Abstract
Background
Information concerning the effects of HIV-1 infection, disease progression and antiretroviral therapy (ART) on male genital white blood cell (WBC) profiles could provide important insight into genital immune defense in HIV-infected men and seminal HIV transmission mechanisms.
Objective
To compare concentrations of WBC populations in semen from HIV-1-seronegative (HIV−) and seropositive (HIV+) men, and determine whether HIV disease stage and ART are associated with alterations in seminal WBC profiles.
Subjects and Methods
Subjects were 102 HIV− men, 98 ART-naive (ART−) HIV+ men, and 22 HIV+ men on dual nucleoside ART, before and six months after addition of indinavir. Seminal WBCs, macrophages (MØ), and T lymphocyte subpopulations were enumerated by immunohistology technique.
Results
Seminal CD4+ and CD8+ T cell populations were severely depleted in ART− HIV+ men regardless of peripheral blood CD4+ cell count; seminal MØ counts were also reduced. HIV+ men on dual nucleoside ART had significantly higher seminal MØ, CD4+ and CD8+ T cell counts than ART− HIV+ men; addition of indinavir led to a dramatic (>25-fold, p<0.001) increase in seminal CD4+ T cell counts which paralleled an increase in blood CD4+ cell counts. Two ART− HIV+ men with notably elevated seminal WBC profiles (>20 × 106 WBCs/ml) and infectious cell-associated HIV in semen are described.
Conclusions
HIV infection severely depletes CD4+ T cells in the male genital tract as it does at other mucosal sites. This provides evidence that ART− HIV+ men have depressed T cell-dependent genital immune defense functions, and are vulnerable to other genital infections that could promote HIV transmission. Seminal CD4+ T cell counts rebounded following treatment with a viral-suppressing ART regimen, indicating that ART may reverse HIV-associated genital immunosuppression. The relative abundance of seminal MØ in HIV+ men suggests that these cells are important HIV host cells in the male genital tract and vectors of HIV transmission. A subgroup of HIV+ men with exceptionally elevated seminal MØ and CD4+ T cell counts and HIV titers may be highly infectious and contribute disproportionately to HIV transmission.
Keywords: Semen, HIV-1, antiretroviral therapy, protease inhibitor, white blood cells, CD4+ lymphocytes, macrophages
INTRODUCTION
The Human Immunodeficiency Virus Type 1 (HIV-1) is transmitted primarily by sexual intercourse in the U.S.A. and worldwide 1-3. Transmission of HIV is inefficient relative to many other sexually transmitted disease (STD) pathogens, and a number of covariates such as concomitant STDs and acute and advanced HIV disease stage, have been associated with elevated titers of HIV-1 in genital secretions and enhanced HIV transmission 4-6.
Male genital tract organs and secretions are populated with white blood cells (WBCs) which participate in immune defense functions (reviewed in Anderson and Pudney 7). HIV-infected WBCs have been detected in genital organs of HIV+ men 8. HIV-infected CD4+ T lymphocytes and macrophages migrate from male genital tissues into semen 9, and recent studies have implicated infected WBCs as vectors of HIV transmission 10-13. Therefore, seminal WBC profiles provide important information concerning numbers and types of HIV-host cells in the male genital tract, potential cellular vectors of HIV transmission, and immune defense of the male genital tract.
Mucosal epithelia are populated with memory CD4+ CCR5+ T cells which are prime targets of HIV infection 14. Memory CD4+ T cells in the gastrointestinal tract are dramatically depleted during the early stages of HIV-1 infection before effects are seen on CD4+ T cells in the peripheral blood 15, 16. Several theories have been presented to explain this effect: 1) memory CD4+ T cells are preferentially infected and killed by HIV during acute infection because they express high levels of CCR5 (HIV co-receptor), 2) memory CD4+ T cells are activated and their lifespan is shortened by HIV infection 17, 3) these cells are targeted by HIV through an interaction between gp120 and the integrin α4β7, a mucosal homing receptor for peripheral blood T cells 18, and/or 4) the migration of memory T cells from peripheral blood to mucosal sites is disrupted 19. CD4+ T cells are also depleted in the female genital tract following HIV and SIV infection 20, 21. However, the effects of HIV-infection, disease stage and antiretroviral therapy (ART) on WBC profiles in the male genital tract have not been described. The male genital tract is also populated with memory mucosal T cells 22, 23, and this cell population is targeted by SIV during acute infection in male macaques24. We therefore hypothesize that HIV infection leads to depletion of CD4+ T lymphocytes in the male genital tract. To test this hypothesis, we compared concentrations of CD4+ T cells, as well as other WBC populations, in archived semen samples from HIV− and untreated HIV+ men. Since recent studies have shown that highly active antiretroviral therapy (HAART) partially reconstitutes peripheral blood and mucosal CD4+ T cell populations and immune function in immunosuppressed HIV-infected subjects 25, 26, we also enumerated seminal CD4+ T cells and other WBC populations in archived semen samples from HIV+ men on dual nucleoside therapy, and in the same cohort 6 months after addition of a protease inhibitor (PI) (indinavir) to their ART regimen, to determine effects of these classes of antiretroviral drugs and HIV suppression on WBC profiles in semen.
