indeed true, one can quantitate the constrictor function of the pelvic floor by measuring vaginal pressures. The constrictor function of the pelvic floor is likely to be important in the fecal and urinary continence mechanism and possibly other pelvic floor disorders. Recent studies indicate that 20–30% of multiparous women have disruption of the pelvic floor muscle, even though it is not clear if the disruption is in the puborectalis or the pubococcygeus muscle19, 21. Future studies should investigate the relationship between anatomical disruptions with the function of the muscle. We propose that the vaginal pressures can assess the constrictor function of the pelvic floor hiatus or puborectalis muscle.
Acknowledgments
Financial supported by: NIH RO1 grant DK60733
Equipment loan from General Electric Healthcare and Philips Medical Systems
Footnotes
Condensation
Three dimensional ultrasound images of the pelvic floor reveal information about the vaginal high pressure zone
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LIU J, GUADERRAMA N, NAGER CW, PRETORIUS DH, MASTER S, MITTAL RK. Functional correlates of anal canal anatomy: puborectalis muscle and anal canal pressure. Am J Gastroenterol. 2006;101:1092–7. doi: 10.1111/j.1572-0241.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 2.PADDA BS JS, PRETORIUS DH, NAGER CW, DEN BOERD, MITTAL RK. The effect of pelvic floor muscle contraction on the anal canal pressure. Am J Physiol. doi: 10.1152/ajpgi.00250.2006. (submitted) [DOI] [PubMed] [Google Scholar]
- 3.DIAMANT NE, KAMM MA, WALD A, WHITEHEAD WE. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735–60. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 4.LOCKE GR, 3RD, PEMBERTON JH, PHILLIPS SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology. 2000;119:1761–6. doi: 10.1053/gast.2000.20390. [DOI] [PubMed] [Google Scholar]
- 5.WHITEHEAD WE, SCHUSTER MM. Anorectal physiology and pathophysiology. Am J Gastroenterol. 1987;82:487–97. [PubMed] [Google Scholar]
- 6.BO K, SHERBURN M. Evaluation of female pelvic-floor muscle function and strength. Phys Ther. 2005;85:269–82. [PubMed] [Google Scholar]
- 7.GUADERRAMA NM, NAGER CW, LIU J, PRETORIUS DH, MITTAL RK. The vaginal pressure profile. Neurourol Urodyn. 2005;24:243–7. doi: 10.1002/nau.20112. [DOI] [PubMed] [Google Scholar]
- 8.AZPIROZ F, ENCK P, WHITEHEAD WE. Anorectal functional testing: review of collective experience. Am J Gastroenterol. 2002;97:232–40. doi: 10.1111/j.1572-0241.2002.05450.x. [DOI] [PubMed] [Google Scholar]
- 9.DIETZ HP. Ultrasound imaging of the pelvic floor. Part II: three-dimensional or volume imaging. Ultrasound Obstet Gynecol. 2004;23:615–25. doi: 10.1002/uog.1072. [DOI] [PubMed] [Google Scholar]
- 10.GUADERRAMA NM, LIU J, NAGER CW, et al. Evidence for the innervation of pelvic floor muscles by the pudendal nerve. Obstet Gynecol. 2005;106:774–81. doi: 10.1097/01.AOG.0000175165.46481.a8. [DOI] [PubMed] [Google Scholar]
- 11.DIETZ HP, SHEK C, CLARKE B. Biometry of the pubovisceral muscle and levator hiatus by three-dimensional pelvic floor ultrasound. Ultrasound Obstet Gynecol. 2005;25:580–5. doi: 10.1002/uog.1899. [DOI] [PubMed] [Google Scholar]
- 12.YANG JM, YANG SH, HUANG WC. Biometry of the pubovisceral muscle and levator hiatus in nulliparous Chinese women. Ultrasound Obstet Gynecol. 2006;28:710–6. doi: 10.1002/uog.3825. [DOI] [PubMed] [Google Scholar]
- 13.MILENA MWJS, PRETORIUS DH, NAGER CW, DEN BOER D, MITTAL RK. Puborectalis muscle in nulliparous women: functional assessment using 3-dimensional ultrasound imaging. Gastroenterology. 2006;130:A114. [Google Scholar]
- 14.PADDA BSJS, PRETORIUS DH, NAGER CW, DEN BOER D, MITTAL RK. Length tension relationship of puborectalis muscle in women. Gastroenterology. 2006;130:A725. [Google Scholar]
- 15.MORIN M, DUMOULIN C, BOURBONNAIS D, GRAVEL D, LEMIEUX MC. Pelvic floor maximal strength using vaginal digital assessment compared to dynamometric measurements. Neurourol Urodyn. 2004;23:336–41. doi: 10.1002/nau.20021. [DOI] [PubMed] [Google Scholar]
- 16.MORGAN DM, KAUR G, HSU Y, et al. Does vaginal closure force differ in the supine and standing positions? Am J Obstet Gynecol. 2005;192:1722–8. doi: 10.1016/j.ajog.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 17.KERREMANS RP. A new method of objective examination in proctology. Gut. 1968;9:243–5. doi: 10.1136/gut.9.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.KEARNEY R, SAWHNEY R, DELANCEY JO. Levator ani muscle anatomy evaluated by origin-insertion pairs. Obstet Gynecol. 2004;104:168–73. doi: 10.1097/01.AOG.0000128906.61529.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DELANCEY JO, KEARNEY R, CHOU Q, SPEIGHTS S, BINNO S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101:46–53. doi: 10.1016/s0029-7844(02)02465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SINGH K, REID WM, BERGER LA. Magnetic resonance imaging of normal levator ani anatomy and function. Obstet Gynecol. 2002;99:433–8. doi: 10.1016/s0029-7844(01)01743-4. [DOI] [PubMed] [Google Scholar]
- 21.DIETZ HP, LANZARONE V. Levator trauma after vaginal delivery. Obstet Gynecol. 2005;106:707–12. doi: 10.1097/01.AOG.0000178779.62181.01. [DOI] [PubMed] [Google Scholar]