Abstract
Maintaining essential fatty acid (EFA) homeostasis during pregnancy is critical for fetal development. As the organ that controls the maternal-to-fetal supply of nutrients, the placenta plays a significant role in guiding EFA transfer to the fetus. Many EFA homeostasis proteins are regulated by peroxisome proliferator-activated receptors (PPARs). The metabolites of di-(2-ethylhexyl)-phthalate (DEHP), a ubiquitous environmental contaminant, might influence EFA homeostasis via trans-activation of PPARs with subsequent downstream effects on EFA transporters and enzymes. To investigate DEHP’s effect on placental/fetal EFA homeostasis, female Sprague-Dawley rats were orally gavaged with either vehicle or DEHP at 750 or 1500 mg/kg/day from gestational day (GD) 0 to GD 19. Changes in the expression of several EFA homeostasis regulating proteins were determined in the junctional (JXN) and labyrinthine (LAB) zones of the placenta, including PPAR isoforms (α, β and γ), fatty acid translocase (FAT/CD36), fatty acid transport protein 1 (FATP1), plasma membrane fatty acid binding protein (FABPpm), heart cytoplasmic fatty acid binding protein (HFABP), cytochrome P450 (CYP) 4A1, and cyclooxygenase (COX)-1 and -2. Additionally, effects of DEHP maternal exposure on the placental transfer and fetal distribution of representative EFAs, arachidonic acid (AA) and docosahexaenoic acid (DHA), and the placental production of prostaglandins (PGs) were investigated. Expression of PPARα, PPARγ, FAT/CD36, FATP1, HFABP and CYP4A1 was up-regulated in JXN and/or LAB while COX-2 was down-regulated in JXN. PPARβ, FABPpm, and COX-1 demonstrated variable expression. Reduced directional maternal-to-fetal placental transfer and altered fetal distribution of AA and DHA were observed in concordance with a decreased total placental PG production. These results correlate with previous in vitro data, suggesting that DEHP could influence placental EFA homeostasis with potential downstream effects in the developing fetus.
Keywords: DEHP, Rat, Placenta, Fatty acid, Transport, Metabolism, PPAR
1. Introduction
The placenta regulates the nutrients and xenobiotics transfer between the mother and the fetus [1]. As a multi-functional organ, the placenta expresses a wealth of proteins for the transport and metabolism of xenobiotics and endogeneous compounds. Alterations to placental function, therefore, can potentially lead to adverse effects on the developing fetus. One particularly important family of compounds that pass through the placenta is fatty acids, especially essential fatty acids (EFAs) and their derivatives, long chain polyunsaturated fatty acids (PUFAs). These compounds play multiple, critical roles in fetal development such as constituents of biological membranes, participants in energy storage and precursors to intra– and intercellular signaling molecules [2,3]. The placenta expresses several EFA transport proteins [e.g., fatty acid translocase (FAT/CD36); fatty acid transport protein 1 (FATP1); plasma membrane fatty acid binding protein (FABPpm); and heart cytoplasmic fatty acid binding protein (HFABP)] [1,3,4] and metabolic enzymes [e.g. cytochrome P450 4A isoforms (CYP4A) and cyclooxygenase (COX)] [5] that participate in FA homeostasis. Many of these proteins are regulated, directly or indirectly, by the peroxisome proliferator-activated nuclear hormone receptor family (PPAR), which has 3 isoforms (α, β and γ) expressed in the placenta [1,5, 6].
Exposure to the widely utilized industrial plasticizer and ubiquitous environmental contaminant, di-(2-ethylhexyl)-phthalate (DEHP), has demonstrated toxicity in rodent reproduction and development [7] and raises concerns about its effects on humans. DEHP’s ability to cross the placenta and accumulate in the fetus [8–10] heightens those concerns. Furthermore, it is not clear if DEHP and its major metabolites, i.e. mono (2-ethylhexyl)-phthalate (MEHP) and ethylhexanoic acid (EHA), potentially act either directly on the fetus and/or indirectly on the placental barrier, resulting in its teratogenic effects. It is worth noting that the conversion of DEHP to MEHP and EHA can occur fairly rapidly through several mechanisms during in vivo exposure, potentially confounding the results and conclusions drawn from the experimentation. Since the current study focused primarily on DEHP exposure, it should be recognized that there is a potential for at least one of the metabolites to actually be the root cause for the observations. While the underlying teratogenic mechanisms of DEHP and its metabolites remain to be elucidated, proposed mechanisms include functional zinc deficiency, hyperoxidative stress, and impaired signal transduction pathways [7]. As a peroxisome proliferator chemical (PPC) [11, 12], DEHP, as well as its MEHP and EHA metabolites, may also affect placental/fetal EFA homeostasis through PPAR agonism. Toward this point, our laboratory has demonstrated the potential of DEHP, MEHP and EHA to affect placental handling and fetal supply of EFAs via PPAR trans-activation and subsequent EFA homeostasis protein regulation in the rat HRP-1 trophoblast [12]. Moreover, we recently reported a significant change in the rat fetal brain lipid profile induced by maternal exposure to DEHP [13].
The aim of the present study was to investigate the effect of DEHP on placental/fetal EFA homeostasis upon maternal exposure. A rat placental model was selected due to its broad application in studying placental development and reproduction. It has two developing stages, choriovitelline and chorioallantoic placenta, which represent the early and middle-late stage of gestation respectively [14]. Furthermore, the rat chorioallantoic placenta begins differentiating into two distinct zones from mid-gestation to parturition: the maternal-facing junctional zone (JXN) for the endocrine/invasive functions and the adjacent fetal-facing labyrinthine (LAB) responsible for maternal-fetal exchange [14]. DEHP was administrated at two doses and contrasted with the control vehicle by daily oral gavage to dams from gestational day (GD) 0 to GD19. The DEHP doses of 750 and 1500mg/kg/day, which were chosen based on previous literature, may be considered relatively high, but they are still within the range of most published studies [7 and reference therein, http://www.cdc.gov/niosh/rtecs/ti55730.html]. Expression of the PPAR isoforms (α,β,γ) and several EFA transporters/enzymes in placental tissue after DEHP exposure was examined, as well as the corresponding functional influence of DEHP on placental EFA transfer and fetal distribution and the placental production of total prostaglandins (PGs).
2. Materials and methods
2.1 Reagents
DEHP, unlabeled fatty acids and TRIzol® Reagents for RNA extraction were obtained from Sigma Chemical Company (St. Louis, MO). Reverse transcriptase-polymerase chain reaction (RT-PCR) kits were obtained from Invitrogen Life Technologies (Gaithersburg, MD). Reagents for gel electrophoresis and Western blot were purchased from either Bio-Rad (Hercules, CA) or Pierce Chemical Company (Rockford, IL). Polyclonal antibodies specific for PPARα, β, and γ, FAT/CD36, FATP1, HFABP and monoclonal antibodies for COX-1 and COX-2 were purchased from Santa Cruz Biotechnology (San Francisco, CA), while the polyclonal antibody to CYP4A1 was obtained from Gentest (Woburn, MA). An antibody to FABPpm was generously provided by Dr. Joseph Mattingly (University of Missouri, Kansas City, Missouri). 3H-arachidonic acid (180 mCi/mmol, AA) and 14C-docosahexaenoic acid (58 mCi/mmol, DHA) were purchased from Moravek Biochemicals (Brea, CA). Unless otherwise noted, all other chemicals and reagents were purchased from Fisher Scientific (Pittsburgh, PA) and used as received.
2.2 Animal treatment and tissue collection
Sprague–Dawley rats (Hilltop Laboratory Animals, Scottdale, PA) of both sexes weighing 300–324 g (males) or 275–299 g (females) were maintained at 20–22°C and 35–50% humidity with a dark/light cycle of 12/12 h. They were housed in steel cages and allowed free access to food and water. Mating was performed as previously described [13]. Pregnant female rats were randomly assigned to a control vehicle (0.5% sodium carboxymethyl cellulose: dimethyl sulfoxide: ethanol = 5:3:2) or DEHP at 750 mg/kg/day or 1500 mg/kg/day from gestational day (GD) 0 to GD 19. DEHP or vehicle were prepared freshly and orally administrated at a volume of 5 ml/kg/day. At GD 20, dams were anesthetized by intraperitoneal injection of a mixture of 180 mg/kg ketamine and 3.6 mg/kg xylazine. The placental, maternal and fetal tissues were immediately isolated, dissected, frozen in liquid nitrogen and then stored at –80°C until further analysis [13]. Please note that DEHP effects on fetus might be sex-related [7], however, such parameters have not been monitored in the current study.