MATERIALS AND METHODS
Patient Populations
HIV and ART-naive HIV+ men
Subjects were 102 HIV-seronegative and 98 ART-naïve (ART−) HIV-seropositive men who have sex with men (MSM), receiving medical care at Fenway Community Health in Boston, MA between 1988 and 1993, prior to the widespread use of ART. Fenway Community Health is the largest center caring for sexual and gender minority patients in New England 27. Peripheral blood CD4+ cell counts in the HIV+ men ranged from undetectable to 1,290 cells/mm3 (median=410).
ART-treated HIV+ men
Twenty-two asymptomatic HIV+ MSM attending Fenway Community Health (Boston, MA) for primary medical care at the beginning of the HAART era (1996−1997) provided semen and blood samples for this study after treatment for a minimum of 6 months with dual nucleoside ART, and then again 6 months after addition of a PI (indinavir) to their ART regimen. Details of the study population and effects of this treatment on seminal and blood HIV-1 levels are reported elsewhere 28. At the start of the study, 15 participants were receiving zidovudine/lamivudine; 4 stavudine/lamivudine; and 3 zidovudine/didanosine. Following provision of blood and semen specimens for the first (pre-PI) time point, men received indinavir (800 mg three times a day) in conjunction with dual nucleoside analog therapy. Peripheral blood CD4+ cell counts in these men prior to indinavir therapy ranged from 81 to 632 cells/mm3 (median=238); six months after addition of indinavir, their CD4+ cell counts ranged from 122 to 672 cells/mm3 (median=314).
General Methods
Semen collection and preliminary analysis
This study was approved by the Institutional Review Board, and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000. Men provided written informed consent for participation in the study. Semen was obtained after a minimum of 48 hours of abstinence by masturbation into sterile specimen containers. Samples were sent on ice packs immediately to the laboratory and processed within 2 hours. Semen volume was measured, and concentration of seminal “round cells” (a combination of WBCs and immature germ cells, indistinguishable by phase microscopy 29) was assessed microscopically on a hemocytometer by a trained technician. Semen was diluted 1:1 in sterile phosphate-buffered saline (PBS), and semen cells were pelleted by centrifugation at 400 × g for 10 minutes. Semen cells were washed 2× in PBS prior to use in immunohistology assays.
Immunohistology Assay
The low numbers of WBC subpopulations in semen preclude routine quantitative analysis by flow cytometry 22. WBCs in semen were enumerated by an immunohistology assay as previously described 30 with slight modifications. The following monoclonal antibodies (MAbs) were used: anti-HLe-1 (CD45) for simultaneous identification of all WBCs, anti-Leu 4 + 5b (CD3) for all T cells, anti-Leu 3a + 3b for detection of CD4+ cells (including T helper/inducer lymphocytes, monocytes and macrophages), anti-Leu-2a for detection of CD8+ T cytotoxic/suppressor lymphocytes (all from Becton Dickinson, Mountain View, CA), Dako IL-2R for detection of interleukin-2 receptor-α (CD25) on activated T lymphocytes, and Dako Macrophage for detection of CD68+ monocytes/macrophages (both from Dako Corporation, Santa Barbara, CA).
An aliquot of the washed semen cell fraction (about 1/5 of the original sample) was adjusted to a concentration of 106 round cells/ml saline, and five microliters of washed semen cells or PBMCs (positive control) were applied to individual spots of Teflon-coated multiwell microscope slides (Roboz Surgical Instruments, Washington, DC), dried, fixed in absolute acetone and stored at −70° C. For use in the immunohistology assay, slides were thawed and rehydrated in TRIS buffer (0.05 TRIS, 0.15M NaCl, pH 7.6). MAbs from the panel were applied to individual spots on the multiwell slides, incubated at 37° C for 30 minutes, and rinsed in TRIS buffer. Antibody-positive cells were visualized using an alkaline phosphatase anti-alkaline phosphatase kit (Dako Corporation, Santa Barbara, CA). All cells reactive with a MAb developed a red precipitate, whereas immature germ cells, spermatozoa and other MAb-negative cells appeared blue due to the hematoxylin counterstain. After microscopically counting both antibody-positive and -negative round (non-sperm) cells in a minimum of 10 reticle fields, the cell number was calculated based on the known round cell count determined previously from the fresh sample. Testing of semen samples was conducted without knowledge of serostatus, peripheral blood CD4+ cell count or therapy status.
