Abstract
Regulatory T cells (Treg) are a subpopulation of CD4+ lymphocytes that maintain immunological self-tolerance in the periphery. T reg also regulate or suppress other classes of immune response such as allograft rejection, allergy, tumor immunity, and responses to microbes. Treg express the Foxp3 transcription factor and CD25, the high affinity interleukin-2 receptor (IL-2R). T reg are divided into two types: naturally occurring Treg derived from thymus (natural Treg) and Treg induced from Foxp3− CD4+ T cells in the periphery (induced Treg). It would be valuable to understand how to control the generation of antigen-specific Treg, which could also provide a new approach to treat autoimmunity, allergy or allograft rejection without suppressing immune responses to tumor and microbes. In this review, we will discuss the role of dendritic cells (DCs) in controlling antigen-specific natural Treg and induced Treg. Natural Treg are anergic upon T cell receptor stimulation generally, however, we found that the antigen-specific natural Treg can be expanded by antigen-presenting mature bone marrow-derived dendritic cells (BM-DCs). Furthermore, recent studies showed that antigen-specific Treg can be induced from Foxp3− CD25− CD4+ T cells by antigen-presenting DCs, particularly select subsets of DCs in the periphery. These findings need to be pursued to develop novel immune suppressive therapies using antigen-specific Treg educated by DCs.
Keywords: dendritic cells, Foxp3+ regulatory T cells
1. Introduction
Regulatory T cells (Treg) are a subpopulation of CD4+ T cells that maintain immunological self-tolerance and control the development of autoimmune diseases [1]. S. Sakaguchi and his colleagues discovered that Treg express high affinity interleukin-2 (IL-2) receptor (CD25) and transcription factor, forkheard box P3, Foxp3, which plays a critical role in the function and development of Treg [1–3]. Mutation of the human gene FOXP3 results in immune disregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) [4,5]. IPEX patients suffer autoimmune enteropathy, diabetes and thyroiditis, hemolytic anemia, thrombocytopenia, food allergy and dermatitis [4,5]. Dermatitis in IPEX can present with features of psoriasis or atopic dermatitis with hyper IgE [6,7]. Treg defects are also reported in patients with multiple sclerosis [8], psoriasis [9] and systemic lupus erythematousus [10]. Foxp3 is currently the most specific marker of Treg [2,3].
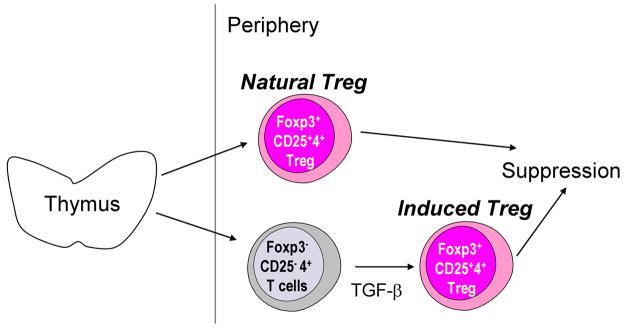
There are at least two types of Foxp3+ CD25+ CD4+ Treg: i) natural Treg are naturally occurring from thymus, while ii) induced Treg are derived from Foxp3− CD25− CD4+ T cells in the periphery by stimulation with transforming growth factor (TGF)-β [2,11] (Fig. 1). At present, there are no markers to distinguish these two types of Treg. Both types are Foxp3+ and CD4+ and the majority are CD25+. Treg represent about 5–10 % of peripheral CD4+ T cells in normal rodents and humans.
Fig. 1. Natural Treg vs induced Treg.
Natural Treg are derived from thymus. The development and function of Treg is determined by the transcription factor, Foxp3. Treg are also induced from Foxp3− cells in the periphery by TGF-β (induced Treg). Natural Treg and induced Treg help maintain immunological self-tolerance in the periphery.
Foxp3+ CD25+ CD4+ Treg control not only autoimmunity, but also allergy, transplant rejection, tumor immunity, and responses to microbes [1]. It would be exciting to treat autoimmune diseases, allergy, and transplant rejection by Treg-based immune therapy. However, polyclonal Treg might have the potential to induce global immune suppression. If medicine is to learn how to suppress unwanted immune responses without blocking the immune responses to tumor and microbes, it will be crucial to understand how to control antigen-specific Treg so that these can specifically suppress a given clinical problem like autoimmunity, allergy and transplant rejection.
