Abstract
Theories of animal defensive behavior postulate that imminent, predictable threat elicits highly focused attention toward the threat source, whereas remote, unpredictable threat elicits distributed attention to the overall environment. We used threat of shock combined with measurement of prepulse inhibition of the startle reflex to test these claims in humans. Twenty-seven participants experienced periods of threat and safety. Threat and safe periods were short or long, with the short threat periods conveying relatively predictable, imminent shocks and the long threat periods conveying unpredictable shocks. Startle reflexes were elicited with equal numbers of acoustic probes presented alone, preceded by a tactile prepulse, or preceded by an auditory prepulse. We observed enhanced tactile relative to auditory prepulse inhibition during short threat periods only. This finding supports the notion that imminent threat, but not remote threat, elicits attention focused toward the relevant modality, potentially reflecting preparatory activity to minimize the impact of the noxious stimulus.
Whether aversive stimuli are predictable or unpredictable in time is a critical determinant of how an organism will adaptively respond to, and what neural structures will be activated by, threatening conditions. Studying rodents, Walker and Davis (1997) have distinguished between phasic fear, which is mediated by the amygdala in response to cues predicting imminent noxious stimulation, and sustained anxiety, which is mediated by the bed nucleus of the stria terminalis (see Davis, 1998, 2006). Along these lines, abundant evidence suggests that humans can be instructed or conditioned to fear discrete cues that predict aversive stimuli (e.g., shock) and to show anticipatory anxiety when aversive stimuli are presented unpredictably (i.e., without a discrete warning cue; Grillon, Ameli, Woods, Merikangas, & Davis, 1991; Grillon, Baas, Lissek, Smith, & Milstein, 2004). Recent studies of humans have demonstrated that these responses or states are dissociable pharmacologically (Baas et al., 2004; Grillon, Baas, et al., 2006; Grillon, Cordova, Morgan, Charney, & Davis, 2004). In the present study, we intended to elaborate the distinction between fear and anxiety by demonstrating differences in cognitive processes under conditions of predictable versus unpredictable shock. In particular, we examined how attention is allocated under short (10 s) periods with relatively predictable timing of shock delivery and long (60 s) periods with more unpredictable timing of shock delivery.
The concept of threat imminence has figured prominently in theories of animal defensive behaviors (Blanchard & Blanchard, 1989; Fanselow, 1994; Gray & McNaughton, 2000) and human psychophysiological responses (Lang, Bradley, & Cuthbert, 1997; Öhman, Hamm, & Hugdahl, 2000). One common theme is that when potential threat exists but is not imminent, attention is heightened, but not necessarily directed toward specific aspects of the environment (Blanchard & Blanchard, 1989; Fanselow, 1994). An organism may show cautious approach and be highly sensitive to any stimuli that may be signaling threat (Gray & McNaughton, 2000). When threat is actualized (e.g., a predator is detected), freezing is a prototypical response as attention is further heightened and directed toward the threat (Blanchard & Blanchard, 1989; Fanselow, 1994). Accordingly, whereas remote threat may elicit a distributed attentional stance, imminent threat may elicit an attentional stance focused toward the specific source of threat. To our knowledge, there is no empirical evidence that these claims are true for humans. A straightforward way to test for these alternative attentional stances is to present neutral stimuli in different sensory modalities and assess the extent to which attention is biased toward one modality over another while subjects anticipate shocks. We would expect that as shock imminence increases, attentional resources would be allocated to the tactile modality over other modalities. The recognition that shock is imminent is directly related to how predictable shock delivery is in time. Thus, short threat periods should convey greater shock imminence than long threat periods to the extent that the former constrain the timing of shock delivery more than the latter.
To test whether threat of predictable, imminent shocks elicits focused attention to tactile stimuli, we measured the startle reflex (eyeblink response) to sudden, loud bursts of white noise (startle probes) and, critically, the degree of modulation of the startle reflex by tactile and auditory stimuli that preceded the startle probes (prepulses). The startle reflex, a defensive cascade of muscle contractions, has proved to be an invaluable tool for studying fear and anxiety in animals and humans (Davis, 1998; Grillon, 2002). Paradigms that experimentally elicit the startle reflex can be conceptualized as probing on-line the affective-motivational state of an organism (Lang et al., 1997). Fear-potentiated startle refers to the augmentation of startle (i.e., larger eyeblinks; see Fig. 1) under fearful and anxiety-provoking conditions (Grillon, 2002). Startle responses are larger when elicited during presentation of a foreground stimulus that has been paired with an aversive stimulus (e.g., shock) than when elicited during presentation of a stimulus that has not been so paired. This finding was first reported for rodents (Brown, Kalish, & Farber, 1951) and has since been replicated in humans (Grillon & Davis, 1997; Hamm, Greenwald, Bradley, & Lang, 1993). Startle responses are also potentiated during extended periods of risk of shock at any time without warning, relative to periods of safety (Bitsios, Philpott, Langley, Bradshaw, & Szabadi, 1999; Grillon et al., 1991). Thus, startle responses are potentiated when elicited under conditions of both imminent, predictable threat and remote, unpredictable threat.