For HIV-1-infected men, the concentration of peripheral blood CD4+ cells was determined by flow cytometry at an off-site clinical laboratory certified by the AIDS Clinical Trial Group.
Statistical Analysis
StatView (version 5.0.1, SAS Institute, Cary, NC, USA) statistical software was utilized to perform the statistical computations. The various WBC measures did not satisfy the assumptions of normal distribution and/or homogeneity of variance. Therefore, the non-parametric Mann-Whitney U test was performed to determine differences between two independent samples, whereas the nonparametric Wilcoxon signed ranks test was used for comparing two related samples. For three group comparisons, one factor analysis of variance (ANOVA) was performed on log-transformed data. Statistically significant ANOVA (p<0.05) was followed by Fisher's protected least significant difference (PLSD) post hoc tests for pairwise comparison of groups. Correlations between variables were determined by the Spearman rank-order correlation coefficient. 31.
RESULTS
Comparison of seminal WBC subpopulations in HIV− and ART-naive HIV+ men
ART− HIV+ men had significantly lower concentrations of total WBCs (p=0.0008), macrophages (p=0.0026), total T lymphocytes (p=0.0001), CD4+ cells (p=0.0001), CD8+ T lymphocytes (p=0.0063) and activated (IL-2 receptor-α+) T lymphocytes (p=0.0001) in semen compared to HIV− men (Table 1). Because the anti-CD4 monoclonal antibody recognizes CD4+ monocytes/macrophages 32, 33, seminal CD4+ T lymphocyte counts were determined by subtracting the number of CD8+ lymphocytes from the total number of T lymphocytes. The seminal CD4+ cell count was highly correlated with the seminal CD4+ T lymphocytes count obtained using this subtraction method (rho=+0.50, p<0.0001); as was the case with the total CD4+ cell count, CD4+ T lymphocyte counts were also dramatically reduced in ART− HIV-1-infected men (median counts: 5,700/ml in HIV− men vs. 0/ml in ART− HIV+ men, p=0.0001).
Table 1.
Total White Blood Cells, Macrophages and T Lymphocyte Subpopulations in Semen from HIV-1 Seronegative and ART-naive Seropositive Men
| Variable1 | HIV− (N=102) | ART− HIV+ (N=98) | Significance2 |
|---|---|---|---|
| Total WBCs | 243,600 (80,960−612,000)3 | 104,286 (30,000−328,960) | 0.0008 |
| Macrophages | 53,077 (21,780−152,288) | 22,162 (2,284−120,606) | 0.0026 |
| T Lymphocytes | 11,566 (2,713−27,827) | 0 (0−13,815) | 0.0001 |
| CD4+ Cells | 10,492 (0−27,576) | 0 (0−11,250) | 0.0001 |
| CD4+ T Lymphocytes4 | 5,700 (0−21,851) | 0 (0−5,600) | 0.0001 |
| CD8+ T Lymphocytes | 1,889 (0−9,261) | 0 (0−5,889) | 0.0063 |
| IL-2 Receptor-α+ T Lymphocytes | 5,855 (0−22,192) | 0 (0−122) | 0.0001 |
Per ml semen
Difference between HIV− and HIV+ groups; Mann-Whitney U test
Median (Interquartile Range)
Number of total T cells minus the number of CD8+ T cells
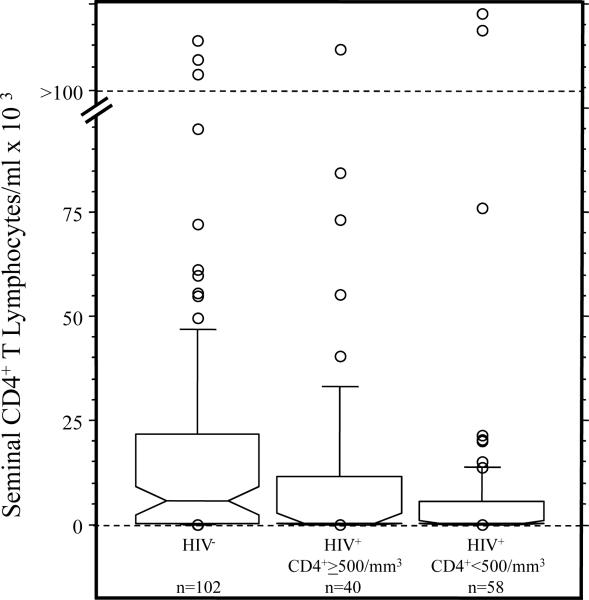
Peripheral blood CD4+ cell counts did not significantly correlate with seminal CD4+ T cell counts or any of the other seminal WBC measures in ART− HIV+ men. To further elucidate the relationship between peripheral blood CD4+ cell and seminal WBC counts, ART− HIV+ men were stratified into high (≥500/mm3) and low (<500/mm3) peripheral blood CD4+ cell groups. ANOVA indicated that both ART− HIV+ groups had significantly lower concentrations of all seminal WBC variables, except for CD8+ T lymphocytes, than HIV− men (p's<0.01, Fisher's PLSD tests), and did not differ from each other (p's>0.10). The results for seminal CD4+ T lymphocytes are shown