The Animal Care and Facilities Committee of Rutgers, The State University of New Jersey, approved all protocols for the care and use of these animals.
2.3 Semiquantitative RT-PCR
Total RNA isolation, RT-PCR and gel electrophoresis were performed as previously described [12] with optimized conditions and normalized to β-actin expression. Gene specific primers were described previously [12,15,16] and the primers for FAT/CD36 (Gene bank accession number NM_031561) were as follows: sense 5’- TCT GAC ATT TGC AGG TCC ATC TAT GCT G-3’; antisense 5’- ATC TCA ACC AGG CCC AGG AGC TTT ATT T-3’.
2.4 Western blot
Whole cell protein extraction and Western blot analyses were performed as previously described [12]. To confirm equal loading, β-actin protein expression was analyzed, demonstrating that the band intensities exhibited no significant change among the samples studied.
2.5 Placental transfer and fetal distribution of essential fatty acids
At GD20, 1.25 µmol/kg (2 µCi/kg) of 3H-AA and 14C-DHA were injected into dams via tail vein in a volume of 0.3 mL. The injection vehicle was freshly prepared and comprised of saline with fatty acid and BSA mixture at a molar ratio of 1:1 (pH = 6.0 to 6.5). The placental and fetal tissues (brain, liver, heart, and intestine) were isolated 3 hr after fatty acid administration upon sacrificing as described in Section 2.2. Blood samples from rat dams and pups of each group were also collected by cardiac puncture. Plasma was separated within 1 hr by centrifugation at 1,000 g for 10 min at 4°C. Plasma and tissue samples from four fetuses of one dam were pooled together. Radioactivity associated with the samples was quantified with scintillation counting after solubilization with Solvable (PerkinElmer Life Sciences, Boston, MA).
2.6 Placenta production of total prostaglandins (PGs)
Upon tissue isolation at GD 20, fresh placentas (four per dam) were pooled for each treatment group. PG production assay was performed as previously described with minor modifications [18]. Briefly, the placental tissues were minced and homogenized in 50 mM Tris-HCl buffer containing protease inhibitor cocktail (pH 7.4) in a ratio of 5:1 (v/w) at 4°C. Placental tissue homogenates were then incubated with COX reaction buffer (50mM Tris-HCl, 2mM EDTA, 1mM glutathione, 1mM tryptophan, pH 8.0) containing AA (50 µM final concentration) at a total reaction volume of 500 µl at 37°C for 1 hr. The samples were boiled for 2–3 min to stop the reaction and then centrifuged at 10,000 g for 30 min at 4°C, where the supernatant fractions were collected. An enzyme linked immunosorbent assay (ELISA) was used to determine the total PG concentration (Prostaglandin Screen Kit, Cayman Chemical, Ann Arbor, MI). The antiserum used in this assay has a 100% cross-reactivity with PGE1, PGE2, PGF1α, and PGF2α; 51.3% with PGF3α; 43.6% with 6-keto-PGF1α; 38.4% with 8-iso PGF2α; 28.5% with 8-iso PGE2; 26.6% with PGD2; 20% with 8-iso-2, 3-dinor PGF1α; 9.5% with PGE3; 5% with thromboxane B2; and <0.01% each with PGA1, PGA3, PGB1, 15-keto PGE2, and PGF-M. The activities of COX-1 and COX-2 were differentiated by adding indomethacin (10 µM), an inhibitor for both COX-1 and COX-2, or the selective COX-2 inhibitor, NS-398 (5 µM) [17], to the reaction buffer and then incubated with the tissue homogenates for 30 min before the addition of the substrate, AA.
2.7 Data analysis
All experiments were repeated a minimum of three times. Data generated from RT-PCR and Western blot were quantitated by densitometry (Gel Expert™ software program, NucleoTech Corp., San Mateo, CA) and normalized to β-actin expression. All data were presented as means ± standard deviation (SD). Statistical significance relative to vehicle controls, shown in the figures, was determined using a one-way ANOVA followed by the Student t-test, where p values < 0.05 were considered to be statistically significant.
3. Results
3.1 Expression of PPAR isoforms in rat placenta
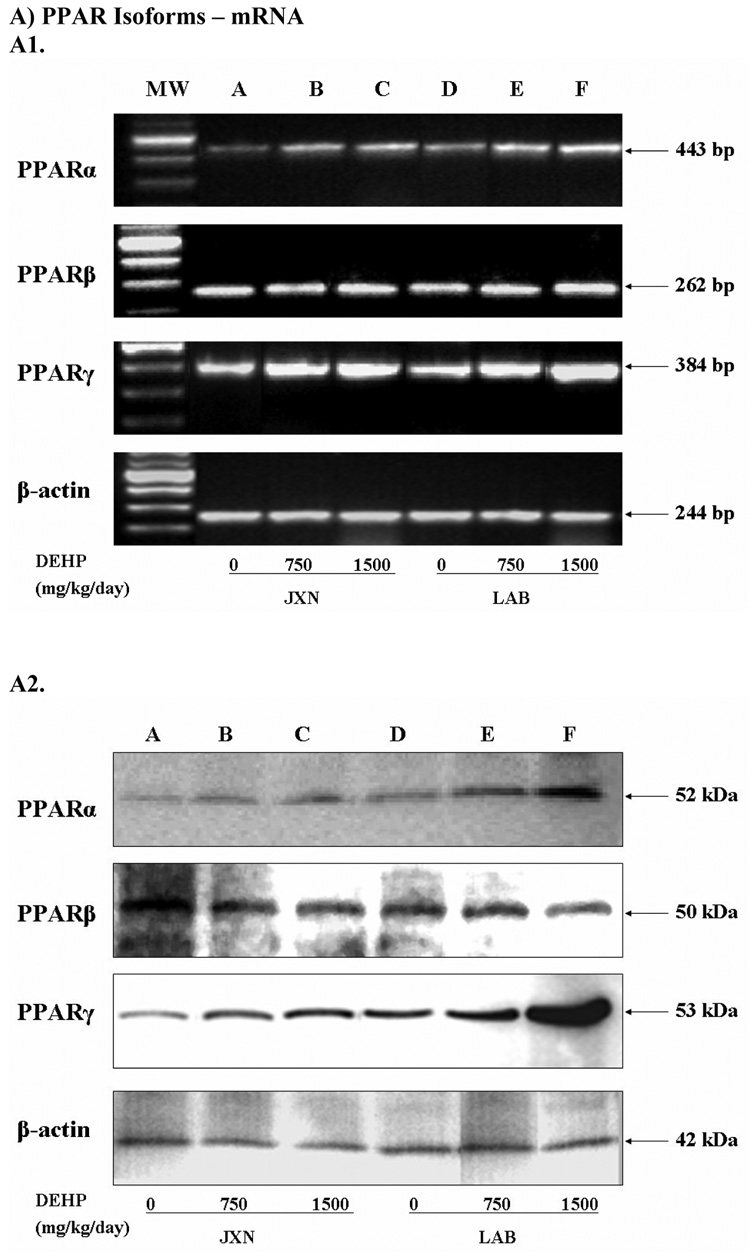
The mRNA and protein expression of PPARα, β and γ were determined by semi-quantitative RT-PCR (Figure 1, Panel A) and Western blot (Figure 1, Panel B), respectively. The maternal facing JXN and the fetal facing LAB from placental tissues at GD 20 were investigated separately to elucidate the potential spatial dependent responses. The expected PCR product size and protein MW were observed for each PPAR isoform. In order to normalize mRNA/protein expression across different samples, β-actin was used as an internal control.
