Abstract
Ultrasonic vocalization at 55 kHz (55 kHz-USVs) by rodents has been proposed to be a behavioral manifestation of affectively positive incentive motivation. To examine the extent to which 55 kHz-USV emissions correlate with cocaine-induced locomotor activity, we measured cocaine-induced 55 kHz-USVs and their relationship to cocaine-induced locomotor sensitization in rats. We demonstrate that similar to locomotor responses, 55 kHz-USVs are also sensitized by exposure to cocaine. Furthermore, we show that the magnitude of cocaine-induced 55 kHz-USV sensitization is positively correlated with that of locomotor sensitization. Moreover, we demonstrate that rats selectively bred for high rates of 55 kHz-USVs exhibit higher levels of cocaine-induced 55 kHz-USV sensitization than animals selectively bred for low levels of 55 kHz USVs. These results suggest that the neural circuits underlying 55 kHz-USV, which may directly reflect affective experience/motivation, can be sensitized by cocaine in a way that resembles locomotor sensitization.
Keywords: locomotor sensitization, ultrasonic vocalization, emotion, motivation, addiction, cocaine
When rodents experience or anticipate positive affective stimuli (e.g. rewards), they emit frequent short (~50 msec) ultrasonic chirps typically at a frequency of ~55 kHz [3, 9, 11, 14]. Since such ultrasonic vocalizations (55 kHz-USVs) are observed at maximal levels in the midst of rough-and-tumble play or during manual stimulation (“tickling”), and secondarily in anticipation of positive rewards, they have been interpreted as an ancestral form of ‘laughter’ or anticipatory eagerness; accordingly USVs have been suggested to be unconditional indicators of positive emotional and motivational states that can be rapidly conditioned to reward predictive stimuli [4, 10, 15, 16]. Therefore, 55 kHz-USVs may serve as a potential direct measure of affective states that are produced by drugs of abuse [6, 12, 17]. To explore this possibility, we evaluated whether 55 kHz-USVs behave in a manner similar to locomotor responses, a widely used measure for the changing sensitivity of the neural substrates upon exposure to addictive drugs.
Drug addiction is mediated by normal as well as pathological emotional and motivational internal states, which are difficult to directly monitor in animal models [14, 18]. The addictive state has been predominantly assessed by drug-induced measurable behavioral output, including locomotor responses, conditioned place preference behavior, and operant conditioning behavior [21]. More recently, 55 kHz-USV as a relatively new and direct measure has been used in detecting addiction-related emotional state [6, 12, 17]. For example, it has been shown that chronic administration of methylphenidate or self administration of amphetamine produces a long-lasting sensitization of 55 kHz-USVs [1, 18]. Here, we report a novel form of cocaine-induced sensitization of 55 kHz-USVs in juvenile rats (postnatal 22 – 23 days, Sprague Dawley and Long Evans).
We contrasted cocaine-induced sensitization of 55 kHz-USVs and cocaine-induced locomotor sensitization to explore the potential connections as well as disconnections between these two measurements. It has been hypothesized that the neural circuits mediating drug-induced locomotor sensitization are overlapping to a great extent with the neural substrates that mediate drug-associated emotional and motivational states [19–21]. As such, sensitization is typically interpreted to reflect a gradual heightening of drug-desire with repeated experiences with addictive drugs. Thus, drug-associated affective drives, when up-regulated, may be manifested in sensitized 55 kHz-USVs and parallel changes in sensitized locomotor responses [19–21]. By determining the relationship between the cocaine effects on 55 kHz-USVs and locomotor sensitization, we sought to determine whether 55 kHz-USVs can be used as a convenient behavioral variable to gauge drug-elicited affective drive.
Using a cocaine-induced locomotor sensitization paradigm, we concurrently measured cocaine-induced 55 kHz-USVS in Sprague Dawley rats. Briefly, we used a 5-day cocaine paradigm, in which rats received an intraperitoneal injection of cocaine (15 mg/kg) once daily for five consecutive days. Rats were placed into locomotor boxes (16″ L × 16″ W × 12″ H, Accuscan, Inc., Columbus, OH) prior to cocaine injections to measure their basal (spontaneous) locomotor activity (scored as photocell beam breaks during the entire 5-min baseline period) and 55 kHz-USVS (scored as the number of 55 kHz-USVs within the same period using the Avisoft Bioacoustic Ultrasoundgate, system 116 – 200,, Berlin, Germany). Rats were then treated with either cocaine or saline and placed into the same boxes to measure cocaine-induced locomotor responses (scored as the number of photocell beam breaks over the entire 15-min period following cocaine injection) and 55 kHz-USVs (scored as the number of 55 kHz-USVs within the same time period; the duration of 55 kHz-USVs was 50.2 ± 4.0 ms among rats of different lines/treatments). Following two days of withdrawal during which all animals received saline injections, a challenge cocaine injection (12 mg/kg) was given to all animals (cocaine treated and saline control alike). All animals entered the test series at the age of 22 – 23 days.