Our research has shown that antigen-specific natural Treg and induced Treg can be controlled by antigen-presenting dendritic cells (DCs) [12–17]. Recently, it has also been shown that there are DC subsets specialized to induce antigen-specific Foxp3+ Treg in peripheral tissues like the intestine and spleen [17–19]. In this review, we will mainly discuss the role of DCs in inducing antigen-specific Foxp3+ Treg.
2. Antigen-specific natural Treg can be expanded by DCs
Natural Treg are anergic and non-proliferative upon T cell receptor stimulation ex vivo [1,2]. However, the natural Treg can be expanded when they are cultured with mature antigen-presenting DCs [12,20]. Among the antigen-presenting cells (APCs) that were tested, mature bone marrow-derived DCs (BM-DCs) and draining lymph node DCs from complete Freund’s adjuvant-treated mice were the most potent for expanding Treg [12]. This expansion was partially dependent on CD80/CD86 on the DCs and IL-2 [12]. IL-2 is known as an essential factor for the survival and homeostasis of Treg in vivo [2].
Natural Treg that had been expanded with antigen presenting DCs plus IL-2 supplementation blocked the development of type 1 diabetes in non-obese diabetic (NOD) mice, using Treg derived from BDC2.5 CD4+ TCR transgenic NOD mice [13]. The expanded natural Treg from BDC2.5 transgenic mice were much more effective than polyclonal T reg from non-transgenic NOD mice. Furthermore, the non-transgenic Treg expanded by mature allogeneic DCs plus IL-2 suppressed graft versus host disease (GVHD) in an alloantigen-specific manner [14]. Thus, DCs are important APCs to expand antigen-specific natural Treg (reviewed in [21], [22]).
While antigen-specific natural Treg can be expanded by DCs, purification of Treg from peripheral CD4+ T cells is necessary before culturing with DCs, which would unavoidably also expand contaminated CD25+ Foxp3− effector T cells. This creates a substantial obstacle for the future of Treg-based clinical therapy. An alternative is to generate antigen-specific Treg from conventional CD4+ T cells or non-regulatory CD4+ T cells as a starting population. As we discuss next, DCs are also superior in inducing antigen-specific Foxp3+ Treg from non-regulatory Foxp3− precursors.
3. DCs are specialized APCs to induce Foxp3+ Treg from Foxp3− precursors
Foxp3 is induced in non-regulatory CD25− CD4+ T cells with combined anti-CD3 and anti-CD28 monoclonal antibody (mAb) stimulation along with supplementation by TGF-β3 [23]. TGF-β plus antigen-presenting spleen DCs can also induce Foxp3+ Treg from CD25− CD4+ NOD BDC2.5 transgenic T cells, and the induced Treg suppressed type 1 diabetes in NOD mice [15].
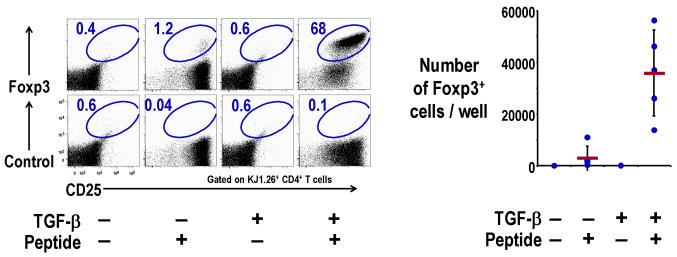
However, the question still remained whether a small population of Foxp3+ T cells in the CD25− CD4+ T cells may have contributed to the induction of the Treg [11]. To overcome this possibility, we used DO11.10 ovalbumin (OVA)-specific, CD4+ TCR transgenic, RAG-2 deficient mice, which lack Foxp3+ Treg, as the starting population. TGF-β along with spleen DCs and antigen were able to differentiate Foxp3+ Treg from Foxp3− precursors (Fig. 2) [16]. When DO11.10 OVA-specific CD4+ transgenic RAG-2 deficient T cells were cultured with antigen-presenting DCs plus TGF-β, the induced Foxp3+ Treg from Foxp3− precursors were as suppressive in vitro as natural Treg, and also suppressed the rejection of OVA-expressing tumor in vivo [16]. This shows that Foxp3− precursors can be differentiated into functional Treg by antigen-presenting DCs plus TGF-β without any contamination of Foxp3+ T cells in the starting population of CD25− CD4+ T cells.