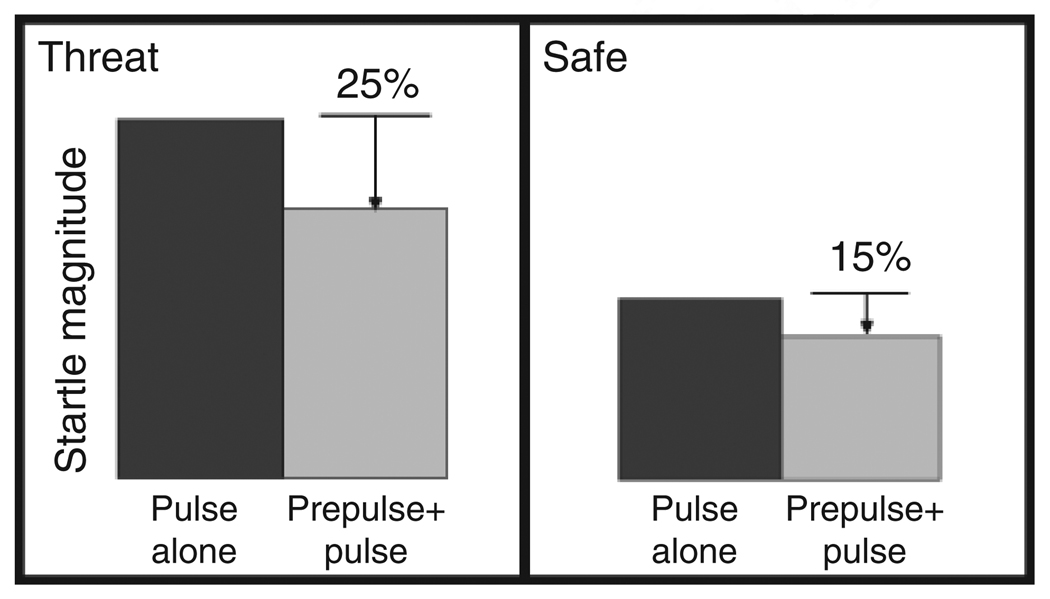
Fig. 1.
Predicted startle modulation by threat (fear-potentiated startle) and prepulse stimuli (prepulse inhibition of startle), as well as their interaction. Startle magnitudes are potentiated under threatening conditions, relative to safe conditions. Startle magnitudes are smaller when weak, nonstartling stimuli (or prepulses) are presented shortly before startle probes (or pulses) than when startle probes are presented alone. One hypothesis tested in the present study is that in addition to having independent effects, prepulses and threat have an interactive effect, such that prepulse inhibition of startle is greater under threat relative to safe conditions (e.g., 25% vs. 15% reduction from probe-alone baseline responses, as illustrated here).
Prepulse inhibition, in contrast, is the reduction of the startle reflex caused by a nonstartling prepulse stimulus (e.g., a 60-dB tone; see Fig. 1) that precedes a startle probe by a short interval (e.g., 120 ms; Blumenthal, 1999; Graham, 1992). Prepulse inhibition is observed when the prepulse and startle probes are presented in the same modality, as well as when they are presented in different modalities. That is, auditory, visual, and tactile prepulses can reduce the magnitude of the acoustically elicited startle reflex (Blumenthal & Gescheider, 1987; Cornwell, Echiverri, & Grillon, 2006; Wynn, Dawson, & Schell, 2004). Prepulse inhibition is thought to be driven mainly by a protective gating mechanism in the primary neural circuit mediating the reflex; thus, the magnitude of prepulse inhibition of startle may reflect the extent to which the prepulse stimulus is processed (Graham, 1992). Although prepulse inhibition is not necessarily mediated by higher cognition, ample evidence indicates that attention modulates the degree of startle inhibition produced by prepulses. Attention-to-prepulse studies have shown that prepulses that are relevant to performing a concurrent task, and therefore those to which participants attend, modulate startle significantly more than task-irrelevant prepulses (Filion, Dawson, & Schell, 1993, 1994; Filion & Poje, 2003). Attentional enhancement of prepulse inhibition is observed under within-modality and cross-modality conditions (Elden & Flaten, 2003).
We measured prepulse inhibition of startle by both tactile (airpuffs) and auditory (tones) prepulse stimuli under threat and safe conditions. The current study builds on previous work (Grillon & Davis, 1997) showing greater auditory prepulse inhibition of startle in healthy participants during long periods of threat (80 s), relative to safety. The authors concluded that threat of shock may have increased alertness (or general vigilance), leading to enhanced processing of the auditory prepulse stimuli and, consequently, greater startle inhibition. Accordingly, we hypothesized that prepulse inhibition of the acoustic startle reflex by auditory and tactile prepulses would be increased under threat of shock relative to safe conditions (Fig. 1). We also addressed how attention is allocated under short, predictable threat conditions versus long, unpredictable threat conditions (~10 s vs. ~60 s) by using auditory and tactile prepulses. We predicted not only that prepulse inhibition of startle would increase under threat regardless of the kind of prepulse, but also that prepulse inhibition would be selectively increased for tactile prepulses under short but not long threat periods. The latter prediction was based on the hypothesis that imminent, predictable threat elicits a focused attentional stance toward the modality in which threat is anticipated, leading to increased processing of modality-specific stimuli, and that a more distributed attentional stance is elicited by remote, unpredictable threat.