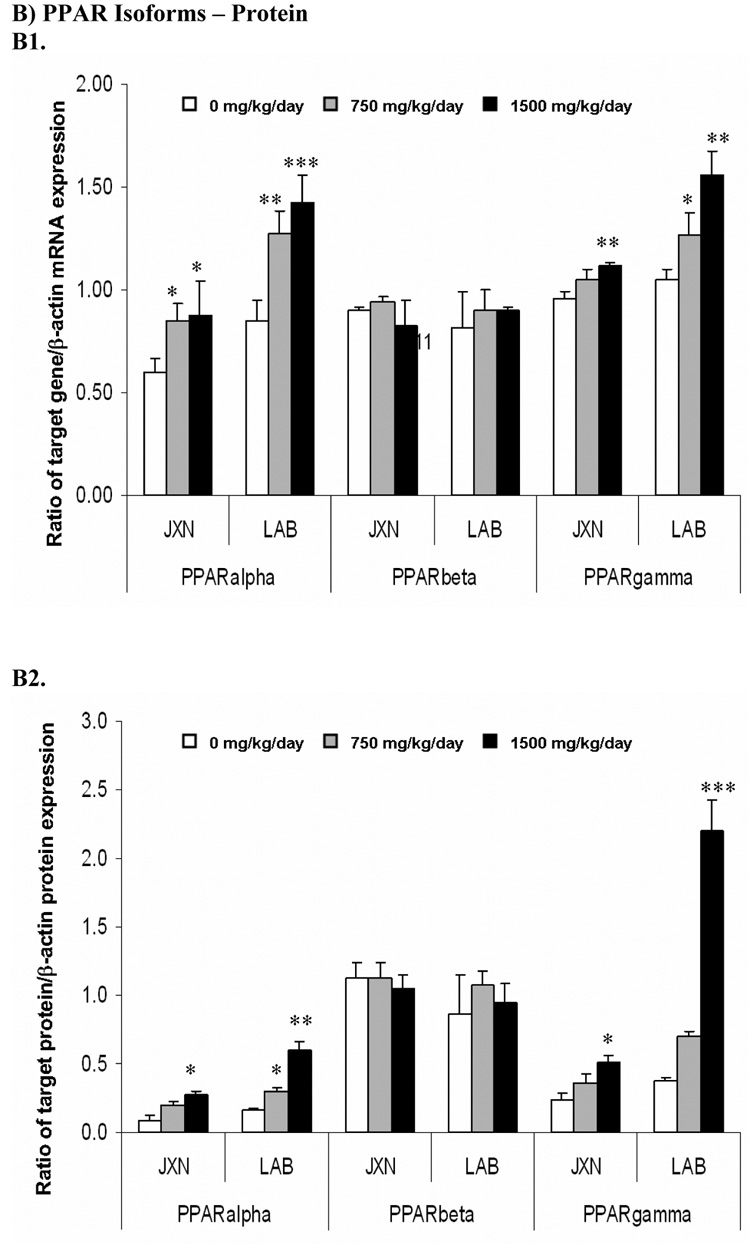
Figure 1. Expression of PPAR isoforms mRNA (Panel A) and protein (Panel B) in rat placenta upon maternal exposure to DEHP.
Dams were dosed with DEHP (750 mg/kg/day or 1500 mg/kg/day) or vehicle from GD 0 through GD 19. Total RNA and whole cell protein were isolated at GD 20 and expressions of PPARα, PPARβ and PPARγ mRNA and protein were analyzed using semi-quantitative RT-PCR and Western blot, respectively. A1 and B1, representative RT-PCR gel electrophoresis and Western blot image. Lane A–C: JXN, junctional zone; Lane D–F: LAB, labyrinthine zone. A2 and B2, densitometry analysis of the RT-PCR gel and Western blot image. Data shown were relative arbitrary values after normalized with β-actin (means ± SD, n =3 or 4). *, p<0.05; **, p<0.01; ***, p<0.001.
A pronounced increase in the mRNA level of PPARα upon exposure to DEHP versus control was observed at 1500 mg/kg (p<0.01), and to a slightly lesser extent at 750 mg/kg (p<0.05). These effects were more obvious in LAB (1.76 fold for 1500 mg/kg, p<0.01) when compared to JXN (1.47 fold for 1500 mg/kg, p<0.01), as shown in Figure 1, Panel A. PPARγ mRNA was also induced dramatically by DEHP administration in both the JXN and LAB (p<0.05) and exhibited dose dependency; however, the increase was relatively lower in JXN and did not reach statistical significance for the low dose (Figure 1, Panel A). In contrast, the mRNA expression of PPARβ seemed to be largely unchanged in placental tissue (p>0.1) by DEHP treatment (Figure 1, Panel A).
Consistent with the mRNA studies, the protein expression of both PPARα and PPARγ increased significantly (p<0.05) in a dose-dependent manner upon DEHP exposure (Figure 1, Panel B). The magnitude of the induction in LAB was much higher than that in JXN, especially for PPARγ, where a more than 4 fold increase was observed with 1500 mg/kg DEHP treatment (p<0.001). Little or no difference was shown for PPARβ (Figure 1, Panel B).
3.2 Expression of EFA transporters in rat placenta
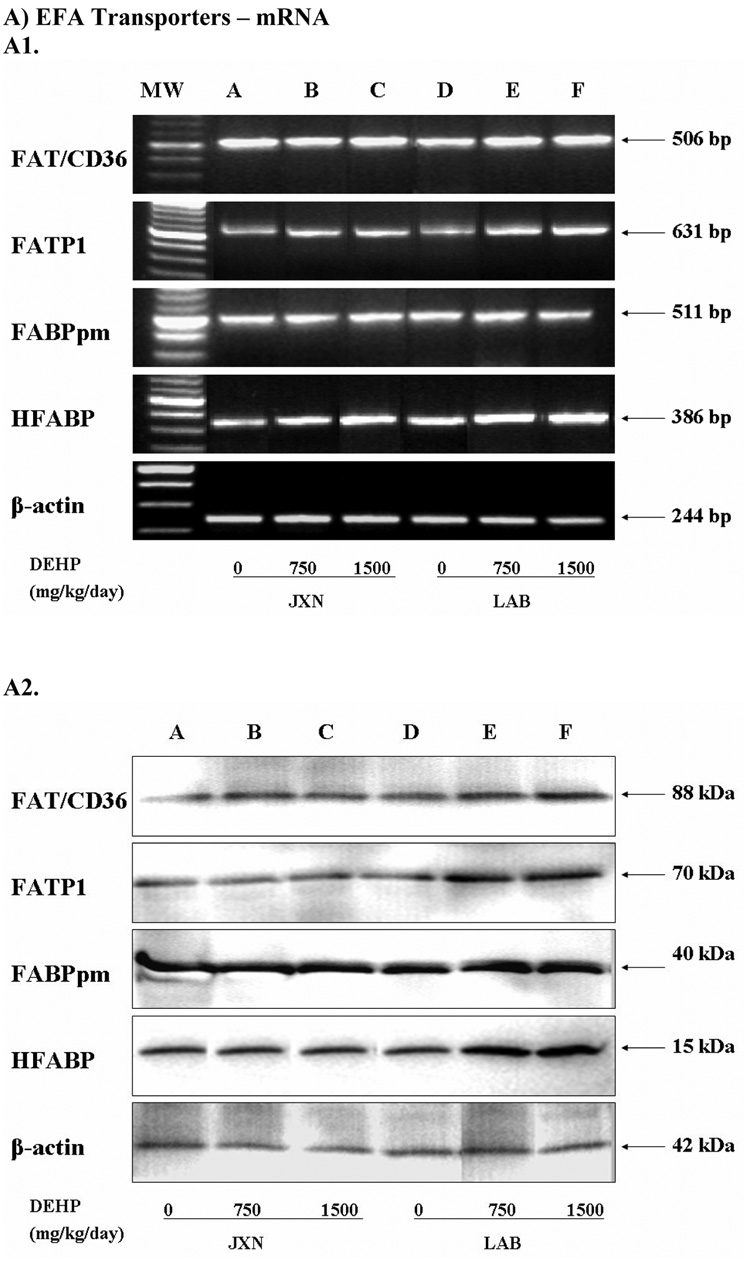
Four long chain EFA transporters were examined: FAT/CD36, FATP1, FABPpm and HFABP. The former three belong to the membrane-associated transporters while HFABP is found in the cytoplasm [1–4]. As illustrated in Panel A of Figure 2, the mRNA expression of FAT/CD36 was increased in LAB by DEHP treatment versus control, with a statistically significant difference observed (p<0.05) for the high dose DEHP group. Similar to the mRNA expression pattern, DEHP also elicited a significant induction of FAT/CD36 protein level in LAB (Figure 2, Panel B, p<0.05). In contrast, no significant change was observed in JXN.