Fig. 2. Induction of Foxp3+ Treg from Foxp3− CD25− CD4+ T cells by DC plus TGF-β.
Foxp3− CD25− CD4+ T cells (2×104) from DO11.10 RAG−/− mice were cultured for 7 days with spleen CD11c+ DCs (2×104) and 0.3 μg/ml OVA peptide in the presence of TGF-β (2 ng/ml). Cells were stained with anti-CD4, KJ1.26 clonotype, CD25, and Foxp3 or isotype control Abs, and analyzed by fluorescence-activated cell sorting (FACS) (Left). Absolute numbers of Foxp3+CD4+KJ1.26 clonotype+ T cells per culture at day 7 are also shown (Right).
Induced Treg have also been shown to be suppressive by E. Shevach’s group. They induced Foxp3+ Treg from 5CC7 CD4+ transgenic RAG deficient Foxp3− precursors using TGF-β plus IL-2 along with anti-CD3 and anti-CD28 mAbs; the Treg were suppressive in vitro [24]. Likewise induced Treg from H+/K+ ATP-ase CD4+ transgenic T cells were suppressive in vitro and inhibited autoimmune gastritis [25]. TGF-β induced Treg also inhibited the development of colitis [26].
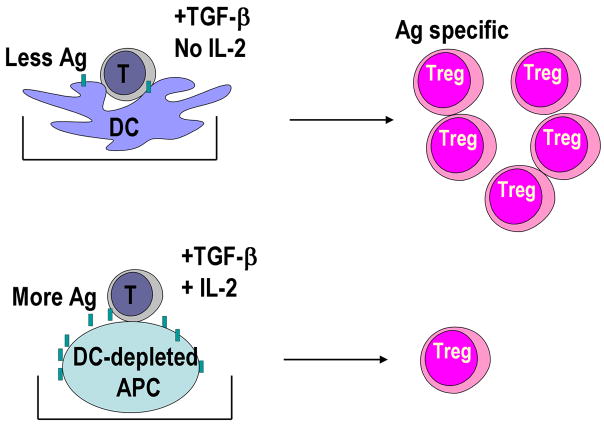
Another question was how DCs differ in inducing Foxp3+ Treg by TGF-β compared to non-DC APCs? To address this question, the induction of Foxp3+ Treg from Foxp3− cells in the presence of TGF-β was compared with CD11c+ DC-enriched and DC-depleted CD11c− splenic APCs. We found that low numbers of CD11c+ DCs were able to induce more Foxp3+ Treg and with smaller doses of antigen (Fig. 3) [16]. We also found that IL-2 was required for the induction of Foxp3+ Treg by TGF-β [16]. The required IL-2 was produced by T cells when stimulated with DCs but not with CD11c− APCs. DCs needed to express CD80/CD86 on the surface in the presence of TGF-β, since DCs from CD80/CD86 double knockout mice did not make T cells produce IL-2 and induced smaller numbers of Foxp3+ Treg [16].
Fig. 3. Lower doses of DCs induce greater number of induced Treg with lower doses of antigen than DC-depleted APCs.
Foxp3− CD25− CD4+ T cells from DO11.10 RAG−/− mice were cultured as in Fig. 2 in the presence of TGF-β. Lower numbers of spleen CD11c+ DCs induced more Foxp3+ Treg and with lower doses of antigen than DC-depleted spleen APCs. The DCs did not require supplementation by IL-2, whereas DC-depleted spleen APCs required IL-2 supplementation to induce Foxp3+ Treg.