METHOD
Participants
Participants were 27 healthy individuals (13 women, 14 men; mean age = 25 years, range = 18–42 years) who gave written informed consent for experimental procedures approved by the National Institute of Mental Health Human Investigation Review Board and received monetary compensation for their involvement. Inclusion criteria were as follows: (a) no past or current psychiatric disorders as per the Structured Clinical Interview for DSM-IV (First, Spitzer, Williams, & Gibbon, 1995); (b) no medical condition that interfered with the objectives of the study, as established by a physician; and (c) no use of illicit drugs or psychoactive medications, as determined by a urine screen. Six participants were excluded from the startle analyses for having small responses (i.e., mean probe-alone startle across all conditions < 10 µV). Sample size for the startle analyses was thus 21 (10 women, 11 men; mean age = 25 years).
Stimuli
Stimulus presentation was controlled by Psylab 7 software (Contact Precision Instruments, London, United Kingdom). Acoustic stimuli were presented binaurally through headphones (Bose-Triport, Framingham, MA). Startle probes were 40-ms bursts of broadband white noise presented at 105 dB(A) with nearly instantaneous rise and fall times. Auditory prepulses were 1000-Hz pure tones presented at 60 dB(A) with nearly instantaneous rise and fall times; their duration was 40 ms. Tactile prepulses were 40-ms puffs of compressed air at 4 psi (controlled by a solenoid); they were delivered to the dorsal surface of the right hand through a plastic tube. Shocks were delivered by a constant-current stimulator through two Ag/AgCl electrodes filled with 0.5% saline/neutral base electrode gel (BioPac Systems, Inc., Goleta, CA) and attached to the right wrist. Electrical current ranged between 3 and 5 mA for 100 ms, depending on levels established during the shock workup (see the next paragraph).
Design and Procedure
After attachment of electromyographic (EMG) electrodes, participants were presented with nine startle probes to habituate the startle response. Next, participants were presented with each type of isolated prepulse stimulus (six airpuffs and six tones) for habituation. During habituation, startle probes and prepulse stimuli were presented every 18 to 22 s. Shock electrodes were subsequently attached to the right wrist, and a shock workup procedure was conducted to set shock intensity at an unpleasant but not painful level.
Participants were informed that they would be at risk of receiving 6 to 12 shocks by the end of the experiment and that shocks would be administered during threat but not safe periods, which would be signaled by the words “THREAT” and “SAFE” on a computer monitor. The duration (short or long) of the threat or safe condition was indicated by the length of a bar underneath the word. These stimuli remained on the screen for the relevant duration, 9 to 11 s for short periods and 54 to 65 s for long periods. Retrospectively, participants reported their distress level for each condition and duration (on a scale from 1, not at all distressed, to 100, highly distressed). Using a 100-point scale allowed participants to conceptualize their distress level as percentages.
The experimental session was divided into four runs of approximately 15 min each, with a 3- to 5-min break between runs. Threat and safe periods alternated within each run (Fig. 2). The two duration conditions (short vs. long period) were pseudorandomly distributed across the experimental session with the constraint that a long period never followed a long period and none of the four runs began or ended with a long period. Each run included nine short threat, nine short safe, three long threat, and three long safe periods. Two runs began with a short threat period, and two runs began with a short safe period. Approximately half the subjects received the trials within each run in a given order, and the order was reversed for the other half of the subjects; orthogonally, approximately half the subjects received each of two different run orders (i.e., ABCD, CDAB).
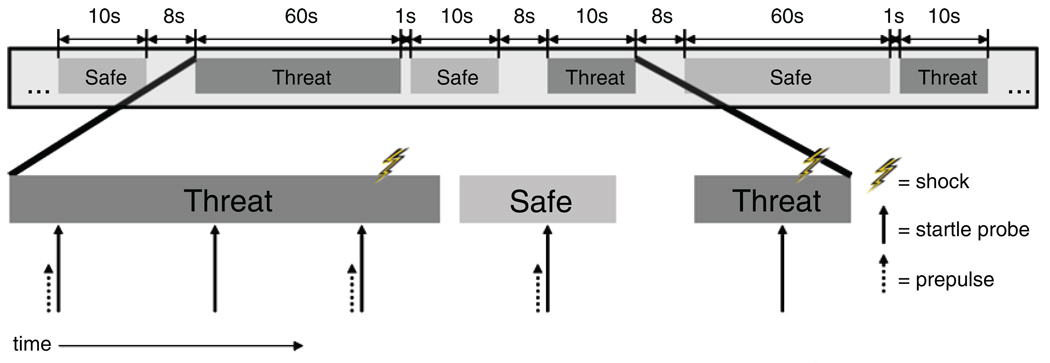
Fig. 2.
Schematic diagram of alternating threat and safe periods within one run. Note that only a portion of a run is depicted. Threat and safe periods had either a short (~10 s) or long (~60 s) duration, and interperiod intervals were variable (1 s or 8 s). Startle probes were delivered during both threat and safe periods, with one presented 4 to 6 s into short periods, and three presented during long periods (4–6 s, 22–28 s, and 40–50 s into long periods). Equal numbers of startle probes were presented alone (probe-alone or baseline startle trials), preceded by an airpuff (tactile prepulse trials), and preceded by a pure tone (auditory prepulse trials). Shocks were administered during threat periods only, and were delivered approximately 1 s after startle probes.
Each run began with two startle probes presented before the first visual cue to rehabituate the startle response. Responses to these probes were not analyzed. One acoustic startle probe was presented during each short period, and three were presented during each long period. Startle probes were presented 4 to 6 s after the onset of short periods. During long periods, startle probes were presented at three intervals: at latencies of 4 to 6 s, 22 to 28 s, and 40 to 50 s (see Fig. 2).