To investigate the effects of repeated drug administration, type of treatment (cocaine/saline) and selectively bred rat lines (high line and low line; see Fig. 2), repeated measures analyses of variance (ANOVA) were computed with test session (day) as the within-subjects variable and treatment condition (cocaine/saline) and line (high line/low line) as the between-subjects variables. Greenhouse Geyser adjustment was employed when the assumption of sphericity was violated (for ease of reading corrected degrees of freedom were rounded down to whole numbers and the Greenhouse Geyser epsilon (ε) is reported. Post hoc comparisons were controlled with the Bonferroni method. T-tests were employed for simple comparisons. Actual p values are reported throughout, however, p values smaller than 0.001 are reported as p < 0.001. Bonferroni controlled comparisons are reported as p < 0.05.
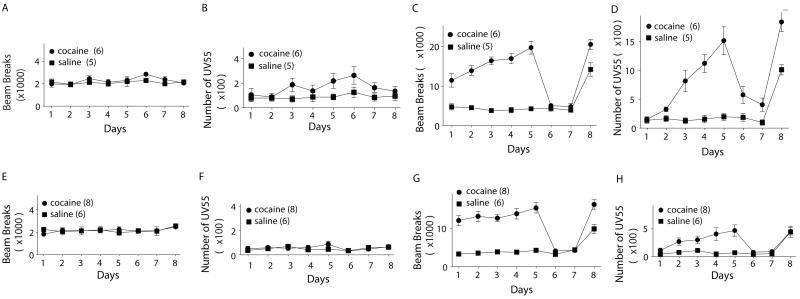
Figure 2.
Cocaine-induced locomotor response and 55 kHz-USVs in HL and LL rats. (A) and (B) Summaries showing that the basal locomotor activity (A) and 55 kHz-USVs (B) were not significantly altered in HL rats along the course of cocaine exposure. (C) and (D) Summaries showing cocaine-induced locomotor sensitization (C) and 55 kHz-USVs sensitization (D). (E) and (F) Summaries showing that the basal locomotor activity (E) and 55 kHz-USVs (F) were not significantly altered in LL rats along the course of cocaine exposure. (G) and (H) Summaries showing cocaine-induced locomotor sensitization (G) and 55 kHz-USVs sensitization (H).
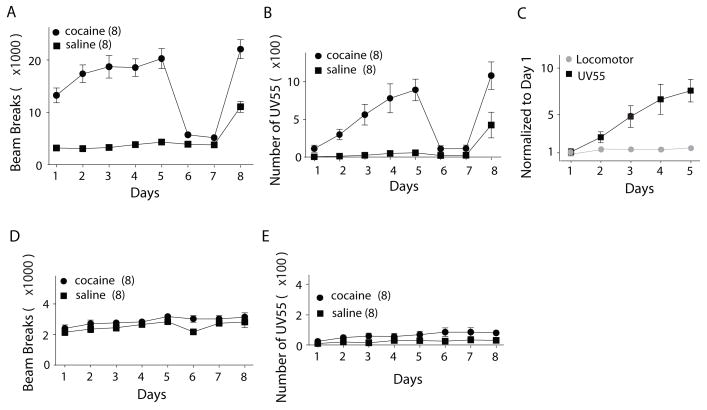
Consistent with previous results, the five-day cocaine paradigm induced locomotor sensitization (Fig. 1) when compared to the saline treated control group (two-way ANOVA with “day” as within and “treatment” (cocaine/saline) as between subjects factors (day: F (2, 38) = 6.0, p = 0.002; treatment: F (1, 14) = 100.2, p < 0.001, day X treatment: F (2, 38) = 4.0, p = 0.016; ε = 0.69; n = 8 for each group; Fig. 1A). When challenged by a cocaine injection following two days of withdrawal, cocaine-pretreated rats exhibited a clearly sensitized locomotor response compared to saline-pretreated animals (t-test: t (14) = 5.3, p < 0.001). Usually, USV started ~3 minutes after the onset of the increase in locomotor activity. In addition, whereas the increased locomotor activity lasted for the entire 15 minute session, USVs did not. The repeated cocaine injections from day 1 to day 5 also produced a gradual increase in the number of 55 kHz-USVs with significant main effects for day (F (2, 29) = 15.9, p < 0.001), treatment ( F (1, 14) = 23.2, p<0.001) and a significant interaction of day X treatment (F (2, 29)=12.0, p < 0.001, ε = 0.53; Fig. 1B). Similar to locomotor activity, 55 kHz-USVs after 2 days of drug withdrawal increased significantly in response to a single challenge dose of cocaine (t-test cocaine/saline: t (14) = 2.64, p = 0.019). Although both the locomotor response and 55 kHz-USVS were sensitized, by cocaine their developmental time courses were substantially different. Whereas we observed a robust acute effect of cocaine on locomotor activity (t-test: t (14) = 6.84, p < 0.001) on the first day of treatment, the acute effect of cocaine treatment on 55 kHz-USVs appeared to be more subtle (t (14) = 2.25, p = 0.041, Fig. 1A, B). Furthermore, when data were normalized (average locomotor activity and 55 kHz-USVs were normalized to the averages on day 1 of cocaine injection), the differential developmental time courses between locomotor and 55 kHz-USV sensitization became even more evident (Fig. 1C).