in Figure 1. The majority of HIV+ men in both groups had undetectable seminal CD4+ T cell counts. Although we did not study men during acute HIV infection, six out of seven subjects with the highest peripheral blood CD4+ cell counts (>1,000/mm3) had undetectable seminal CD4+ T cells, providing further evidence that genital T cell depletion occurs early in HIV disease, before profound reduction in peripheral CD4+ cell counts. For CD8+ T lymphocytes, although both ART− HIV+ groups had a median of 0, only ART− HIV+ men with peripheral blood CD4+ counts <500/mm3 showed a significantly lower concentration compared to HIV− men (p=0.0004).
Figure 1.
Notched box plots representing CD4+ T lymphocyte concentrations in semen of HIV− men and HIV+ men with peripheral blood CD4+ cell counts ≥ and < 500/mm3. For each box, the horizontal lines, from bottom to top, represent 25th, 50th (median) and 75th percentiles, the whiskers delineate the 10th and 90th percentiles, the notch defines the 95% confidence interval around the median and the open circles identify outlying values. Statistically significant group comparisons: HIV− vs. HIV+ ≥ 500/mm3 (p=0.0017) and HIV− vs. HIV+ < 500/mm3 (p=0.0001).
Although ART− HIV+ men as a group had lower seminal WBC counts than HIV− men, two ART− HIV+ men with advanced disease stage had extremely high seminal WBC concentrations (Table 2). One ART− HIV+ man with a peripheral blood CD4+ count of 40/mm3 had a seminal WBC concentration of 55.68 × 106/ml, with 15.5 × 106/ml macrophages and 6.2 × 106/ml CD4+ T lymphocytes. Another ART− HIV+ subject with a peripheral blood CD4+ T lymphocyte count of 23/mm3 had a seminal WBC concentration of 29.00 × 106/ml, with 24.35 × 106/ml macrophages and 2.2 × 106/ml CD4+ T lymphocytes. In both of these cases, seminal WBC concentrations were >100-fold higher than the median values for HIV− and ART− HIV+ groups. When assessed for HIV-1 using a microculture technique 34, both samples had high titers of infectious HIV-1 in the cellular fraction, but not in the cell-free seminal plasma fraction. Neither of these men had recent or concurrent symptomatic STDs as assessed by clinical history and physical exam.
Table 2.
Elevated Concentrations of White Blood Cells in Semen from Two ART− HIV+ Men with AIDS
| Subject | Total WBCs1 | Macrophages | CD4+ T Lymphocytes2 | CD8+ T Lymphocytes | IL-2 Receptor-α+ T Lymphocytes |
|---|---|---|---|---|---|
| D096 | 55,680,000 | 15,467,000 | 6,186,665 | 1,546,666 | 9,666,663 |
| 19950 | 29,000,000 | 24,349,055 | 2,188,676 | 0 | 0 |
Semen variables per ml semen
All T cells minus CD8+ cells
The effect of ART on seminal WBC profiles in HIV+ men
HIV+ men receiving dual nucleoside ART had significantly higher concentrations of seminal WBCs including total, CD8+ and activated T lymphocytes, and macrophages than did ART− HIV+ men (p=0.0003, 0.0001, 0.0001 and 0.03, respectively). HIV+ men on dual nucleoside ART also had modestly higher concentrations of seminal CD4+ cells (p=0.056) and seminal CD4+ T lymphocytes (p=0.03) than ART− HIV+ men. Six months following the addition of indinavir to the ART regimen, substantial increases in CD4+ cell counts were observed in both semen and blood [seminal CD4+ cells, p=0.03; seminal CD4+ T lymphocytes, p=0.001; peripheral blood CD4+ cells, p=0.05 (Table 3)]. As was the case with ART− HIV+ men, none of the seminal WBC measures was significantly correlated with peripheral blood CD4+ T lymphocyte count either before or after addition of indinavir therapy in the ART cohort.