One third (48) of the startle probes were presented alone (i.e., probe-alone startle), one third were preceded by an airpuff at a stimulus onset asynchrony (SOA) of 120 ms (tactile prepulse), and one third were preceded by a pure tone at an SOA of 120 ms (auditory prepulse).Airpuff timing was adjusted by a 50-ms delay to account for the length of the tube. Short and long periods were followed by 8-s and 1-s interperiod intervals, respectively, to maintain an overall schedule of startle-probe delivery every 18 to 22 s.
Eight shocks were administered, four during short threat periods and four during long threat periods. In one long threat period, two shocks were administered, so that 7 of the 48 threat periods (4 short, 3 long) contained shock. Of the shocks during the long threat periods, two occurred after the first startle probe (i.e., ~5–7 s into the period), and two occurred after the last startle probe (i.e., ~41–51 s into the period), to reinforce the belief that shocks could be administered at any time during threat. Shocks were administered approximately 1 s after startle-probe delivery (or ~19 s before the next probe) to limit potential effects of shock sensitization on subsequent startle responding.
Physiological Recording and Scoring
Physiological recordings were made with a commercial system (Contact Precision Instruments, London, United Kingdom). Two 2-mm Ag/AgCl electrodes were attached below the left eye to measure EMG for startle-response analysis. A third Ag/AgCl electrode attached to the right forearm served as ground. Electrodes were filled with electrode gel, and impedances were kept below 20 kΩ. Continuous EMG activity was digitized at 1000 Hz using a 30- to 500-Hz band-pass filter. Scoring of EMG data was done with Psylab 7. EMG data were rectified and smoothed with a 20-ms time constant. Startle responses were scored by measuring the peak value of the first deflection beginning 20 to 100 ms following probe onset and subtracting the onset value from this peak value. Trials with response amplitudes below 1 µV were classified as nonresponse trials, and response amplitudes on these trials were converted to zero. These nonresponse trials were included in the averages to compute magnitudes.
Statistical Analysis
Prepulse inhibition was quantified in two alternative ways: percentage change from probe-alone startle magnitudes and standardized difference from probe-alone startle magnitudes; probe-alone means were taken within each combination of condition (threat, safe) and duration (short, long). Some researchers prefer to use percentage change to control for the influence of probe-alone (baseline) magnitudes on prepulse-inhibition values (Blumenthal, Elden, & Flaten, 2004). Descriptive analyses with raw and log10-transformed percentage-change values showed 200 to 300% more variance in the safe condition than in the threat condition. Standardized differences demonstrated