Therefore, DCs are specialized APCs to induce Foxp3+ Treg from Foxp3− precursors in the presence of exogenous TGF-β. This suggests that DCs can actively induce Foxp3+ Treg using TGF-β provided in the periphery and thereby DCs have the potential to bring about immunological self-tolerance through this pathway.
4. The CD103+DC subset in the gut uses retinoic acid (RA) to maintain oral tolerance by inducing Foxp3+ Treg
The groups of Y. Belkaid and F. Powrie found that a subset of gut DCs expressing CD103, the αEβ7 integrin, is specialized to induce Foxp3+ Treg in the absence of any exogenous factors and participate in maintaining oral tolerance [18,19]. CD103+ DCs from mesenteric lymph nodes or gut-associated lymphoid tissue used not only endogenous TGF-β to induce Foxp3+ Treg, but also retinoic acid (RA) [18,19]. RA acts as a co-factor for TGF-β to induce Foxp3+ Treg [18,19,27,28]. Bioactive RA is converted from retinal by CD103+ gut DCs using retinal dehydorogenase [18,19]. The group of B. Pulendran reported that Lamina propria macrophages also induce Foxp3+ Treg by a mechanism dependent on IL-10, RA and TGF-β [29]. Their experimental condition showed that TGF-β needs to be added to the inductive cultures, which indicates that the intestinal environment might provide TGF-β to macrophages.
With respect to mechanism, RA alone cannot induce Foxp3+ Treg from Foxp3− cells [18,19], however, the Treg induced by TGF-β plus RA are more stable and can function in a more efficient way than the Treg induced by TGF-β alone [19]. In the presence of TGF-β, RA blocked the induction of IL-17 producing T cells, which also require TGF-β to develop, and enhanced the induction of Foxp3+ Treg [27]. D. Mathis, C. Benoist and coworkers recently reported that RA reduced the formation of Foxp3− CD4+ effector memory cells, and may not induce Foxp3+ Treg directly [30]. They showed that the effector memory cells, which produce IL-4, IL-21 and interferon (IFN)-γ, actively inhibited the induction of Foxp3+ Treg by TGF-β. Further studies should clarify whether RA induces Foxp3+ Treg directly or reduces the effector T population.
CD103+ DCs are tissue-derived migratory DCs that present feeding antigens to T cells [31]. Consequently, it is possible that intestine-derived CD103+ DCs present feeding antigen to induce Foxp3+ Treg to maintain oral tolerance in the gut. It is of interest that dietary intake of vitamin A, which is a precursor of RA, might affect the inducing of dietary-antigen specific Foxp3+ Treg. The induced Treg in the gut could then become part of the Treg pool in the periphery [32].
5. CD8+ DEC-205+ DCs from spleen induce antigen-specific Foxp3+ Treg using endogenous TGF-β
In addition to oral tolerance, systemic tolerance to tissue-specific antigen might be mediated by a subset of DCs in the periphery. Conventional DCs are divided into two major subsets in mouse spleen [33–35]. One is CD8+ DEC-205+ and the other is CD8− dendritic cell inhibitory receptor-2 (DCIR-2)+ [36]. DEC-205 (CD205), which was first recognized by the NLDC-145 mAb, is a type I transmenbrane protein with multiple C-type lectin domains and mainly expressed on CD8+ DCs [33]. DCIR-2, which is recognized by the 33D1 mAb, is a type II transmenbrane protein with a single external C-type lectin domain and is mainly expressed on CD8− DCs. [36]. We have recently reported that CD8+ DEC-205+ spleen DCs can induce Foxp3+ Treg in the absence of added IL-2 or TGF-β in vitro and in vivo [17].