Figure 1.
Cocaine-induced sensitization of locomotor response and 55 kHz-USVs (first 5 days, followed by 2 vehicle control days, and a final cocaine test for all animals). (A) A summary showing cocaine-induced locomotor sensitization. (B) A summary showing cocaine-induced sensitization of 55 kHz-USVs. (C) Normalized data showing the differential time courses of cocaine-induced locomotor sensitization and 55 kHz-USVs sensitization. (D) and (E) Summaries showing that the basal locomotor activity (D) and 55 kHz-USVs (E) were not significantly altered along the course of cocaine and vehicle exposure in non-treated animals.
Moreover, while basal locomotor activity showed a steady increase over the entire course of the experiment (day 1 – day 8) in both cocaine and saline treated animals, no significant group differences were observed between treatment and control groups (2-way ANOVA with “day” as within and “treatment” as between subjects factors (day: F (3, 47) = 4.59, p = 0.005; treatment: F (1, 14) = 2.9, p = 0.11, day X treatment F (3, 47) = 0.78, p = 0.53; ε = 0.49). Similarly the basal rate of 55 kHz-USVs increased over the course of the experiment but group differences, again, did not reach statistical significance (day: F (3,43) = 5.91, p = 0.002; treatment: F (1, 14) = 3.45, p = 0.084, day X treatment: F (3,43) = 1.67, p = 0.19; ε = 0.49).
Taken together, these results indicate that with differential temporal properties, both 55 kHz-USVs and locomotor activity are sensitized by repeated exposure to cocaine. Baseline calling rates and locomotor activity significantly increased over time, yet remained unaltered by cocaine exposure. The increase in baseline activity and calling may be related to handling, accumulating odor cues or increasing familiarity with the test environment. It should be noted that over the first 6 days of treatment a non-significant trend towards increased 55 kHz-USVs was observed in the cocaine baseline, which may reflect gradual development of anticipatory vocalizing (Fig. 1 E).
In a second experiment, we compared, within the same paradigm, two genetic lines of Long Evans rats. These rat lines differ in the number of 55 kHz-USVs emitted in response to socially rewarding ‘tickling’ stimulation, which was either substantially higher (high line, or HL) or modestly lower (low line, or LL) than that observed in normal unselected Long Evans rats [8]. 55 kHz-USVs from HL and LL exhibited similar frequency, duration, and other characteristics as observed in Sprague Dawley rats.
As summarized in Fig. 2B & F, the spontaneous basal 55 kHz-USVs did not differ between HL and LL animals. An ANOVA comparing the baseline calling rates between the saline treated control groups of both, high and low line failed to show significant main effects for day (F (7, 63) = 0.45, p = 0.87) and line (F (1, 9) = 1.92, p = 0.2). As summarized in Fig 2A & E, there were also no baseline locomotor differences between the two lines (day: F (7, 63) = 0.73, p = 0.65; line: F (1, 9) = 0.03, p= 0.866).