more homogeneity of variance across the threat and safe conditions and were entered into a repeated measures analysis of variance (ANOVA) to test our hypotheses (Grillon & Davis, 1997). We used a T-score conversion of raw scores (M = 50, SD = 10) over the entire data set (excluding habituation), and subtracted (auditory or tactile) prepulse-startle T scores from probe-alone startle T scores for each of the four cells.1 Greater differences reflect greater prepulse inhibition. It should be noted that the critical comparison was between auditory prepulse inhibition and tactile prepulse inhibition within the same condition (i.e., derived from the same probe-alone baseline), which obviated the need to control for probe-alone startle in this comparison.
RESULTS
Subjective Distress and Probe-Alone Startle
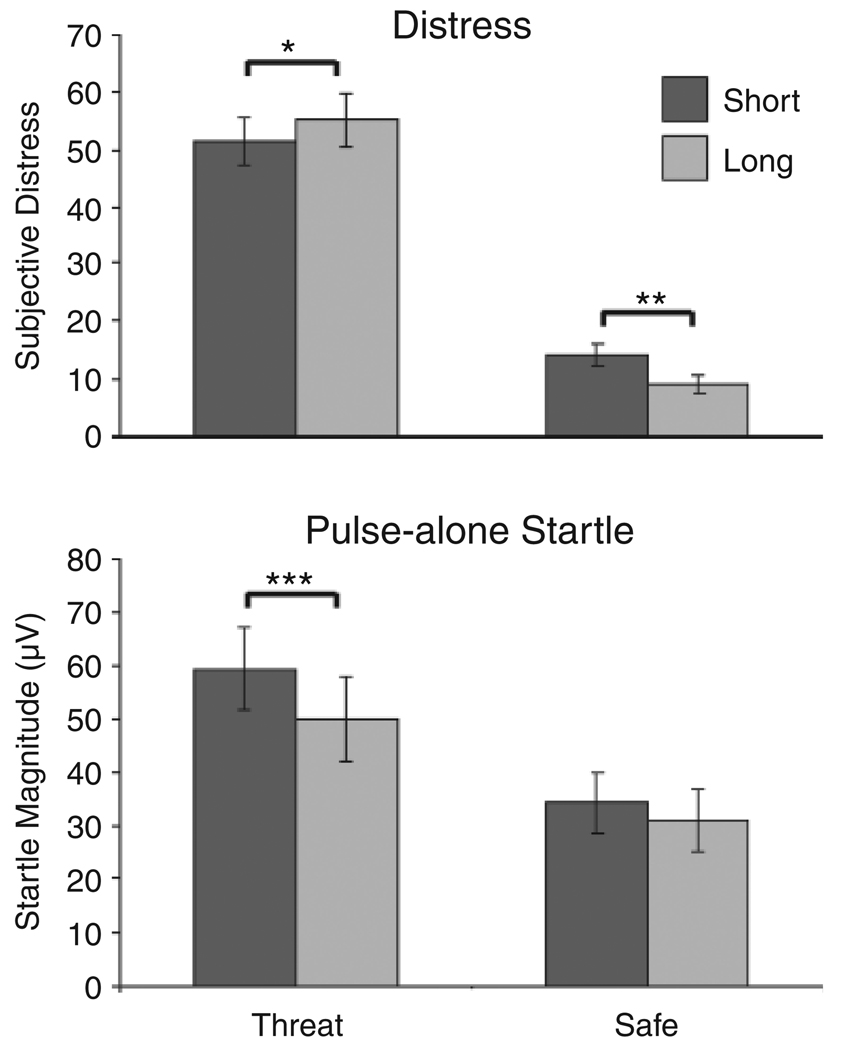
Two-way Condition (safe, threat) × Duration (short, long) ANOVAs revealed significant interactions for distress ratings, F(1, 26) = 11.49, p < .005, ηp2 = .31, and raw probe-alone startle magnitudes, F(1, 20) = 6.62, p < .05, ηp2 = .25 (Fig. 3). Participants reported greater distress during long threat relative to short threat periods, F(1, 26) = 5.51, p < .05, ηp2 = .18, and greater distress during short safe relative to long safe periods, F(1, 26) = 12.20, p < .005, ηp2 = .32. Probe-alone raw startle magnitudes were higher during short threat relative to long threat periods, F(1, 26) = 21.59, p < .001, ηp2 =.52, but did not differ between short and long safe periods, F(1, 20) = 2.20, n.s., ηp2 = .10 (Fig. 3). Several main effects were also significant. Both measures showed greater responses for threat relative to safe periods—distress: F(1, 26) = 114.85, p < .001, ηp2 = .82; probe-alone startle, F(1, 20) = 31.59, p < .001, ηp2 = .62. Probe-alone startle magnitude was greater for short than for long periods, F(1, 20) = 21.59, p < .001, ηp2 = .52, but duration did not have a significant effect on subjective distress, F < 1.
Fig. 3.
Subjective distress and raw probe-alone startle magnitude as a function of condition (threat vs. safe) and period duration (short vs. long). Asterisks indicate a significant difference between short and long periods, *p < .05, **p < .005, ***p < .001.
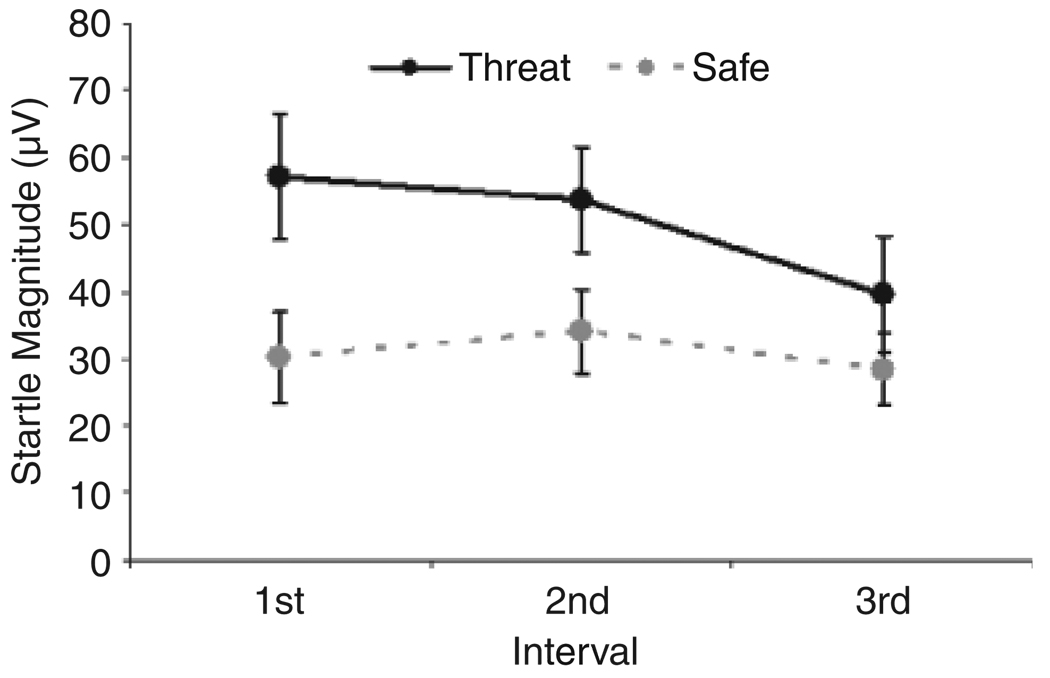
The long periods were divided into intervals (i.e., three presentations of startle probes), and startle magnitudes showed a significant linear decrease in the long threat periods, but not in the long safe periods (i.e., a Condition × Interval interaction), Flinear(1, 20) = 11.17, p < .005, ηp2 = .36 (Fig. 4). Post hoc tests revealed that startle magnitudes during short threat periods were significantly greater than those in the third interval (i.e., 40–50 s following period onset) of the long threat periods only, t(20) = 5.68, p < .001.
Fig. 4.
Raw probe-alone startle magnitudes for each interval during long threat and safe periods.