Table 3.
Effect of Dual Nucleoside and Protease Inhibitor (PI) (Indinavir) Antiretroviral Therapy on WBC Populations in Semen and Blood (n=20)
| Variable | Body Fluid | Dual Nucleoside Therapy, Pre PI ART | Dual Nucleoside + Indinavir (6 Mos.) | Significance1 |
|---|---|---|---|---|
| Total WBCs | Semen2 | 213,440 (69,008−295,653)3 |
312,000 139,808−567,410) |
NS |
| Macrophages | Semen | 77,510 15,518−158,615) |
73,470 13,823−307,758) |
NS |
| T Lymphocytes | Semen | 12,180 4,955−34,715) |
110,000 35,968−200,213) |
0.0004 |
| CD4+ T Lymphocytes | Semen | 2,610 0−15,060) |
67,435 1,640−111,850) |
0.001 |
| CD8+ T Lymphocytes | Semen | 5,340 700−5,340) |
12,000 5,150−45,000) |
NS |
| IL-2 Receptor-α+ T Lymphocytes | Semen | 1,380 450−30,485) |
10,000 0−43,113) |
NS |
| CD4+ Cells4 | Peripheral Blood | 238 157−425) |
314 241−441) |
0.05 |
Difference between pre- and post-PI ART values; Wilcoxon signed ranks test
Semen WBC variables per ml semen
Median (Interquartile Range)
Per mm3
DISCUSSION
Results from this study indicate that CD4+ T cells are depleted in the male genital tract during HIV infection. Seminal CD4+ T cell counts were significantly decreased in ART− HIV+ men, but were restored following treatment with combination ART. These findings are consistent with clinical studies that have documented depletion of CD4+ T cells at other mucosal sites during early HIV infection, and reconstitution of both peripheral blood and mucosal CD4+ T cells in HIV+ subjects receiving HAART. A number of reports have documented depletion of CD4+ T lymphocytes in the gastrointestinal mucosa of HIV-infected individuals 15, 35, and their partial restoration following HAART 25, 36, 37. An earlier study from our group also documented depletion of WBCs and CD4+ T lymphocytes in the endocervix of HIV+ women in comparison to uninfected women 20, and Veasey and coworkers reported a similar effect following SIV infection of macaques 21. Because CD4+ T cells play an important helper role in both cellular and humoral acquired immune defense functions, the results of these earlier studies suggest that HIV infection has a broad suppressive effect on mucosal immune defense functions at various mucosal sites. A small study performed by Denny and associates 22 showing a 50% reduction in the proportion of CD4+ T cells in semen from HIV+ men provided the first evidence that seminal CD4+ T lymphocytes may be depleted following HIV infection. The results of the present study showing decreased concentrations of CD4+ T cells in semen from HIV+ men with high peripheral CD4+ cell counts provide evidence that selective CD4+ T cell depletion also occurs in the male genital tract. These findings suggest that genital T-cell dependent immune defense functions may be impaired in HIV-infected men. If so, HIV+ men may be more vulnerable to genital infections, some of which are co-factors for HIV transmission 5. Our observation that seminal CD4+ T cell counts rebound in HIV+ men following treatment with a viral suppressing ART regimen provides evidence that genital CD4+ T cell populations are restored following suppression of viral replication.
ART− HIV+ men in this study also had reduced seminal CD8+ T lymphocyte concentrations, suggesting that HIV infection impairs anti-viral cellular immune defense mechanisms in the male genital tract. This differs from earlier reports that showed increased concentrations of CD8+ T cells in the endocervix 20 and gastrointestinal mucosa 15 following HIV infection. This discrepancy may be related to the time course of HIV disease and treatment, or to differential effects of HIV infection on the various compartments of the body. The men enrolled in this study tended to have highly advanced HIV disease, and may have initially manifested increases in mucosal CD8+ cells that declined over time as they became more immunocompromised. This is supported by data from the present study indicating that seminal CD8+ T cells were significantly reduced only in men with peripheral blood CD4+ cell counts less than 500/mm3. Lim et al. 16 found that gut mucosal CD8+ T lymphocyte counts initially increased following HIV infection, but decreased following depletion of CD4+ blood counts. CD8+ T cell counts in semen were dramatically increased following administration of combination ART suggesting that ART also reconstitutes CD8-mediated cellular immune functions in the male genital tract.