Both, HL and LL rats showed increased and sensitized 50 kHz calling and locomotor responses after cocaine treatment (Figs 2, C,D,G,H). High line animals emitted an increasing number of 55 kHz-USVs over the course of the experiment illustrated by a significant main effect for day of treatment (F (1, 15) = 21.1, p < 0.001) and treatment condition (cocaine/saline: F (1,9) = 20.2, p = 0.001) which was accompanied by a significant interaction (day X treatment: F (1, 15) = 20.2, p = 0.001; ε = 0.44). The effect of repeated cocaine injections on locomotor activity in HL rats was similar with a significant main effect for day (F (1,13) = 7.4, p = 0.01), a significant effect of treatment (cocaine/saline: F (1,9) = 76.6, p < 0.001) and a significant interaction (day X treatment: F (1, 15) = 10.3, p = 0.003; ε = 0.38). Repeated administration of cocaine to low line animals resulted in a superficially similar pattern. Low line animals responded to repeated cocaine administration with increased calling rates over days (F (4, 48) = 6.14, p < 0.001), a significant main effect for treatment (cocaine/saline: F (1,12) = 8.6, p = 0.013) and a significant interaction of day and treatment (F (4, 48) = 5.4, p = 0.001). The locomotor response of low line animals was also amplified by cocaine treatment yielding a significant main effect for day (F (4, 48) = 5.1, p = 0.002) and treatment condition (cocaine/saline: F (1,12) = 51, p < 0.001).
However, the magnitude of 55 kHz-USV sensitization differed dramatically between high line and low line animals (Figure 2D, H). Cocaine-treated HL rats averaged 1519 (± 242) calls on the 5th day of treatment whereas LL rats averaged only 471 (±102, X ± SEM) calls (t-test: t (12) = 4.39, p = 0.001). At the same time, the HL and LL rats differed only marginally in locomotor activity (day 5, beam breaks: LL, 15215 ±1326; HL, 19705 ± 1613, t-test: t (12) = 2.17, p = 0.051). In other words, a more than 200% terminal difference in 55 kHz-USVs between HL and LL rats, corresponded to a less than 25% difference in locomotor activity.
Upon the final cocaine challenge, locomotor sensitization was observed in both, HL and LL rats. An ANOVA yielded significant effects for both “line“ and “treatment” (line: F (1, 21) = 10.9, p = 0.003; treatment: F (1, 21) = 22.31, p < 0.001). In contrast to the similar locomotor response, the two lines differed substantially in their 50 kHz USV response to cocaine challenge. A 2-way ANOVA between the lines yielded a significant main effect for “line” (F (1,21) = 81.48, p < 0.001 ) illustrating the above differences in absolute calling rates between high line and low line animals after cocaine stimulation. The comparison also yielded a significant main effect for “treatment” (F (1,21) = 14.82, p = 0.001) indicating a differential reaction of cocaine and saline pretreated animals to cocaine challenge. Unlike the locomotor activity data, the analysis of 50 kHz calling also resulted in a significant treatment X line interaction (F (1,21) = 14.3, p = 0.001). Follow-up testing revealed that this interaction was due to a lack of 50 kHz USV sensitization in the low line. The number of 50 kHz vocalizations emitted by low line animals did not differ statistically between saline and cocaine pre-treated animals (t-test: t (12) = 0.07, p >0.05). Within the high line, however, cocaine pretreated animals called significantly more than saline pretreated controls (t-test: t (9) = 4.15, p < 0.05; Bonferroni). This difference is also illustrated in Fig. 2D and Fig. 2 H.
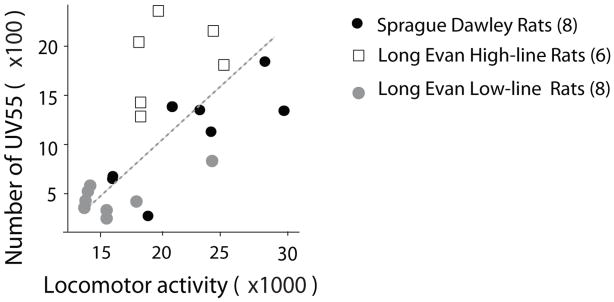
Finally, when the data from all three rat lines (HL/LL Long Evans, and Sprague Dawley) were pooled, we found a positive correlation between cocaine-induced 55 kHz-USVs and the associated locomotor responses (Spearman: r = 0.67, p < 0.001,, n = 22; Fig. 3). On the other hand, the saline-pretreated rats showed a more modest positive correlation after cocaine challenge (r= 0.46, p = 0.049, n = 19).