Prepulse Inhibition of Startle
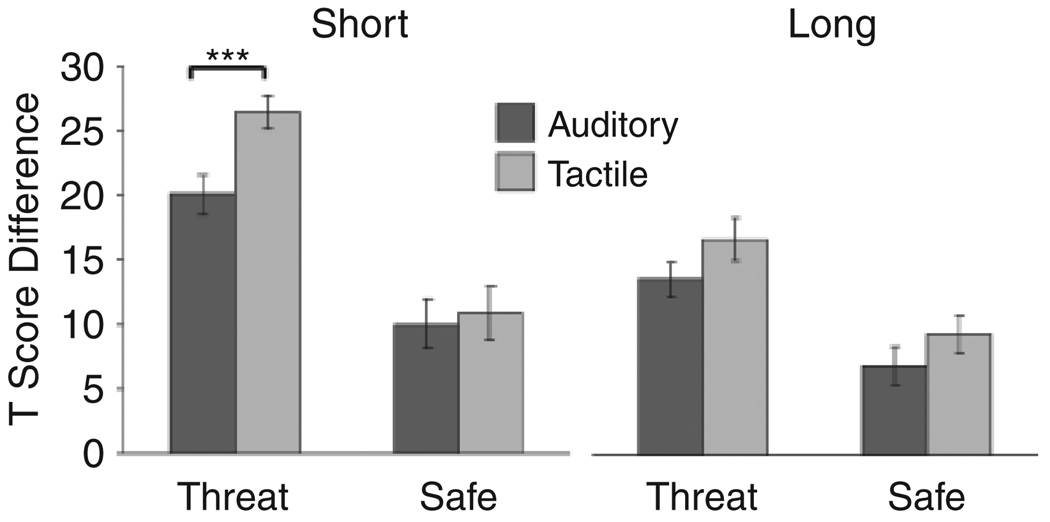
A Condition (threat, safe) × Duration (short, long) × Prepulse Modality (tactile, auditory) ANOVA on prepulse-inhibition magnitude revealed a significant three-way interaction, F(1, 20) = 5.54, p < .05, ηp2 = .22 (Fig. 5). During short periods, there was a significant Condition × Prepulse Modality interaction, F(1, 20) = 9.32, p < .01, ηp2 = .32. Prepulse inhibition was greater for tactile prepulses than for auditory prepulses during short threat periods, F(1, 20) = 14.38, p < .001, ηp2 = .42, but not during short safe periods, F < 1. From short safe periods to short threat periods, the increase in the T-score difference was 15.6 with tactile prepulses and 10.1 with auditory prepulses; thus, prepulse inhibition was 55% greater for tactile prepulses. During long periods, condition and prepulse modality did not have an interactive effect on prepulse-inhibition magnitudes, F < 1. Prepulse inhibition was greater for long threat periods than for long safe periods, F(1, 20) = 19.50, p < .001, ηp2 = .49, and was greater for tactile prepulses than for auditory prepulses, F(1, 20) = 7.99, p < .01, ηp2 = :29. From long safe periods to long threat periods, the increase in the T-score difference was 6.9 for tactile prepulses and 6.3 for auditory prepulses.
Fig. 5.
Prepulse inhibition of acoustic startle as a function of period duration (short vs. long), condition (threat vs. safe), and prepulse modality (tactile vs. auditory). Asterisks indicate a significant difference between modalities, ***p < .001.
Prepulse inhibition also showed main effects of the independent variables. Prepulse inhibition was greater during threat than safe periods, F(1, 20) = 40.26, p < .001, ηp2 = .67; was greater during short than long periods, F(1, 20) = 20.24, p < .001, ηp2 = .50; and was greater for tactile prepulses than for auditory prepulses, F(1, 20) = 9.06, p < .01, ηp2 = .31.
DISCUSSION
The aim of this study was to characterize attentional processes under conditions in which the timing of shocks is relatively predictable versus unpredictable. Participants experienced alternating threat and safe periods, with both kinds of periods varying in duration. We measured prepulse inhibition of the acoustic startle reflex by both tactile and auditory prepulses to assess modality-specific attentional processes. Based on theories relating defensive responses to threat imminence, our primary hypothesis was that tactile prepulses would generate greater prepulse inhibition than auditory prepulses during short threat periods only, given that short threat periods signaled imminent threat and attention would be biased toward the source (i.e., modality) of threat. Our physiological and subjective measures (Fig. 3 and Fig. 4) converge in demonstrating that threat of shock elicited fear and anxiety. In addition, our results support our hypothesis of increased modality-specific attention toward imminent but not remote threat: We found selective enhancement of tactile prepulse inhibition under short but not long threat periods (Fig. 5). As in a previous study (Grillon & Davis, 1997), we also observed, in general, greater prepulse inhibition under threat of shock relative to safe conditions.
Probe-alone startle magnitudes and subjective distress ratings were significantly higher during threat than safety, a finding that supports previous studies (Grillon et al., 1991; Grillon, Baas, et al., 2004). In addition, duration modulated the effect of threat on probe-alone startle and subjective distress. Whereas probe-alone startle was greater during short threat periods relative to long threat periods, it did not differ between short and long safe periods. Greater startle during short threat than during long threat periods may reflect intense aversive arousal associated with a phasic fear response following the onset of threat and more moderate arousal associated with sustained anxiety over long threat periods. During long threat periods, unlike during long safe periods, startle magnitudes decreased linearly from an initial level that was comparable to startle magnitudes during short threat periods. Participants reported higher distress during long threat periods relative to short threat periods, and during short safe periods relative to long safe periods. This pattern of distress ratings suggests that long threat periods were experienced as the most stressful because of the unpredictable timing of shock and is consistent with the results of previous studies and with the prolonged startle potentiation we observed during long threat periods (Grillon & Baas, 2003; Grillon, Baas, Cornwell, & Johnson, 2006). That short safe periods were more distressing than long safe periods may reflect less complete recovery from the preceding threat period in the short safe periods.