The effect of ART on semen WBC populations was most pronounced after the addition of the PI, indinavir, to combination therapy. Since PIs suppress HIV viral load in blood and semen 28, 38-40, and indinavir penetrates the male genital tract very effectively 41-43, this observation provides evidence that male genital tract immune reconstitution occurs when HIV replication is suppressed, and suggests that genital immune depletion is directly related to HIV viral load. The results of this study are limited by the small sample size, the short duration of therapy, and the use of a narrow panel of ART drugs. The effects of longer periods of HAART, as well as other combinations of antiretroviral drugs on seminal WBC populations, should be studied.
Peripheral blood CD4+ cell counts in HIV+ men were not associated with CD4+ T lymphocyte or other WBC concentrations in semen. Although this study did not include men with acute HIV infection, decreased numbers of seminal CD4+ T cells in men with high peripheral blood CD4+ cell counts suggest that this cell population is depleted during early stages of HIV disease, and remains so until viral replication is fully suppressed by ART. Normally, HIV− and HIV+ men have much higher concentrations of CD4+ cells in blood than semen 44, 45. However, two men in the ART− HIV+ cohort were extreme outliers. These men, who had very low peripheral blood CD4+ cell counts (40 and 23 CD4+ cells/mm3 blood), had extraordinarily high concentrations of seminal CD4+ T cells (6.2 × 106 and 2.1 × 106/ml) and macrophages (15.5 × 106 and 24.4 × 106/ml). This dramatic dissociation between CD4+ cell concentrations in blood and genital secretions can occur because mucosal tissues and especially the male genital tract are compartments with distinct immunological microenvironments. Regions of the male genital tract are immunologically privileged sites due to immunological barriers and high concentrations of immunosuppressive factors 7, and immune cell numbers and their activation are usually tightly controlled. However, as the two outlier subjects demonstrate, normal immune regulation in the male genital tract can be disrupted. A number of studies have documented discordance between blood and semen viral load in some subjects 46-48. Elevated seminal WBC counts are associated with high seminal HIV viral loads 34, 49, 50, and the two individuals in this study with dramatically elevated seminal WBC counts were no exception. It is possible that such men are highly infectious. Factors that have been associated with elevated concentrations of seminal WBCs and HIV include genital infections with organisms such as cytomegalovirus 50 and Neisseria gonorrhea 49. The men in this study with highly elevated seminal WBC counts did not have a documented symptomatic STD, but it is possible that they had an asymptomatic genital infection.
In summary, data from this study demonstrate that CD4+ T cell counts decline in the male genital tract before they decrease in the peripheral circulation of HIV-infected men. This provides evidence that genital tract T cell-dependent acquired immune functions are impaired in HIV-infected men, perhaps rendering them more susceptible to concomitant STD infections that can increase HIV transmission rates. Data from this study also show that treatment with a viral suppressive ART regimen is associated with significant restoration of CD4+ T cells in the genital tract, and thus could improve acquired immune function in the genital tract. Because the relative concentration of seminal macrophages compared to CD4+ T lymphocytes was considerably higher in HIV+ men regardless of disease stage, macrophages are likely primary HIV host cells in the male genital tract and vectors of HIV transmission. The numbers of WBCs capable of transmitting HIV (macrophages and CD4+ T cells) were reduced overall in semen from HIV+ men, but were strikingly elevated in a small subset (2 out of 98 ART-naive HIV+ men in this study). It is possible that men such as these with apparent genital immune activation and elevated HIV titers in semen are highly infectious, and may contribute disproportionately to HIV transmission.
ACKNOWLEDGMENTS
The authors acknowledge Lynne Tucker and Adriana Martinez for excellent technical support, and J.D. Zipkin for assistance with the statistical analysis. We also gratefully acknowledge the fine service provided by the staff of the Research Department of Fenway Community Health, and the altruism of the study participants.
Sources of support:
This work was supported by the National Institutes of Health (AI035564-10, DK072933-11A1 and AI071909-01A2) and the Lifespan-Tufts-Brown Center for AIDS Research (P30 AI42853).
REFERENCES
- 1.AIDS epidemic update. Joint United Nations Programme on HIV/AIDS and World Health Organization; Geneva: 2006. [Google Scholar]
- 2.Fowler MG, Melnick SL, Mathieson BJ. Women and HIV. Epidemiology and global overview. Obstet Gynecol Clin North Am. 1997 Dec;24(4):705–729. doi: 10.1016/s0889-8545(05)70340-5. [DOI] [PubMed] [Google Scholar]
- 3.HIV/AIDS Surveillance Report Centers for Disease Control and Prevention.