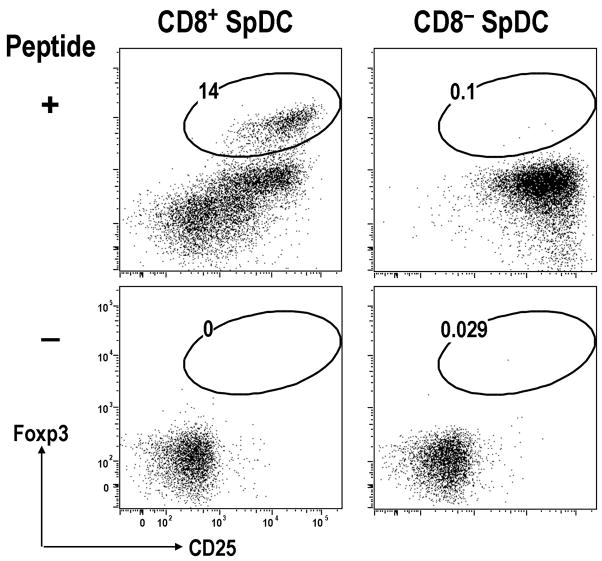
When CD8+ DEC-205+ or CD8− DCIR-2+ DCs from spleen were compared for the capacity to induce Foxp3+ Treg in vitro, CD8+ DEC-205+ DCs were able to induce Foxp3+ Treg from Foxp3− DO11.10 RAG-2 deficient CD4+ T cells with low doses of antigen but without any exogenous TGF-β (Fig. 4) [17]. The induction of Foxp3+ Treg by CD8+ DEC-205+ DCs was blocked by neutralizing anti-TGF-β mAb, indicating an endogenous source of TGF-β. In contrast, CD8− DCIR-2+ DCs induced more Foxp3+ Treg from Foxp3− DO11.10 RAG-2 deficient CD4+ T cells in the presence of low doses of antigen when TGF-β was added into the culture [17]. In these experiments, the induced Foxp3+ Treg showed suppressive capacity in vitro.
Fig. 4. CD8+ spleen DCs induce Foxp3+ T reg from Foxp3− CD25− CD4+ T cells in the absence of exogenous TGF-β in vitro.
As in Fig. 1, but Foxp3− CD25− CD4+ T cells from DO11 RAG−/− mice were cultured with CD8− or CD8+ spleen DCs plus peptide in the absence of added TGF-β. After 5-days of culture, cells were analyzed by FACS. Cells were gated on CD4+ CD11c− T cells.
The group of R. Noelle has also studied the function of DC subsets in inducing Treg. They reported that CD8+ spleen DCs preferentially express Programmed death 1 ligand (PDL-1) and that this is essential to induce Foxp3+ Treg in the presence of exogenous TGF-β [37]. They also found that CD8+ DCs were able to induce more Foxp3+ Treg in the presence of exogenous TGF-β than CD8− DCs, which is contrary to our data described above. However, this might be due to the different experimental conditions. In contrast to Wang et al, we used lower doses of antigen, lower numbers of T cells and no exogenous IL-2 in the culture [17].
CD8+ DEC-205+ spleen DCs produced more of the latent form of TGF-β than CD8− DCIR-2+ spleen DCs [17], which would need to be activated, e.g., by integrin αvβ8 on the surface of DCs [38]. We also noted that the production of the latent form of TGF-β was reduced if the CD8+ DEC-205+ DCs were matured by poly IC, a toll like receptor (TLR)3 ligand. The CD8+ DEC-205+ DCs matured by poly IC also showed a reduced capacity to induce Foxp3+ Treg in vitro [17].
These findings suggest that the production of latent form of TGF-β by CD8+ DEC-205+ spleen DCs is required for the induction of Foxp3+ Treg, and that CD8+ DEC-205+ DCs might contribute to systemic tolerance in the periphery by inducing tissue-antigen specific Foxp3+ Treg in the steady state. CD8+ DEC-205+ spleen DCs can capture apoptotic dying cells and induce Treg to antigen in the dying cells [39]. Apoptotic cell death can induce the production of TGF-β [40]. Therefore, an interesting possibility would be that capture of apoptotic cells might help CD8+ DEC-205+ DCs produce TGF-β in the steady state. On the other hand, CD8− DCIR-2+ spleen DCs might use TGF-β that is provided by the other types of cells to induce Foxp3+ Treg to maintain systemic tolerance in the periphery.