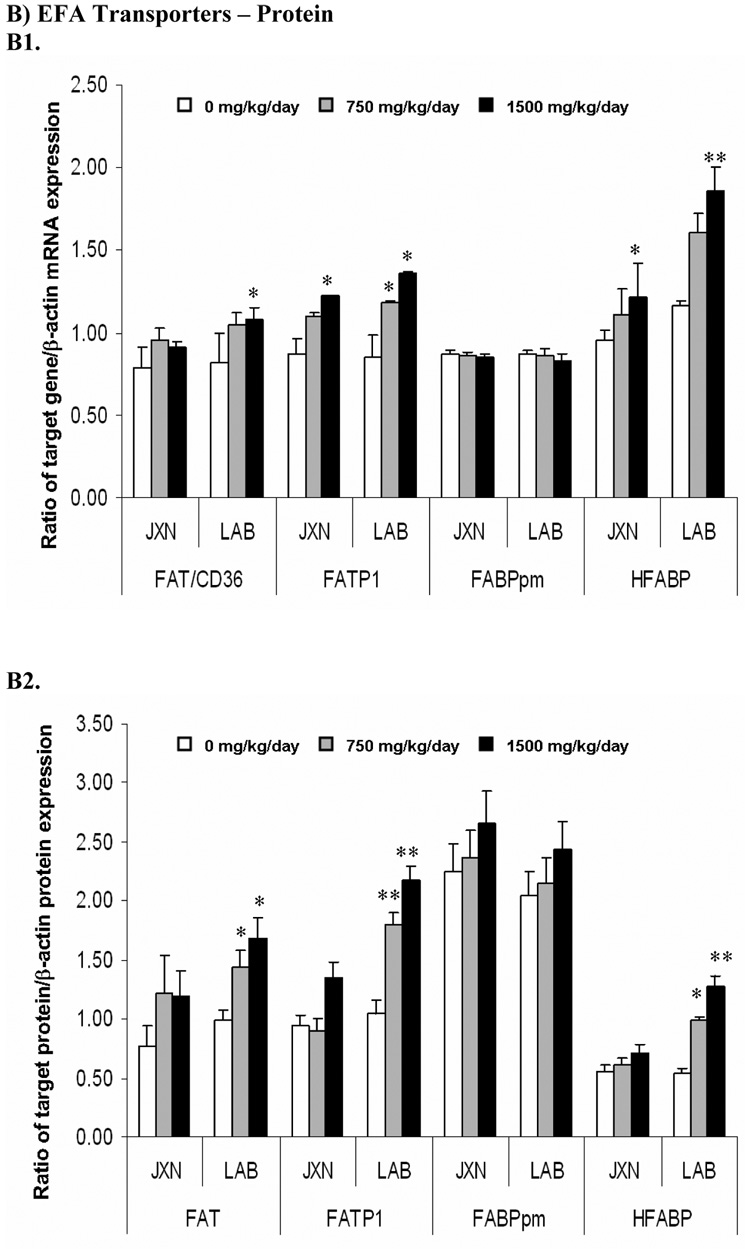
Figure 2. Expression of fatty acids transporters mRNA (Panel A) and protein (Panel B) in rat placenta upon maternal exposure to DEHP.
Dams were dosed with DEHP (750 mg/kg/day or 1500 mg/kg/day) or vehicle from GD 0 through GD 19. Total RNA and whole cell protein were isolated at GD 20 and expressions of FAT/CD36, FATP1, FABPpm and HFABP mRNA and protein were analyzed using semi-quantitative RT-PCR and Western blot, respectively. A1 and B1, representative RT-PCR gel electrophoresis and Western blot image. Lane A-C: JXN, junctional zone; Lane D-F: LAB, labyrinthine zone. A2 and B2, densitometry analysis of the RTPCR gel and Western blot image. Data shown were relative arbitrary values after normalized with β-actin (means ± SD, n =3 or 4). *, p<0.05; **, p<0.01; ***, p<0.001.
The expression patterns of FATP1 and HFABP upon DEHP exposure were quite similar. Significant mRNA induction (p<0.05) was demonstrated for these two transporters in both the LAB and JXN by 1500 mg/kg DEHP administration (Figure 2, Panel A), with a slightly greater induction observed for HFABP. Interestingly, concurrent significant elevations for the FATP1 and HFABP proteins (Figure 2, Panel B) were demonstrated only in LAB (p<0.05), but not JXN (Figure 2, Panel B). In contrast to the other three investigated EFA transporters, placental FABPpm expression was not altered in a statistically significant manner upon DEHP exposure.
3.3 Expression of EFA metabolic enzymes in rat placenta
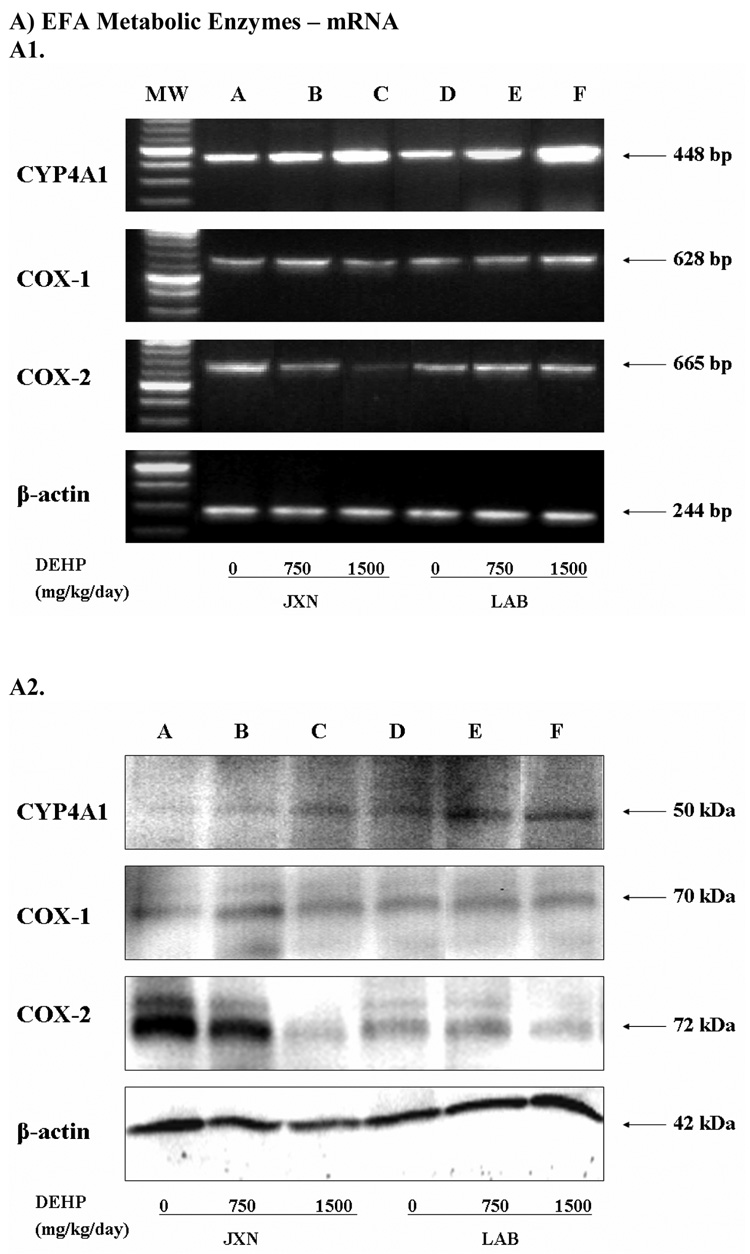
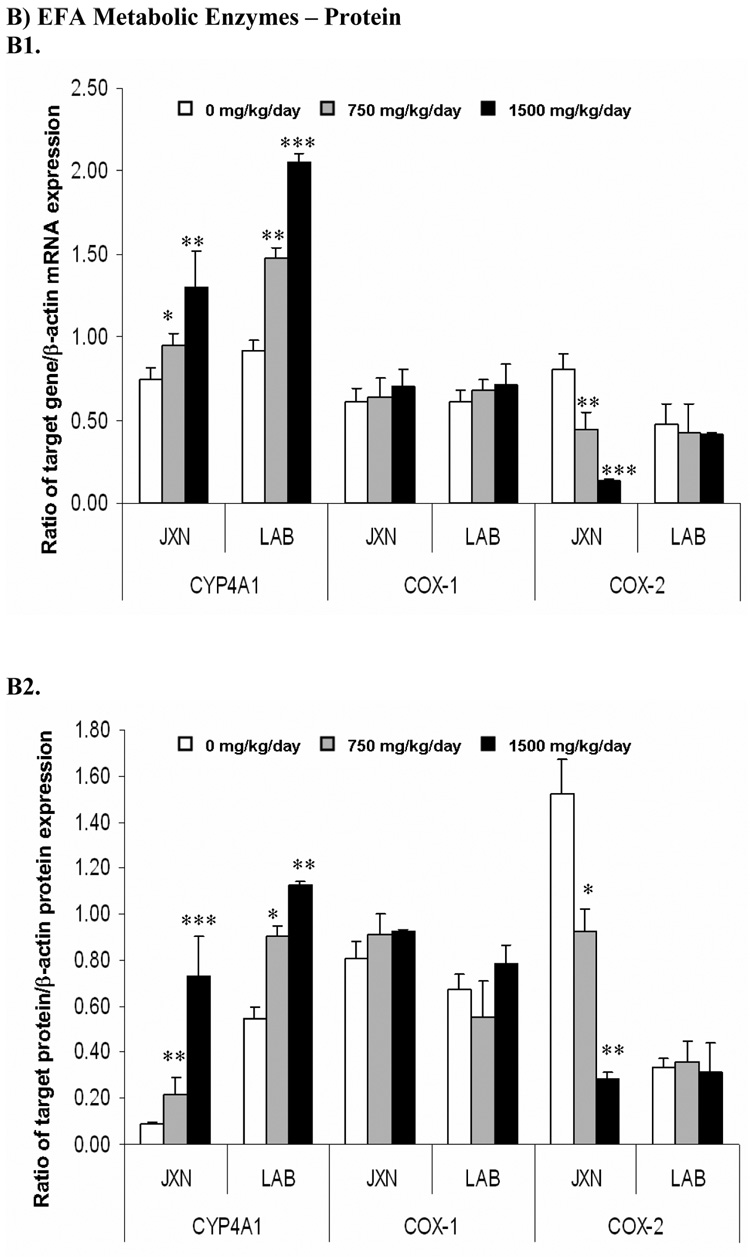
CYP4A1 mRNA expression in late term placenta was significantly increased in both the JXN (p<0.05) and LAB (p<0.01) upon exposure to low or high dose of DEHP (Figure 3, Panel A). Consistently, marked elevation of CYP4A1 protein level was also observed in these placental tissues by DEHP treatment (Figure 2, Panel B, p<0.05).