- 4.Beyrer C. HIV epidemiology update and transmission factors: risks and risk contexts--16th International AIDS Conference epidemiology plenary. Clin Infect Dis. 2007 Apr 1;44(7):981–987. doi: 10.1086/512371. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS. HIV and sexually transmitted diseases: lethal synergy. Top HIV Med. 2004 Oct-Nov;12(4):104–107. [PubMed] [Google Scholar]
- 6.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003 Oct;1(1):25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DJ, Pudney J. Human male genital tract immunity and experimental models. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. Third ed. Vol. 2. Elsevier Academic Press; Boston: 2005. pp. 1647–1660. [Google Scholar]
- 8.Pudney J, Anderson D. Orchitis and human immunodeficiency virus type 1 infected cells in reproductive tissues from men with the acquired immune deficiency syndrome. Am J Pathol. 1991 Jul;139(1):149–160. [PMC free article] [PubMed] [Google Scholar]
- 9.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997 Oct;176(4):960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 10.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000 Apr;6(4):475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 11.Girard M, Mahoney J, Wei Q, et al. Genital infection of female chimpanzees with human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1998 Oct 10;14(15):1357–1367. doi: 10.1089/aid.1998.14.1357. [DOI] [PubMed] [Google Scholar]
- 12.Kaizu M, Weiler AM, Weisgrau KL, et al. Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J Infect Dis. 2006 Oct 1;194(7):912–916. doi: 10.1086/507308. [DOI] [PubMed] [Google Scholar]
- 13.Khanna KV, Whaley KJ, Zeitlin L, et al. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J Clin Invest. 2002 Jan;109(2):205–211. doi: 10.1172/JCI13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picker LJ. Immunopathogenesis of acute AIDS virus infection. Curr Opin Immunol. 2006 Aug;18(4):399–405. doi: 10.1016/j.coi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim SG, Condez A, Lee CA, Johnson MA, Elia C, Poulter LW. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Immunol. 1993 Jun;92(3):448–454. doi: 10.1111/j.1365-2249.1993.tb03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006 Mar;12(3):289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 18.Arthos J, Cicala C, Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008 Mar;9(3):301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 19.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005 Apr 28;434(7037):1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 20.Quayle AJ, Kourtis AP, Cu-Uvin S, et al. T-Lymphocyte Profile and Total and Virus-Specific Immunoglobulin Concentrations in the Cervix of HIV-1-Infected Women. J Acquir Immune Defic Syndr. 2007 Mar;44(3):292–298. doi: 10.1097/QAI.0b013e31802c5b3a. [DOI] [PubMed] [Google Scholar]
- 21.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J Infect Dis. 2003 Mar 1;187(5):769–776. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 22.Denny TN, Skurnick JH, Garcia A, et al. Lymphocyte immunoregulatory cells present in semen from human immunodeficiency virus (HIV)-infected individuals: a report from the HIV Heterosexual Transmission Study. Cytometry. 1996 Mar 15;26(1):47–51. doi: 10.1002/(SICI)1097-0320(19960315)26:1<47::AID-CYTO7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 23.Pudney J, Anderson DJ. Immunobiology of the human penile urethra. Am J Pathol. 1995 Jul;147(1):155–165. [PMC free article] [PubMed] [Google Scholar]
- 24.Le Tortorec A, Le Grand R, Denis H, et al. Infection of semen-producing organs by SIV during the acute and chronic stages of the disease. PLoS ONE. 2008;3(3):e1792. doi: 10.1371/journal.pone.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006 Aug;80(16):8236–8247. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torre D, Speranza F, Martegani R. Impact of highly active antiretroviral therapy on organ-specific manifestations of HIV-1 infection. HIV Med. 2005 Mar;6(2):66–78. doi: 10.1111/j.1468-1293.2005.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer K, Appelbaum J, Rogers T, Lo W, Bradford J, Boswell S. The evolution of the Fenway Community Health model. Am J Public Health. 2001 Jun;91(6):892–894. doi: 10.2105/ajph.91.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer KH, Boswell S, Goldstein R, et al. Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin Infect Dis. 1999 Jun;28(6):1252–1259. doi: 10.1086/514775. [DOI] [PubMed] [Google Scholar]