6. Antigen delivery to DC subsets in vivo and the induction of Foxp3+ Treg
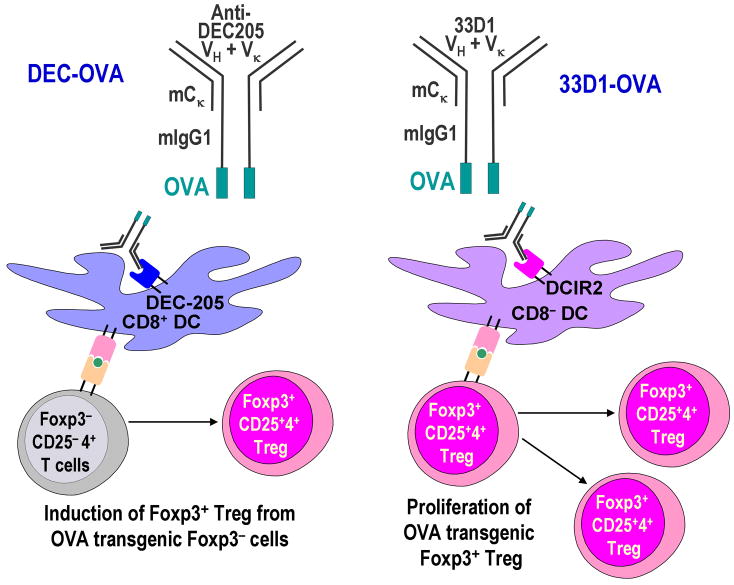
To investigate the importance of CD8+ DEC-205+ DCs in inducing Foxp3+ Treg in vivo, we used a targeting strategy to deliver antigen in vivo within anti-DC subset mAb [36]. OVA protein was engineered into anti-DEC-205 mAb (DEC-OVA) or anti-DCIR2 33D1 mAb (33D1-OVA) (Fig. 5) to deliver antigen selectively to each DC subset in vivo [36].
Fig. 5. Antigen targeting to DC subsets by anti-DC subset mAb and Foxp3+ Treg.
OVA was engineered into anti-DEC-205 mAb (DEC-OVA) or anti-DCIR-2 33D1 mAb (33D1-OVA). In vivo injection of these chimera Abs delivers antigen to each DC subset selectively [36]. We found that antigen targeting via DEC-205 led to the induction of Foxp3+ Treg from Foxp3− precursors, whereas antigen targeting within 33D1 mAb expanded preformed Foxp3+ Treg (natural Treg) in vivo [17].
Using adoptive transfer of Foxp3− DO11.10 RAG-2 deficient OVA transgenic CD4+ T cells, we found that in vivo OVA delivery to CD8+ DEC-205+ DCs by DEC-OVA induced Foxp3+ Treg more efficiently than OVA delivery to CD8− DCIR-2+ DCs by 33D1-OVA [17], which is consistent with the data in vitro as described above. This also confirmed prior reports showing that antigen delivery by anti-DEC-205 mAb induced Treg [41,42], although antigen delivery to CD8− DCIR-2+ DCs was not studied. In contrast, when natural Treg from DO11.10 RAG-2 sufficient OVA transgenic mice were adoptively transferred and stimulated with DEC-OVA or 33D1-OVA, we found that the natural Treg proliferated better by 33D1-OVA than DEC-OVA [17].
These results suggest that CD8+ DEC-205+ DCs and CD8− DCIR-2+ DCs may have different roles in inducing Foxp3+ Treg from Foxp3− precursors and maintaining natural Foxp3+ Treg in the periphery (Fig. 5). For application to clinical therapy, it might be possible to use antigen delivery to CD8+ DEC-205+ DCs to induce Foxp3+ T reg from Foxp3− CD4+ T cells, to use the antigen delivery to CD8− DCIR-2+ DCs to expand natural Foxp3+ Treg, or to use a combination of both strategies. However, these experiments were done with transgenic T cells. The targeting of antigens to induce or expand Foxp3+ Treg from the polyclonal non-transgenic T repertoire is an important issue for future clinical therapy.
7. The induction of antigen-specific Foxp3+ Treg by DC subsets in skin
Treg also contribute to the maintainance of immune homeostasis in the skin. CCR4+ CD103high Foxp3+ Treg accumulate in the skin and actively maintain tolerance [43]. In the skin, there are DC subsets such as Langerhans cells (LCs) in the epidermis and dermal DCs, where the latter are currently divided into Langerin+ or Langerin− [44–46]. The skin DCs migrate to skin draining lymph nodes and present antigens from skin to T cells [35,47]. However, the outcome of antigen presentation might differ with each skin DC subset.