Figure 3. Expression of fatty acids metabolic enzymes mRNA (Panel A) and protein (Panel B) in rat placenta upon maternal exposure to DEHP.
Dams were dosed with DEHP (750 mg/kg/day or 1500 mg/kg/day) or vehicle from GD 0 through GD 19. Total RNA and whole cell protein were isolated at GD 20 and expressions of CYP4A1, COX-1 and COX-2 mRNA and protein were analyzed using semi-quantitative RT-PCR and Western blot, respectively. A1 and B1, representative RT-PCR and Western blot. Lane A-C: JXN, junctional zone; Lane D-F: LAB, labyrinthine zone. A2 and B2, densitometry analysis of the RT-PCR gel and Western blot image. Data shown were relative arbitrary values after normalized with β-actin (means ± SD, n =3 or 4). *, p<0.05; **, p<0.01; ***, p<0.001.
No obvious change was demonstrated for COX-1 mRNA and protein (Figure 3, Panel A) by DEHP treatment. In contrast, mRNA expression of the inducible COX isoform, COX-2, decreased significantly in JXN by 55% and 86% for the 750 mg/kg and 1500 mg/kg DEHP doses, respectively (p<0.01), while no apparent change was shown in LAB (Figure 3, Panel A). In accordance with the mRNA expression patterns, COX-2 protein levels decreased significantly in JXN (p<0.01), but not LAB (Figure 3, Panel B).
3.4 Long Chain EFA transfer across rat placenta
In order to determine the functional effect of DEHP on the placental transfer of long chain EFAs into the fetal circulation, amounts of AA and DHA were evaluated in samples from the maternal and fetal plasma as well as the placental tissue (Figure 4). The radioactivity quantified by scintillation counting represents parent fatty acid and any of its potential metabolites. DHA and AA were selected in the current study as representative of the ω-3 and ω-6 EFAs, respectively [2–4].
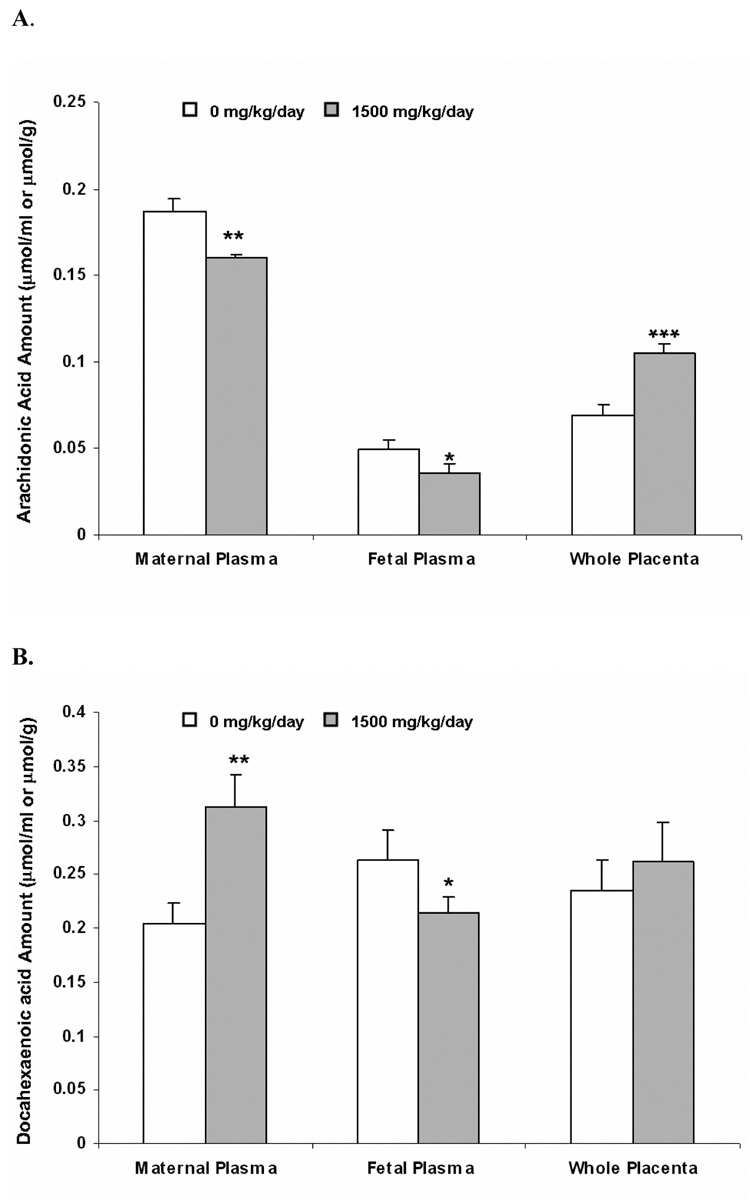
Figure 4. Placental transfer of radioactivity after injection of 3H-arachidonic acid (AA, Panel A) and 14C-docosahexaenoic acid (DHA, Panel B) to the dam as a function of DEHP exposure.
Dams were dosed with DEHP (1500 mg/kg/day) or vehicle from GD 0 through GD 19. 1.25 µmol/kg (2 µCi/kg) of 3H-AA and 14C-DHA were injected to dams from tail vein at GD 20. 3 hr later, placental tissues and plasma samples were collected and amounts of radioactivity (AA and DHA and any potential metabolites) were determined. Data shown were means ± SD, n =3 or 4. *, p<0.05; **, p<0.01, ***, p<0.001.
The total radioactivity associated with AA and any potential metabolites significantly decreased in the DEHP group versus control in both the fetal (about 35% decrease, p<0.05) and the maternal plasma (about 15% decrease, p<0.05) (Figure 4, panel A). In contrast, enrichment of the radioactivity was evident in placental tissue (about 1.5 fold, p<0.001) upon DEHP exposure (Figure 4, panel A). A distinctive placental transfer pattern was observed for DHA. Although there was a pronounced decrease in the DEHP group versus control in fetal plasma (decreased by 20%, p<0.05), the total radioactivity of DHA and its metabolites tended to be higher in the maternal plasma and the placental tissue upon DEHP exposure, and the induction was statistically significant for the maternal plasma (Figure 4, panel B, p<0.05). Notably, ratios of the maternal-to-fetal plasma levels of AA and DHA were both increased (1.20 and 1.85 fold, respectively) in spite of the induced or reduced maternal plasma amount.