Our central finding was that the increase in prepulse inhibition during threat periods relative to safe periods was greater for tactile than auditory prepulses during short periods, but not during long periods. Because the magnitudes of tactile and auditory prepulse inhibition were expressed as differences from the same probe-alone baseline within each condition, this result cannot be attributed to differences in baseline startle across conditions. Actively attending to a prepulse stimulus has been shown to selectively increase prepulse inhibition from that stimulus regardless of sensory modality (Elden & Flaten, 2003; Filion et al., 1993, 1994). Attention-to-prepulse studies have uniformly involved instructing participants to focus on one type of stimulus and ignore others. We found a similar differential pattern even though participants were not instructed to attend to the prepulses, and thus our results suggests that attention was naturally directed toward afferent somatosensory activity rather than auditory activity during short threat periods. This finding fits with the idea that imminent threat increases the relevance of stimuli that are processed by the same modality in which threat is anticipated, such that modality-specific environmental changes receive selective attention; in turn, this selective attention potentially facilitates preparatory processes involved in minimizing the impact of the impending stimulus (Carlsson et al., 2006; Fanselow & Helmstetter, 1988; Ploghaus, Becerra, Borras, & Borsook, 2003). Indeed, a noxious stimulus may be less painful when its timing can be predicted (Carlsson et al., 2006; Rhudy, Williams, McCabe, Rambo, & Russell, 2006), an effect potentially mediated by anticipatory activity in somatosensory cortices (Miltner, Braun, Arnold, Witte, & Taub, 1999).
There was no evidence that tactile prepulse inhibition was differentially enhanced during long threat periods. Rather, both auditory and tactile prepulse inhibition were increased in long threat periods relative to long safe periods. This result is consistent with Grillon and Davis’s (1997) finding of enhanced auditory prepulse inhibition under threat of shock. Long threat periods, therefore, did not elicit an attentional stance focused toward the tactile modality, as short threat periods did. Although selective focus may not be sustainable over long durations, the allocation of attentional resources when shocks are relatively unpredictable may in some instances be similar to the allocation of attentional resources when shocks are more predictable. We measured prepulse inhibition at three intervals within long periods, but averaged these data in the main analysis to have equal numbers of trials for short and long periods. An analysis of the data from the long periods without collapsing across intervals did not reveal differential prepulse inhibition across modalities as a function of threat and interval (i.e., the analysis of prepulse inhibition did not yield a reliable three-way interaction among threat, modality, and interval. In other words, there was no interval during long periods at which tactile prepulses produced stronger inhibition of startle than auditory prepulses in threat periods relative to safe periods. These results suggest that an attentional stance focused toward an anticipated threat source is specific to situations in which the threat is relatively imminent and predictable. When the threat is relatively remote, sustained anxious arousal may lead to general attentional facilitation of sensory processes in all modalities (Baas, Milstein, Donlevy, & Grillon, 2006; Cornwell et al., 2007).
In general, we observed greater prepulse inhibition by tactile prepulses than by auditory prepulses. Multiple factors could have contributed to this effect. Airpuffs may have produced greater prepulse inhibition because they caused more intense sensations (Blumenthal, 1999), but it is not easy to determine relative intensity for stimuli impinging on different sensory modalities. Also, shock administration could have led to selective sensitization of tactile sensory processes, and given that airpuffs were delivered in close proximity to the shock electrodes, they may have received enhanced processing, which might have elicited greater inhibition of startle than found for auditory tones. Although the main effect of prepulse modality could be explained by sensitization processes, the selective increase in startle inhibition by tactile prepulses during short threat periods only cannot.
The present findings support the contention that, depending on its proximity, threat may elicit two distinct cognitive states reflected in defensive behaviors of animals (Blanchard & Blanchard, 1989; Davis, 2006) and psychophysiological responses in humans (Grillon & Bass, 2003; Lang et al., 1997). When threat is imminent, attention may be directed predominantly toward the source, so as to prepare for the impending stimulus, perhaps at the (rightful) expense of attending to other environmental changes. When threat is more remote, attention may be distributed to maximize the potential for identifying any stimulus that may be signaling threat (Baas et al., 2006; Cornwell et al., 2007). When periods of threat and safety are defined explicitly, these two cognitive-attentional states appear to be functionally adaptive responses to predictable and unpredictable aversive stimulation. The approach taken here, investigating attentional processes via modality-specific prepulse inhibition, may offer a new avenue for studying how these adaptive responses differ from pathological ones underlying anxiety disorders.
Acknowledgments
This research was supported by the intramural research program of the National Institute of Mental Health, National Institutes of Health.
Footnotes
Preliminary analyses of the correlation between probe-alone (i.e., baseline) startle magnitudes and prepulse-inhibition magnitudes revealed that out of eight Pearson coefficients (2 Conditions × 2 Durations × 2 Modalities), only one was statistically significant, r(19) = .64, p < .005; the average Pearson r was .27 (range = −.32 to .64). Thus, our standardized-difference method performed well in quantifying prepulse inhibition independently of probe-alone startle.