LCs do not participate in the presentation of pathogens during a viral infection and Leishmaniasis [35]. Nevertheless, it is possible that the major histocompatibility complex (MHC) class II presentation pathway on LCs is used to present antigen for purposes of Treg induction, i.e., that LCs in the steady state are specialized to induce Foxp3+ Treg. Also, LCs produced TGF-β [48], which is an important cytokine in inducing Foxp3+ Treg as described above. It might be possible that LCs play an important role in inducing Foxp3+ T reg in the skin.
Consistent with this idea, the number of Foxp3+ Treg was expanded by epidermal LCs that had been stimulated with transgenic RANKL expression on keratinocytes [49], although in this report it was not determined whether natural Treg or induced Treg were participating. It is of interest to investigate further role of LCs in inducing Foxp3+ Treg.
8. Other DC subsets and the induction of antigen-specific Foxp3+ Treg
Plasmacytoid DCs, which produce large amounts of type I interferons (IFNs) following TLR recognition of nucleic acids, also seem to be capable of inducing Foxp3+ Treg. For example, plasmacytoid DCs are responsible for the induction of antigen-specific Foxp3+ Treg following donor splenocyte transfusion along with anti-CD40 ligand Ab [50]. Plasmacytoid DCs from tumor draining lymph nodes activated Treg through an indoleamine 2,3-dioxygenase (IDO) dependent pathway [51]. TLR-9 ligand, CpG-activated human plasmacytoid DCs induced Foxp3+ Treg from CD25− CD4+ T cells [52]. Liver plasmacytoid DCs also play a role in inducing oral tolerance, although the involvement of induced Treg was not determined [53]. Tolerogenic plasmacytoid DCs can be distinguished by the expression of chemokine receptor CCR9, which is a receptor of CCR9 ligand, CCL25. CCR9+ plasmacytoid DCs, but not CCR9− plasmacytoid DCs, could induce Foxp3+ Treg in vitro and in vivo and suppress GVHD [54].
Moreover, Foxp3+ Treg were induced by a subset of more mature DCs in the human thymus, following stimulation by thymic stromal lymphopoietin (TSLP) [55]. Bone marrow DCs cultured with TGF-β and IL-10 could induce Foxp3+ Treg and suppress GVHD [56].
9. Conclusions
Taken together, antigen-specific Foxp3+ T reg can be induced by DCs. However, many reports to date have been done in transgenic T cells and model antigens such as OVA. For future clinical therapy, it is necessary to identify ways to induce Foxp3+ T reg from non-transgenic polyclonal T cells and clinically relevant antigens. Currently, it would appear important to use DCs to present antigens and to deliver other signals required for the control of Treg.
Acknowledgments
This work was supported by NIH AI 051573 and a program project grant from the Juvenile Diabetes Research Foundation. We thank Christopher Fiorese and Judy Adams for help.
Abbreviations used in this paper
- Treg
regulatory T cells
- DCs
dendritic cells
- APCs
antigen presenting cells
- Ab
antibody
- NOD
non-obese diabetic
- GVHD
graft versus host disease
- TGF-β
transforming growth factor-β1
- OVA
ovalbumin
- RA
retinoic acid
- DCIR2
dendritic cell inhibitory receptor-2
- DEC-OVA
chimeric anti-DEC-205 mAb with OVA protein
- 33D1-OVA
chimeric anti-DCIR2 mAb with OVA protein
- LCs
Langerhans cells
- FACS
fluorescence-activated cell sorting
Biography
Sayuri Yamazaki graduated from School of Medicine, Tokyo Medical and Dental University, and received MD in 1991. She became a graduate student at the Department of Dermatology, Graduate School of Medicine, Tokyo Medical and Dental University, and received PhD in 1995. She worked as a Dermatologist at hospitals affiliated with Tokyo Medical and Dental University, and became Head Dermatologist after receiving the certification of Specialist in Dermatology in 1996. She started research of Treg as Visiting Researcher at Dr. Shimon Sakaguchi’s lab in Tokyo Metropolitan Institute for Gerontology in 1998. Then, she became Research Fellow for Young Scientists, Japan Society for the Promotion of Science at Sakaguchi’s lab at Institute of Frontier Medical Science, Kyoto University from 1999–2001. She has been Research Associate (Instructor) at Dr. Ralph M Steinman’s lab at the Rockefeller University in New York, USA since 2001.