Figure 3.
A summary showing that cocaine-induced 55 kHz-USVs sensitization is positively correlated with cocaine-induced locomotor sensitization.
In summary, the line comparison indicates that 55 kHz-USVs sensitize differentially in HL and LL rats. This is especially interesting because locomotor sensitization appears to be comparable between the two lines. Low line animals called much less than high line animals in response to cocaine stimulation and they lacked the characteristic increase in 55 kHz-USVs observed in both HL Long Evans and typical Sprague Dawley rats when presented with a cocaine challenge. HL and LL rats did not differ statistically in their baseline locomotor or calling activity indicating that the observed differences are related to some aspect of cocaine exposure. Given the accumulating evidence suggesting that 55 kHz-USVs correspond to a positive affective state and our hypothesis that HL and LL rats represent different affective phenotypes [4, 10, 15, 16], it is tempting to interpret the observed differences as differences in affective state that are not reflected to the same extent in locomotor activity.
The mesolimbic dopamine system, which is highly conserved among mammals, mediates basic as well as pathologically altered emotional and motivational urges such as the addictive state [7, 13]. It has been hypothesized that repeated exposure to drugs of abuse causes plastic changes within the mesolimbic dopamine system, resulting in increasingly stronger, or sensitized, drug-associated affective drives [20, 21]. It has long been known that during the course of repeated exposure to drugs of abuse, the enhancing effect of drugs on locomotor activity becomes increasingly stronger [20, 21]. Given its similar temporal and anatomical properties to the development of addiction, drug-induced locomotor sensitization has been hypothesized to be mediated by the same or similar neural changes that mediate the sensitization of drug-related emotional and motivational urges [19–21]. Given that dopamine release in the nucleus accumbens is tightly linked to 50 kHz-USVs [3], it is possible that the neural circuits underlying 50 kHz-USVs may also be in part mediated by the mesolimbic dopamine system.
The most straightforward interpretation of emotional and motivational state would be that our experimental animals express what they experience affectively, especially affect related to arousal of the mesolimbic dopamine system [2, 5, 17]. Although other animals cannot communicate affect in the same way as humans do, they do have a variety of vocalizations that may be putative readouts of their affective states [3, 4]. Indeed, a series of seminal findings indicate that rats emit chirps at ~55 kHz when they are in positive emotional and motivational states [9, 10, 15, 16]. It has been postulated that such vocalizations may directly reflect the underlying positively valanced appetitive states that are relevant for drug addictions [17]. Past work has shown that, the frequency of 55 kHz-USV emission is increased by various positive incentives and reduced by negative incentives [10, 15, 16]. The present findings provide further support that 55 kHz-USVs can be a relatively direct measure for drug-elicited emotional and motivational arousals. Along with this hypothesis, the sensitization-like process of 55 kHz-USVs upon repeated exposure to cocaine (Fig. 1B and Fig 2D) may be interpreted as a gradual increase in the arousal of cocaine-related emotional and motivational drives.
A key question is whether cocaine-induced 55 kHz-USV sensitization is functionally equivalent to locomotor sensitization. Our results suggest that cocaine-induced 55 kHz-USV sensitization shares some core features with locomotor sensitization, but that it also differs in several important aspects. The commonalities include i) both 55 kHz-USVs and locomotor response can be sensitized by repeated exposure to cocaine (Fig. 1 and Fig 2); and ii) the magnitude of 55 kHz-USV sensitization and locomotor sensitization are positively correlated at the single animal level (Fig. 3). On the other hand, the most apparent distinction between cocaine-induced 55 kHz-USV sensitization and locomotor sensitization is their developing time course over repeated trials. Compared with locomotor sensitization, 55 kHz-USV did not promptly increase on the first cocaine test, but exhibited a gradual increase along the entire test series (Figs 2C & 2D).