- 29.Politch JA, Wolff H, Hill JA, Anderson DJ. Comparison of methods to enumerate white blood cells in semen. Fertil Steril. 1993 Aug;60(2):372–375. doi: 10.1016/s0015-0282(16)56116-0. [DOI] [PubMed] [Google Scholar]
- 30.Wolff H, Anderson DJ. Immunohistologic characterization and quantitation of leukocyte subpopulations in human semen. Fertil Steril. 1988 Mar;49(3):497–504. [PubMed] [Google Scholar]
- 31.Siegel S, Castellan NJ. Nonparametric statistics for the behavioral sciences. 2nd ed. McGraw-Hill; New York: 1988. [Google Scholar]
- 32.Lewin SR, Sonza S, Irving LB, McDonald CF, Mills J, Crowe SM. Surface CD4 is critical to in vitro HIV infection of human alveolar macrophages. AIDS Res Hum Retroviruses. 1996 Jul 1;12(10):877–883. doi: 10.1089/aid.1996.12.877. [DOI] [PubMed] [Google Scholar]
- 33.Wood GS, Warner NL, Warnke RA. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]
- 34.Anderson DJ, O'Brien TR, Politch JA, et al. Effects of disease stage and zidovudine therapy on the detection of human immunodeficiency virus type 1 in semen. Jama. 1992 May 27;267(20):2769–2774. [PubMed] [Google Scholar]
- 35.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003 Nov;77(21):11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talal AH, Monard S, Vesanen M, et al. Virologic and immunologic effect of antiretroviral therapy on HIV-1 in gut-associated lymphoid tissue. J Acquir Immune Defic Syndr. 2001 Jan 1;26(1):1–7. doi: 10.1097/00126334-200101010-00001. [DOI] [PubMed] [Google Scholar]
- 38.Leruez-Ville M, Dulioust E, Costabliola D, et al. Decrease in HIV-1 seminal shedding in men receiving highly active antiretroviral therapy: an 18 month longitudinal study (ANRS EP012). Aids. 2002 Feb 15;16(3):486–488. doi: 10.1097/00002030-200202150-00023. [DOI] [PubMed] [Google Scholar]
- 39.Taylor S, Ferguson NM, Cane PA, Anderson RM, Pillay D. Dynamics of seminal plasma HIV-1 decline after antiretroviral treatment. Aids. 2001 Feb 16;15(3):424–426. doi: 10.1097/00002030-200102160-00022. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Dornadula G, Beumont M, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998 Dec 17;339(25):1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 41.Chan DJ, Ray JE. Quantification of antiretroviral drugs for HIV-1 in the male genital tract: current data, limitations and implications for laboratory analysis. J Pharm Pharmacol. 2007 Nov;59(11):1451–1462. doi: 10.1211/jpp.59.11.0001. [DOI] [PubMed] [Google Scholar]
- 42.Solas C, Lafeuillade A, Halfon P, Chadapaud S, Hittinger G, Lacarelle B. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2003 Jan;47(1):238–243. doi: 10.1128/AAC.47.1.238-243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor S, Reynolds H, Sabin CA, et al. Penetration of efavirenz into the male genital tract: drug concentrations and antiviral activity in semen and blood of HIV-1-infected men. Aids. 2001 Oct 19;15(15):2051–2053. doi: 10.1097/00002030-200110190-00022. [DOI] [PubMed] [Google Scholar]
- 44.Chao C, Jacobson LP, Tashkin D, et al. Recreational drug use and T lymphocyte subpopulations in HIV-uninfected and HIV-infected men. Drug Alcohol Depend. 2008 Apr 1;94(1−3):165–171. doi: 10.1016/j.drugalcdep.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson DJ, Politch JA, Tucker LD, et al. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res Hum Retroviruses. 1998 Apr;14(Suppl 1):S43–49. [PubMed] [Google Scholar]
- 46.Coombs RW, Speck CE, Hughes JP, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998 Feb;177(2):320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 47.Sadiq ST, Taylor S, Kaye S, et al. The effects of antiretroviral therapy on HIV-1 RNA loads in seminal plasma in HIV-positive patients with and without urethritis. Aids. 2002 Jan 25;16(2):219–225. doi: 10.1097/00002030-200201250-00011. [DOI] [PubMed] [Google Scholar]
- 48.Xu C, Politch JA, Tucker L, Mayer KH, Seage GR, 3rd, Anderson DJ. Factors associated with increased levels of human immunodeficiency virus type 1 DNA in semen. J Infect Dis. 1997 Oct;176(4):941–947. doi: 10.1086/516539. [DOI] [PubMed] [Google Scholar]
- 49.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997 Jun 28;349(9069):1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 50.Speck CE, Coombs RW, Koutsky LA, et al. Risk factors for HIV-1 shedding in semen. Am J Epidemiol. 1999 Sep 15;150(6):622–631. doi: 10.1093/oxfordjournals.aje.a010061. [DOI] [PubMed] [Google Scholar]