REFERENCES
- Baas JMP, Grillon C, Bocker KBE, Brack AA, Morgan CA, 3rd, Kenemans JL, Verbaten MN. Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology. 2004;161:233–247. doi: 10.1007/s00213-002-1011-8. [DOI] [PubMed] [Google Scholar]
- Baas JMP, Milstein J, Donlevy M, Grillon C. Brainstem correlates of defensive states in humans. Biological Psychiatry. 2006;59:588–593. doi: 10.1016/j.biopsych.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Philpott A, Langley RW, Bradshaw CM, Szabadi E. Comparison of the effects of diazepam on the fear-potentiated startle reflex and the fear-inhibited light reflex in man. Journal of Psychopharmacology. 1999;13:226–234. doi: 10.1177/026988119901300303. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Progress in Neuropsychopharmacology and Biological Psychiatry. 1989;13:S3–S14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD. Short lead interval startle modification. In: Dawson M, Schell A, Boehmelt A, editors. Startle modification: Implications for neuroscience, cognitive science, and clinical science. New York: Cambridge University Press; 1999. pp. 51–71. [Google Scholar]
- Blumenthal TD, Elden A, Flaten MA. A comparison of several methods used to quantify prepulse inhibition of eyeblink responding. Psychophysiology. 2004;41:326–332. doi: 10.1111/j.1469-8986.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Gescheider GA. Modification of the acoustic startle reflex by a tactile prepulse: The effects of stimulus onset asynchrony and prepulse intensity. Psychophysiology. 1987;24:320–327. doi: 10.1111/j.1469-8986.1987.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Brown JS, Kalish HI, Farber IE. Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. Journal of Experimental Psychology. 1951;41:317–328. doi: 10.1037/h0060166. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Öhman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. NeuroImage. 2006;32:1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Baas JMP, Johnson L, Holroyd T, Carver FW, Lissek S, Grillon C. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. NeuroImage. 2007;37:282–289. doi: 10.1016/j.neuroimage.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Echiverri AM, Grillon C. Attentional blink and prepulse inhibition of startle are positively correlated. Psychophysiology. 2006;43:504–510. doi: 10.1111/j.1469-8986.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Elden A, Flaten MA. Similar effects of attention directed to acoustic and tactile stimuli on prepulse inhibition of acoustic startle. Scandinavian Journal of Psychology. 2003;44:363–372. doi: 10.1111/1467-9450.00356. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Helmstetter FJ. Conditional analgesia, defensive freezing, and benzodiazepines. Behavioral Neuroscience. 1988;102:233–243. doi: 10.1037//0735-7044.102.2.233. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. Modification of the acoustic startle-reflex eyeblink: A tool for investigating early and late attentional processes. Biological Psychology. 1993;35:185–200. doi: 10.1016/0301-0511(93)90001-o. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. Probing the orienting response with startle modification and secondary reaction time. Psychophysiology. 1994;31:68–78. doi: 10.1111/j.1469-8986.1994.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Filion DL, Poje AB. Selective and nonselective attention effects on prepulse inhibition of startle: A comparison of task and no-task protocols. Biological Psychology. 2003;64:283–296. doi: 10.1016/s0301-0511(03)00077-2. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RI, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- Graham FK. Attention: The heartbeat, the blink, and the brain. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Hillsdale, NJ: Erlbaum; 1992. pp. 3–29. [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. 2nd ed. Oxford, England: Oxford University Press; 2000. [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Cornwell BR, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: Effect of predictability. Biological Psychiatry. 2006;60:752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Morgan CA, Charney DS, Davis M. Effects of the beta-blocker propanolol on cued and contextual fear conditioning in humans. Psychopharmacology. 2004;175:342–352. doi: 10.1007/s00213-004-1819-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Effects of stress and shock anticipation on prepulse inhibition of the startle reflex. Psychophysiology. 1997;34:511–517. doi: 10.1111/j.1469-8986.1997.tb01737.x. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Greenwald MK, Bradley MM, Lang PJ. Emotional learning, hedonic change, and the startle probe. Journal of Abnormal Psychology. 1993;102:453–465. doi: 10.1037//0021-843x.102.3.453. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation and action. In: Lang PJ, Simons RF, Balaban MF, editors. Attention and orienting: Sensory and motivational processes. Hillsdale, NJ: Erlbaum; 1997. pp. 97–135. [Google Scholar]
- Miltner WHR, Braun C, Arnold M, Witte H, Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397:434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- Öhman A, Hamm A, Hugdahl K. Cognition and the autonomic nervous system: Orienting, anticipation, and conditioning. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2nd ed. New York: Cambridge University Press; 2000. pp. 533–574. [Google Scholar]
- Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: Expectation, hypnosis, placebo. Trends in Cognitive Sciences. 2003;7:197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Williams AE, McCabe KM, Rambo PL, Russell JL. Emotional modulation of spinal nociception and pain: The impact of predictable noxious stimulation. Pain. 2006;126:221–233. doi: 10.1016/j.pain.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. Journal of Neuroscience. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn J, Dawson M, Schell A. The functional relationship between visual backward masking and prepulse inhibition. Psychophysiology. 2004;41:306–312. doi: 10.1111/j.1469-8986.2004.00153.x. [DOI] [PubMed] [Google Scholar]