As demonstrated at the molecular and cellular levels, drug-induced adaptive changes in the mesolimbic dopamine system occur sequentially [22]. If 55 kHz-USVs is a valid index of the affectively experienced aspect of emotional and motivational arousal, the initial dormant phase of 55 kHz-USV activation (i.e., lack of acute effect of cocaine on the first day of testing) may indicate that the first injection has both positive and negative affective properties, which precludes the arousal of a clear elevation of 55 kHz-USVs. If true, the locomotor response appears to be insensitive to this period of mixed affect. Finally, a substantial difference in 55k Hz-USVs between HL and LL rats was accompanied by only a marginal difference in locomotor activity and although both lines showed locomotor sensitization when challenged with a single dose of cocaine on day 8, LL rats did not respond with a corresponding increase in 55 kHz-USVs. Our selectively bred lines are temperamentally disposed to respond to positive stimuli with either high or low rates of 55 kHz-USVs. If the observed differences in calling correspond to differences in affect, these differences are not reflected to the same extent in the locomotor response.
Collectively, the results from this study suggest that 55 kHz-USVS is an independent and relatively direct measure to assess drug-induced emotional and motivational drives, which remain to be adequately characterized at the psychological level [18]. With this novel approach, which may directly tap into the underlying psychoneurobiological processes of addiction [2, 17], future studies may be more able to explore the molecular and cellular basis underlying the gradual enhancement of drug-associated emotional and motivational affective arousals.
Acknowledgments
This work was supported by the Washington State University Alcohol and Drug Abuse Research Program, the Hope Foundation for Depression Research, and NIH (DA023206).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T. Repeated intravenous amphetamine exposure: rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behav Brain Res. 2009;197:205–209. doi: 10.1016/j.bbr.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 2007;56:283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brudzynski SM. Communication of adult rats by ultrasonic vocalization: biological, sociobiological, and neuroscience approaches. ILAR J. 2008;50:43–50. doi: 10.1093/ilar.50.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Brudzynski SM. Ultrasonic calls of rats as indicator variables of negative or positive states: acetylcholine-dopamine interaction and acoustic coding. Behav Brain Res. 2007;182:261–273. doi: 10.1016/j.bbr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behav Brain Res. 2007;182:284–289. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covington HE, 3rd, Miczek KA. Vocalizations during withdrawal from opiates and cocaine: possible expressions of affective distress. Eur J Pharmacol. 2003;467:1–13. doi: 10.1016/s0014-2999(03)01558-9. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y, Zukin RS. Synaptic Plasticity and Addiction. Cognitive Sciences. 2007;1:189–210. [Google Scholar]
- 8.Harmon KM, Cromwell HC, Burgdorf J, Moskal JR, Brudzynski SM, Kroes RA, Panksepp J. Rats selectively bred for low levels of 50 kHz ultrasonic vocalizations exhibit alterations in early social motivation. Dev Psychobiol. 2008;50:322–331. doi: 10.1002/dev.20294. [DOI] [PubMed] [Google Scholar]
- 9.Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- 10.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 11.Mallo T, Matrov D, Herm L, Koiv K, Eller M, Rinken A, Harro J. Tickling-induced 50-kHz ultrasonic vocalization is individually stable and predicts behaviour in tests of anxiety and depression in rats. Behav Brain Res. 2007;184:57–71. doi: 10.1016/j.bbr.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Mutschler NH, Covington HE, 3rd, Miczek KA. Repeated self-administered cocaine “binges” in rats: effects on cocaine intake and withdrawal. Psychopharmacology (Berl) 2001;154:292–300. doi: 10.1007/s002130000646. [DOI] [PubMed] [Google Scholar]
- 13.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 14.Panksepp J. Neuroevolutionary sources of laughter and social joy: modeling primal human laughter in laboratory rats. Behav Brain Res. 2007;182:231–244. doi: 10.1016/j.bbr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Panksepp J, Burgdorf J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav Brain Res. 2000;115:25–38. doi: 10.1016/s0166-4328(00)00238-2. [DOI] [PubMed] [Google Scholar]
- 16.Panksepp J, Burgdorf J. Laughing” rats and the evolutionary antecedents of human joy? Physiol Behav. 2003;79:533–547. doi: 10.1016/s0031-9384(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 17.Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- 18.Panksepp JB, Gordon J, Turner NC. Treatment of ADHD with methylphenidate may sensitize brain substrates of desire. Consc Emotion. 2002;3:7–19. [Google Scholar]
- 19.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 20.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 21.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 22.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]