3.5 Long chain EFA fetal distribution
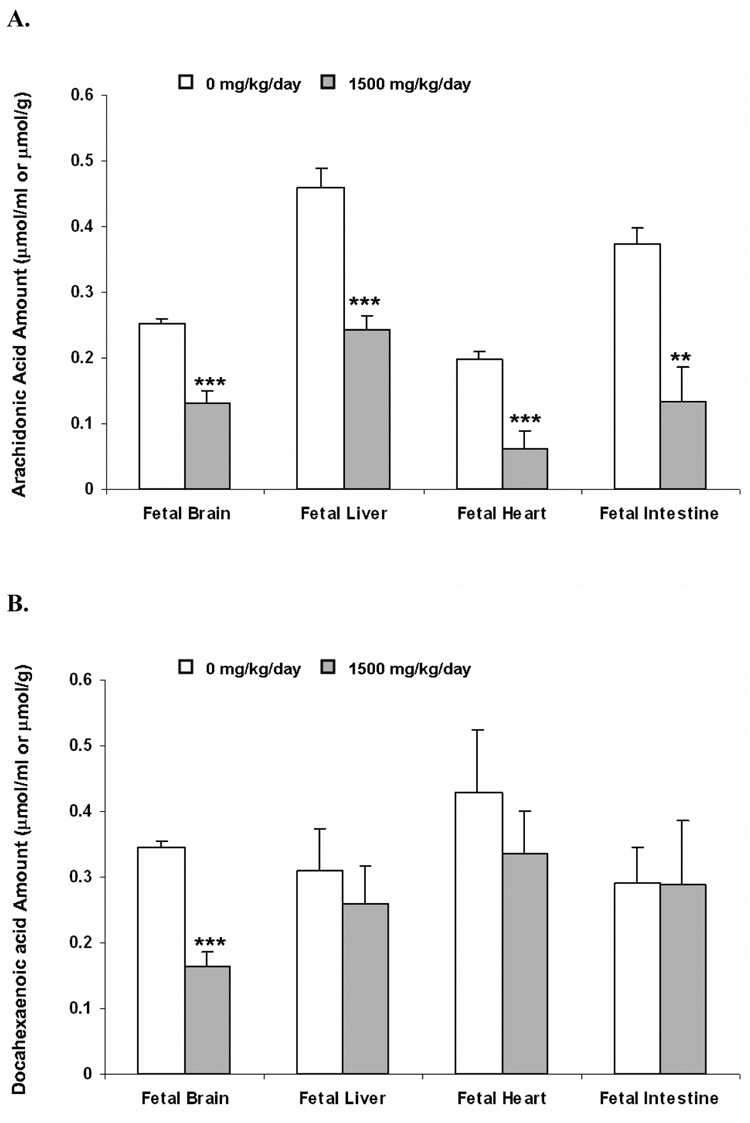
After maternal tail vein dosing of 3H-AA or 14C-DHA, the presence of radioactivity was observed in the fetal organs investigated, i.e. brain, liver, heart and intestine (Figure 5). Maternal exposure to DEHP significantly decreased the radioactivity associated with AA and any potential metabolites in the examined fetal organs, with the greatest reduction in fetal heart and intestine (about 70% decrease, p<0.001), and smaller decrease in fetal brain and liver (about 50% decrease, p<0.01) (Figure 5, panel A). Changes in the fetal DHA distribution by DEHP exposure were similar to that observed for AA. The magnitude of the reduction of DHA and its metabolites, however, was much lower and did not reach statistical significance, except for the fetal brain, where a remarkable 50% decrease (p<0.001) was observed (Figure 5, panel B). Please note that the reduced EFA levels detected in the fetal organs reflected a decreased transfer of these EFAs into the fetal compartment (Figure 4).
Figure 5. Fetal distribution of radioactivity after injection of 3H-arachidonic acid (AA, Panel A) and 14C-docosahexaenoic acid (DHA, Panel B) to the dam as a function of DEHP exposure.
Dams were dosed with DEHP (1500 mg/kg/day) or vehicle from GD 0 through GD 19. 1.25 µmol/kg (2 µCi/kg) of 3H AA and 14C DHA were injected to dams from tail vein at GD20. 3 hr later, fetal tissues were collected and amounts of radioactivity (AA and DHA and any potential metabolites) were determined. Data shown were means ± SD, n =3 or 4. *, p<0.05; **, p<0.01, ***, p<0.001.
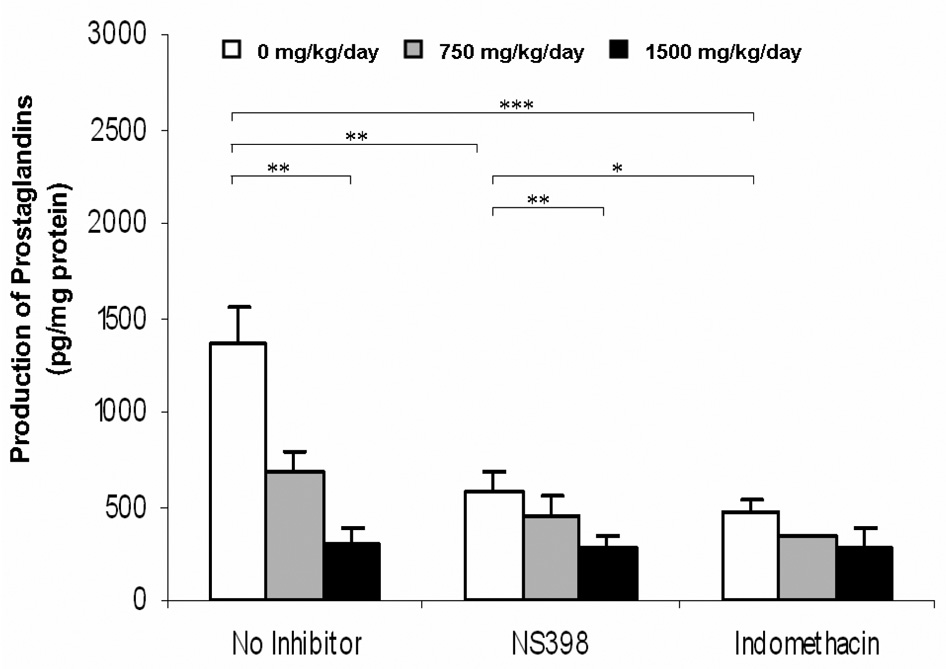
3.6 Placental production of total prostaglandins (PGs)
DEHP treatment resulted in a significant dose dependent decrease (p<0.05) in the formation of total PGs from placental homogenates, as measured by ELISA. This result suggests that COX enzyme activity may be reduced in these placental tissues. When the placental homogenate was incubated with the COX-2 selective inhibitor, NS-398, the total PGs production further decreased, with the greatest reduction observed in the control group (58% reduction, p< 0.01), followed by the 750 mg/kg DEHP group (35% reduction), and the 1500 mg/kg DEHP group (7% reduction) (Figure 6). Similar levels of PGs formation were observed in the presence of indomethacin (non-selective COX inhibitor) (Figure 6). The lack of significant change in placental total PG formation between NS-398 and indomethacin incubation is likely a result of no apparent change in COX-1 activity in placenta.
Figure 6. Production of total prostaglandins (PGs) in rat placenta upon maternal exposure to DEHP.
Dams were dosed with DEHP (750 mg/kg/day or 1500 mg/kg/day) or vehicle from GD 0 through GD 19. Placental tissue were isolated and homogenized at GD 20 and total PGs production were measured by ELISA with an incubation of 50 µM arachidonic acid. Inhibition study with indomethacin (10 µM) and NS-398 (5 µM) were also performed. Data shown were means ± SD, n =3 or 4. *, p<0.05; **, p<0.01; ***, p<0.001.
4. Discussion
The present study demonstrated significant changes in the expression of EFA homeostasis related proteins in the rat placenta upon maternal exposure to DEHP, known PPAR agonists, as well as its MEHP and EHA metabolites [21]. Changes in the EFA maternal-to-fetal transfer and fetal distribution as well as placental production of PGs were also observed. Given that placenta functions prominently in guiding proper fetal development [1–3], such changes to fetus and the placenta suggest that DEHP-mediated alterations to placental and fetal EFA homeostasis may potentially lead to adverse effects in the fetus.
Although the doses used in the current study are relatively high, related toxicokinetic studies demonstrated that the dam DEHP plasma level might be comparable to human exposure. After a single oral gavage dose of DEHP, the, maximum plasma concentrations of DEHP in dams were about 2 µg/mL and 3–4 µg/mL at low (750 mg/kg) and high dose (1500 mg/kg) respectively (data not shown). MEHP was also detected after DEHP dosing in dam plasma, having about a two order of magnitude higher level than those observed for DEHP (65 µg/mL at 750 mg/kg and 136 µg/mL at 1500 mg/kg). This is consistent with what would be expected in in vivo studies conducted with DEHP and could be a possible confounder, as mentioned in the Introduction section. Please note that MEHP and EHA are actually suggested to be the main PPAR agonists that would alter EFA homeostasis [21]. The observed concentrations of DEHP and MEHP were higher than what was reported in the human maternal plasma at term in healthy subjects, where the mean DEHP and MEHP concentrations were 1.15 +/− 0.81 and 2.05 +/− 1.47 µg/ml respectively [32]. It is noteworthy that patients having a long-term exposure to DEHP containing devices were reported to have a whole blood DEHP concentration of about 70 to 80 µg/mL [7]. Furthermore, to correlate toxic effects observed in animals with acceptable levels of exposure for humans, 10 fold increases DEHP exposure are commonly used for the inter- and intraspecies variability.