Footnotes
Conflict of Interest Statement
Sayuri Yamazaki has no conflicting financial interests. Ralph M Steinman has financial interests in Celldex, which is developing anti–DEC-205 antibodies for human use.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–62. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 4.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 6.Nieves DS, Phipps RP, Pollock SJ, et al. Dermatologic and immunologic findings in the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Arch Dermatol. 2004;140:466–72. doi: 10.1001/archderm.140.4.466. [DOI] [PubMed] [Google Scholar]
- 7.Halabi-Tawil M, Ruemmele FM, Fraitag S, et al. Cutaneous manifestations of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Br J Dermatol. 2008 doi: 10.1111/j.1365-2133.2008.08835.x. [DOI] [PubMed] [Google Scholar]
- 8.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, McCormick TS, Cooper KD. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: Mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–73. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 11.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen processing dendritic cells. J Exp Med. 2003;198:235–47. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–77. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki S, Patel M, Harper A, et al. Effective expansion of alloantigen-specific Foxp3+ CD25+ regulatory T cells by dendritic cells during the mixed leukocyte reaction. Proc Natl Acad Sci USA. 2006;103:2758–63. doi: 10.1073/pnas.0510606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo X, Tarbell KV, Yang H, Pothoven K, Bailey SL, Ding R, Steinman RM, Suthanthiran M. Dendritic cells with TGF-β1 differentiate naive CD4+CD25− T cells into islet-protective Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2007;104:2821–6. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki S, Bonito AJ, Spisek R, Dhodapkar M, Inaba K, Steinman RM. Dendritic cells are specialized accessory cells along with TGF-β for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3− precursors. Blood. 2007;110:4293–302. doi: 10.1182/blood-2007-05-088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–33. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehervari Z, Sakaguchi S. Control of Foxp3+ CD25+CD4+ regulatory cell activation and function by dendritic cells. Int Immunol. 2004;16:1769–80. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Dendritic cells expand antigen-specific Foxp3+CD25+CD4+ regulatory T cells including suppressors of alloreactivity. Immunol Rev. 2006;212:314–29. doi: 10.1111/j.0105-2896.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 22.Tarbell KV, Yamazaki S, Steinman RM. The interactions of dendritic cells with antigen-specific, regulatory T cells that suppress autoimmunity. Semin Immunol. 2006;18:93–102. doi: 10.1016/j.smim.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson TS, Dipaolo RJ, Andersson J, Shevach EM. IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–6. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 25.Dipaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFβ-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–93. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 26.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, Shevach EM. CD4+ Foxp3+ regulatory T cells confer infectious tolerance in a TGF-β-dependent manner. J Exp Med. 2008;205:1975–81. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 28.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 30.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–70. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaensson E, Uronen-Hansson H, Pabst O, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–71. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 34.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 35.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–55. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 36.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:9331–6. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Travis MA, Reizis B, Melton AC, et al. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–5. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Larregina AT, Shufesky WJ, Perone MJ, Montecalvo A, Zahorchak AF, Thomson AW, Morelli AE. Use of the inhibitory effect of apoptotic cells on dendritic cells for graft survival via T-cell deletion and regulatory T cells. Am J Transplant. 2006;6:1297–311. doi: 10.1111/j.1600-6143.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 40.Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-β from phagocytes digesting apoptotic T cells. Nat Med. 2008;14:528–35. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 41.Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–9. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 42.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 43.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–47. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ginhouz F, Collin MP, Bogunovic M, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–46. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–56. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–31. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci USA. 2006;103:7783–8. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGFβ1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–52. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372–9. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 50.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–62. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 51.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–60. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe N, Wang YH, Lee HK, Ito T, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 56.Sato K, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–79. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]