DEHP and its metabolites belong to the PPCs that measurably activate PPARs [11]. Accordingly, we demonstrated that the mRNA and protein expressions of PPARα and PPARγ in the JXN and LAB of rat placenta were elevated by DEHP exposure in a dose-dependent manner (Figure 1), suggesting that both PPARα and PPARγ might be involved in the DEHP-controlled regulation in rat placenta, most likely through the formation of MEHP and EHA. Additionally, PPARγ might play a more critical role, since PPARγ activation was dramatically higher compared to PPARα, especially at the protein level (Figure 1, Panel B). It has been reported that PPARγ knockout mice were embryonic lethal due to improper placentation [19], further indicating the importance of this isoform in regulating placental/fetal development. Current studies in our laboratory are focused on investigating the mechanism(s) for the up regulation of the PPARα and PPARγ isoforms.
There was no consistently significant regulation pattern of PPARβ by DEHP treatment (Figure 1), which is in accordance with our in vitro experiment from rat trophoblasts [12] and other literature reports [20,21]. Since PPARβ is determined to be essential in placenta development and giant cell differentiation [6], and its deficiency resulted in frequent embryonic lethality [22], further study to elucidate the regulation of PPARβ by other xenobiotics are warranted. Interestingly, in contrast to PPARβ and PPARγ null mice, no placental abnormality has been observed in PPARα null mice [23].
Of the three cellular surface proteins involved in placental fatty acid uptake and transport, both the mRNA and protein expressions of FABPpm did not change upon DEHP administration (Figure 2), which is consistent with literature report [24] and our previous in vitro data [12]. In contrast, the other EFA transporters investigated, FAT/CD36, FATP1 and HFABP, were significantly induced by DEHP in LAB (Figure 2), the established rat placental transport barrier [14]. Considering the significant PPAR activation in placenta from DEHP treated rats and that regulations of these transporters by PPARs have been established in various other tissues, induced expression of these transporters by DEHP can likely be attributed to the mediation by PPARs. Other potential regulation pathways can not be ruled out, however, e.g. DEHP and its metabolites are known agonists of the orphan nuclear receptors of pregnane X-receptor (PXR) and the constitutive androstane receptor (CAR) [25]. Further mechanistic studies would be helpful to clarify this issue. It should be noted that the expression patterns of these EFA transporters were different and had more significant variation at the protein level versus mRNA, suggesting the involvement of translational and post-translational events.
Fatty acid metabolic enzymes also contribute to fatty acid homeostasis and may be subjected to DEHP regulation, e.g. fatty acyl-Co-A oxidase and 3-ketoacyl-CoA thiolase [5]. We are particularly interested in the enzymes involved in the AA metabolic cascade consisting of CYP4A1, COX-1 and COX-2 [5]. They mediate the metabolism of long chain EFAs to eicosanoids, which can be further converted to other important bioactive molecules critical for fetal development, such as PG, thromboxane and prostacyclin [26]. The current study demonstrated that the expression of CYP4A1, the major CYP4A isoform present in rat placenta [12], was significantly induced in both the LAB and JXN (Figure 3), consistent with previous reports in mouse liver [27] and testis [28]. The induction pattern of CYP4A1 correlated with the observed changes of PPARα by DEHP exposure (Figure 1), which might reflect the fact that CYP4A isoforms are under transcriptional control of PPARs, particularly PPARα [29].
Although there is no apparent change of COX-1 in placental tissues upon maternal exposure to DEHP, COX-2 expression was notably decreased in JXN (Figure 3), the layer responsible for uterine implantation/invasion and hormonal regulation of pregnancy [14]. In addition, COX-2 activity in placental tissue was significantly down-regulated, as indicated from the reduced total PGs production from placental homogenates by DEHP treatment and the similar inhibition patterns of PG formation in the presence of COX-2 selective and non-selective inhibitors (Figure 6). Considering that COXs catalyze the rate-limiting reaction for PG biosynthesis and that PGs are important for the initiation of parturition [30], the reduction of COX-2 and the subsequent decreased PGs production at late term might contribute to the delayed spontaneous term labor observed in DEHP treated dams in another study [31]. However, this is contrary to the reported shorter duration of pregnancy [32] and, as of yet, can not be explained. Inhibition of COX-2 by PPAR ligands has been identified in various tissues and cell lines [33–35]. The placenta might also be a target organ, as suggested from the reduced COX-2 and elevated PPARα/γ expression, although further mechanistic study is needed to confirm this hypothesis. Notably, there is no PPRE identified in the promoter region of COX-2, and thus interference with NF-κB pathway and/or AP-1 mediated transcriptional activation has been suggested [33–35].
In the maternal-placental-fetal unit, efficient transfer of AA and DHA from the maternal plasma is of critical importance for the normal development of fetal visual and brain functions. In the current study, a reduced placental transfer of AA and DHA from the maternal to the fetal compartment by DEHP treatment was shown, as evident from the increase in the maternal to fetal influx ratios as well as the decease in the fetal plasma concentrations. Consequently, the amount of AA and DHA (and/or their metabolites) in major fetal organs demonstrated a similar downward trend, especially in the fetal brain, where a significant reduction of up to 50% was observed (Figure 5). These data are consistent with previous reports, where diverse biochemical changes affecting blood fatty acid/lipid levels have been demonstrated in the brain, liver and other organs of the rat fetus from DEHP-fed dams [13,36]. In addition, a concordant reduction in EFAs was observed in the fetal brain lipid profile after DEHP exposure in the same rat dams used for this study [13]. The reduction in placental EFAs transfer may be partly resulted from DEHP’s alteration of the trophoblastic expression of lipid/fatty acid homeostasis regulating proteins, where an increase in the uptake and transport of selected fatty acids and accumulation of various fatty acid/lipid classes in the placenta might lead to higher placental metabolism/lipid synthesis, and thus reduce the fetal supply of EFAs. Protective mechanism of the placental EFA accumulation has been discussed previously [3]. Interestingly, a higher level of EFAs was observed in the microvillous and basal syncytial trophoblast membranes than in the fetal and maternal plasma and tissues [3]. If microvillous expression of fatty acid transporters were to increase, it is plausible that this would lead to a placental accumulation of fatty acids and may affect the fetal compartment. Clearly, additional studies are required to clarify the exact mechanism that controls the current reported phenomena. In addition,
Actually, the interactions between AA/DHA and EFA transporters and metabolic enzymes have been identified in various cells, including trophoblasts [1,3–5]. It is believed that a reduction in EFAs transfer to the fetus would have significant adverse effects on fetal development and may be associated with an increased risk of certain diseases in adult life, and therefore dietary supplementation with EFAs during the critical perinatal period has been suggested [37]. However, an excess PUFAs supply may also have detrimental effects [3]. For example, a high intake of DHA through fish oil supplementation has been demonstrated to be responsible for the impaired neonatal growth and delayed psychomotor maturation of rat pups [38].
Directional transfer of EFAs across the placenta controls fetal EFA homeostasis through highly regulated processes, and PPAR activation might be one of them. Our study investigated the effects of DEHP maternal exposure on placental/fetal EFA homeostasis via potential trans-activation of PPARs and their subsequent regulation of EFA transporters and enzymes. Expression of PPARα, PPARγ, FAT/CD36, FATP1, HFABP and CYP4A1 were up-regulated in the JXN and/or LAB zones of the placenta while COX-2 was substantially down-regulated in JXN. DEHP treatment also reduced the directional maternal-to-fetal placental transfer of EFAs, decreased the placental production of PGs, and influenced the fetal distribution of EFAs, especially the brain. These results are consistent with our previous in vitro data and suggest that DEHP and its MEHP and EHA metabolites might influence placental/fetal EFA homeostasis and potentially result in abnormal fetal development. Efforts to minimize DEHP exposure, such as avoiding phthalate-containing products, during pregnancy may need increased attention from clinicians. Although there were no data directly demonstrating a transcriptional regulation mechanism for DEHP interactions with PPAR isoforms, current results do indicate that PPAR might play a role, at least partly, in the DEHP induced altered fatty acid homeostasis in rat placenta. Additional studies to delineate the contributions of DEHP's main metabolites, MEHP and EHA, to the observed toxicity during DEHP exposure, are required to fully decipher the underlying mechanisms.
Acknowledgments
This study was supported by a grant from the National Alliance for Autism Research (T.J. Cook and G.T. Knipp) and by the National Institute of General Medical Sciences (R01-GM65448; G.T. Knipp).
Abbreviations
- AA
arachidonic acid
- COX
cyclooxygenases
- CYP4A1
Cytochrome P450 subfamily 4A1
- DEHP
di-(2-ethylhexyl)-phthalate
- EFA
essential fatty acid
- DHA
docosahexaenoic acid
- PG
prostaglandins
- FABPpm
plasma membrane fatty acid binding protein
- FAT/CD36
fatty acid translocase
- FATP1
fatty acid transport protein 1
- HFABP
heart cytoplasmic fatty acid binding protein
- JXN
junctional
- LAB
labyrinthine
- PPAR
peroxisome proliferator-activated receptor
- PUFA
polyunsaturated fatty acid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knipp G, Audus KL, Soares MJ. Nutrient transport across the placenta. Adv Drug Deliv. Rev. 1999;38:41–58. doi: 10.1016/s0169-409x(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 2.Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J. Pediatr. 2003;143:S1–S8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- 3.Haggarty P. Effect of placental function on fatty acid requirements during pregnancy. Eur. J. Clin. Nutr. 2004;58:1559–1570. doi: 10.1038/sj.ejcn.1602016. [DOI] [PubMed] [Google Scholar]
- 4.Dutta-Roy AK. Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am J Clin Nutr. 2000;71:S315–S322. doi: 10.1093/ajcn/71.1.315s. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Wang Q, Cook TJ, Knipp GT. Effects of placental fatty acid metabolism and regulation by peroxisome proliferator activated receptor on pregnancy and fetal outcome. J. Pharm. Sci. 2007;96:2582–2606. doi: 10.1002/jps.20973. [DOI] [PubMed] [Google Scholar]
- 6.Fournier T, Tsatsaris V, Handschuh K, Evain-Brion D. PPARs and the placenta. Placenta. 2007;28:65–76. doi: 10.1016/j.placenta.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Kavloc R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Ty R, Williams P, Zacharewski T. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod. Toxicol. 2002;16:529–653. doi: 10.1016/s0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 8.Singh AR, Lawrence WH, Autian J. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. J. Pharm. Sci. 1975;64:1347–1350. doi: 10.1002/jps.2600640819. [DOI] [PubMed] [Google Scholar]
- 9.Kihlstrom I. Placental transfer of diethylhexyl phthalate in the guinea-pig placenta perfused in situ. Acta. Pharmacol. Toxicol. (Copenh) 1983;53:23–27. doi: 10.1111/j.1600-0773.1983.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 10.Silva MJ, Reidy JA, Herbert AR, Preau JL, Jr, Needham LL, Calafat AM. Detection of phthalate metabolites in human amniotic fluid. Bull Environ. Contam. Toxicol. 2004;72:1226–1231. doi: 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- 11.Dzhekova-Stojkova S, Bogdanska J, Stojkova Z. Peroxisome proliferators: their biological and toxicological effects. Clin. Chem. Lab. Med. 2001;39:468–474. doi: 10.1515/CCLM.2001.076. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Cook TJ, Knipp GT. Effect of di-(2-ethylhexyl)-phthalate and its metabolites on fatty acid homeostasis regulating proteins in HRP-1 cells. Toxicol. Sci. 2005;84:287–300. doi: 10.1093/toxsci/kfi083. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Agrawal S, Cook TJ, Knipp GT. Di-(2-ethylhexyl)-phthalate affects fetal brain lipid profiling in rat upon maternal exposure. Arch Toxicol. 2007;81:57–62. doi: 10.1007/s00204-006-0143-8. [DOI] [PubMed] [Google Scholar]
- 14.Soares MJ. Molecular Mechanisms of Placental Development. In: Battaglia FC, editor. Placental Function and Fetal Nutrition. Vol. 39. Philadelphia, PA: Nestec Ltd. Vevey/Lippincott-Raven Publishers; 1997. pp. 31–45. Nestlé Nutrition Workshop Series. [Google Scholar]
- 15.Xu Y, Knipp GT, Cook TJ. Expression of CYP4A isoforms in developing rat placenta and trophoblast cell models. Placenta. 2005;26:218–225. doi: 10.1016/j.placenta.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Knipp GT, Cook TJ. Expression of COX isoforms in developing rat placenta and trophoblast cell models. Mol. Pharm. 2005;2:481–490. doi: 10.1021/mp0500519. [DOI] [PubMed] [Google Scholar]
- 17.Kawai S. Cyclooxygenase selectivity and the risk of gastro-intestinal complications of various non-steroidal anti-inflammatory drugs: a clinical consideration. Inflamm. Res. 1998;47 Suppl 2:S102–S106. doi: 10.1007/s000110050291. [DOI] [PubMed] [Google Scholar]
- 18.Yaghi A, Webb CD, Scott JA, Mehta S, Bend JR, McCormack DG. Cytochrome P450 metabolites of arachidonic acid but not cyclooxygenase-2 metabolites contribute to the pulmonary vascular hyporeactivity in rats with acute Pseudomonas pneumonia. J Pharmacol Exp Ther. 2001;297:479–488. [PubMed] [Google Scholar]
- 19.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 20.Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol. Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 21.Maloney E, Waxman DJ. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol. Appl. Pharmacol. 1999;161:209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- 22.Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A. 2006;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalik L, Desvergne B, Dreyer C, Gavillet M, Laurini RN, Wahli W. PAR expression and function during vertebrate development. Int J Dev Biol. 2002;46:105–114. [PubMed] [Google Scholar]
- 24.Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J. Biol. Chem. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- 25.Kretschmer XC, Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters? Chem. Biol. Interact. 2005;155:111–128. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002;56:215–222. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 27.Wong JS, Gill SS. Gene expression changes induced in mouse liver by di(2-ethylhexyl) phthalate. Toxicol. Appl. Pharmacol. 2002;185:180–196. doi: 10.1006/taap.2002.9540. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Ishizuka M, Kazusaka A, Fujita S. Alterations of activities of cytosolic phospholipase A2 and arachidonic acid-metabolizing enzymes in di-(2-thylhexyl)phthalate-induced testicular atrophy. J. Vet. Med. Sci. 2004;66:1119–1124. doi: 10.1292/jvms.66.1119. [DOI] [PubMed] [Google Scholar]
- 29.Johnson EF, Hsu MH, Savas U, Griffin KJ. Regulation of P450 4A expression by peroxisome proliferator activated receptors. Toxicology. 2002;181–182:203–206. doi: 10.1016/s0300-483x(02)00282-2. [DOI] [PubMed] [Google Scholar]
- 30.Kniss DA. Cyclooxygenases in reproductive medicine and biology. J. Soc. Gynecol. Investig. 1999;6:285–292. doi: 10.1016/s1071-5576(99)00034-9. [DOI] [PubMed] [Google Scholar]
- 31.Narotsky MG, Weller EA, Chinchilli VM, Kavlock RJ. Nonadditive developmental toxicity in mixtures of trichloroethylene, Di(2-ethylhexyl) phthalate, and heptachlor in a 5 ×5 ×5 design. Fundam. Appl. Toxicol. 1995;27:203–216. doi: 10.1006/faat.1995.1125. [DOI] [PubMed] [Google Scholar]
- 32.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biol Neonate. 2003;83:22–24. doi: 10.1159/000067012. [DOI] [PubMed] [Google Scholar]
- 33.Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- 34.Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPARgamma. J. Biol. Chem. 2000;275:28028–28032. doi: 10.1074/jbc.M001387200. [DOI] [PubMed] [Google Scholar]
- 35.Subbaramaiah K, Lin DT, Hart JC, Dannenberg AJ. Peroxisome proliferator-activated receptor gamma ligands suppress the transcriptional activation of cyclooxygenase-2. Evidence for involvement of activator protein-1 and CREB-binding protein/p300. J. Biol. Chem. 2001;276:12440–12448. doi: 10.1074/jbc.M007237200. [DOI] [PubMed] [Google Scholar]
- 36.Bell FP. Effects of phthalate esters on lipid metabolism in various tissues, cells and organelles in mammals. Environ. Health Perspect. 1982;45:41–50. doi: 10.1289/ehp.824541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE. Essential fatty acids in visual and brain development. Lipids. 2001;36:885–895. doi: 10.1007/s11745-001-0798-1. [DOI] [PubMed] [Google Scholar]
- 38.Amusquivar E, Ruperez FJ, Barbas C, Herrera E. Low arachidonic acid rather than alpha-tocopherol is responsible for the delayed postnatal development in offspring of rats fed fish oil instead of olive oil during pregnancy and lactation. J Nutr. 2000;130:2855–2865. doi: 10.1093/jn/130.11.2855. [DOI] [PubMed] [Google Scholar]