Abstract
FLT3 is mutated in roughly 30% of human AML. We used our model of APL with activated FLT3 to assess the effectiveness of chemotherapy in combination with SU11657, an inhibitor of FLT3. We found that median survival of untreated and doxorubicin-treated mice was not significantly different. While SU11657 alone increased median of survival to 55 days (P=0.01), dual therapy increased median survival to 62 days (P=0.003) when compared to controls. Neither agent alone or in combination increased survival of control mice. These results suggest that the use of targeted therapeutics can overcome resistance to traditional chemotherapies in AML.
Keywords: Acute promyelocytic leukemia, FLT3 inhibitor, chemotherapy
1. Introduction
Initial or acquired resistance to standard chemotherapy remains a critical issue in the treatment and survival of patients with acute myeloid leukemia (AML). This problem increases with age, as adults over 65 rarely tolerate or respond to standard therapy with median survival of approximately 3.5–7 months [1].
Almost 80% of AML blasts express FLT3, a receptor tyrosine kinase (RTK) that is associated with proliferation and differentiation of normal hematopoietic stem cells and survival of AML blasts [2]. Activating mutations in FLT3 have been identified in about 30% of AML blasts. The most frequent mutation is the internal tandem duplication (ITD) that has been shown to be an independent prognostic marker of non-favorable outcome [3]. For these reasons, FLT3 inhibitors are being vigorously studied in preclinical and clinical studies for treatment of new and refractory AML [4, 5]. Several groups have shown that FLT3 inhibitors can be successfully combined with other therapies, such as (i) anthracyclines to induce apoptosis of primary blasts in vitro [6] or (ii) ATRA to induce remission of leukemic mice in vivo [7].
We and others have previously described mouse models in which the ITD mutation of FLT3 was introduced into the bone marrow of PML-RARA (PR) mice [7, 8]. In our model, these mice developed leukemia that is transplantable with onset of 3–6 weeks. SU11657 is a multi-targeted RTK inhibitor targeting FLT3 as well as PDGFR, VEFGR1, VEGFR2 and KIT kinases. We assessed the anthracycline response of PR and PR-FLT3 leukemias and the effect of adding FLT3 inhibitor to such treatment.
2. Material and Methods
2.1. Mice and transplantation of leukemic cells
FVB/N mice were bred and maintained at the University of California, San Francisco in accordance with institutional guidelines. Bone marrow and spleen cells from leukemic animals (PR and PR-FLT3, respectively) were suspended in phosphate buffered saline (PBS). Mice were sublethally irradiated (4.5 Gy), and 1×106 and 0.3×106 cells, respectively, were injected into tail vein of each recipient mouse as described previously [7]. The diet was supplemented with antibiotics for 2 weeks following irradiation.
2.2. Treatment of mice
For survival studies, treatment started on day 7 after irradiation. Doxorubicin (Novaplus) was administered at 3 mg/kg/day by intraperitoneal injection (ip) for 3 days. SU11657 (Sugen, South San Francisco) was administered at 20 mg/kg/day in carboxymethylcellulose suspension by oral gavage, 3 days on, 4 days off for 3 weeks. PBS by ip and water or carboxymethylcellulose vehicle by oral gavage were used for treatment controls. Acute treatment began after disease developed in mice (day 18 for PR-FLT3 and Day 28 for PR mice). The treatment was carried out for 3 consecutive days and the mice were sacrificed the following day.
2.3. Statistical analysis
Standard Kaplan-Meier survival curves were calculated using Prism 3.0.
2.4. Histopathology
Tissues (sternum, liver, kidney, lungs, lymph nodes, spleen) were excised and fixed in buffered formalin solution before embedding in paraffin. Sternum was decalcified for 3 hrs before embedding (11% formic acid, 8% paraformaldehyde). Sections were prepared according to standard protocols and were stained with hematoxylin and eosin [7].
3. Results and discussion
3.1. Resistance to anthracycline intrinsic
Resistance to standard chemotherapy is a large problem in the treatment of leukemia. SU11657 is a small oral multi-targeted RTK inhibitor with similar properties to SU11248 compound, targeting FLT3 among other kinases. We set out to test whether the combination of SU11657 treatment with an anthracycline might increase survival of leukemic mice.
Daunorubicin, first-line chemotherapy in the treatment of human leukemia, is highly toxic when administered ip in mice and thus another anthracycline - doxorubicin - was chosen for this work. Extensive study showed that in this mouse model, the highest tolerated dose of doxorubicin is 3 mg/kg/day. High doses of SU11657 are toxic in mice as well, and preliminary results showed that the optimal dosing in this mouse strain is 3 days on the treatment and 4 days off.
The first goal of this study was to develop a mouse model that would become resistant to anthracyclines. Surprisingly, initial results showed that both the PR and PR-FLT3 leukemias were resistant to doxorubicin treatment in vivo (data not shown).
3.2. Survival experiments
For both PR and PR-FLT3 leukemia, two independent survival experiments with 3–5 mice per each treatment group were performed. Recipients of PR and PR-FLT3 leukemia were randomly divided into 4 groups in each experiment. First group was treated with SU11657 alone, second group with doxorubicin alone, third group was treated with a combination of SU11657 and doxorubicin, and the fourth group was treated with vehicles only. All treatment regimens were started on day 7 to ensure that (i) leukemia was engrafted and (ii) tumor burden in tissues was still low, given the resistance to doxorubicin observed at the maximum tolerated dose.
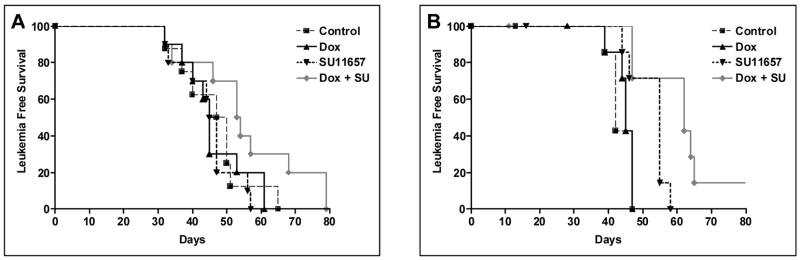
For recipients of PR-FLT3 leukemia, the median survival of vehicle-treated mice was 42 days; for doxorubicin-treated group, it was 45 days. SU11657 increased median of survival to 55 days; combination treatment increased median survival to 62 days. While the difference between control and doxorubicin-treated groups was not significant, difference between SU11657-treated and control group was significant (P=0.01); difference between combination treatment and SU11657-only treatment was significant as well (P=0.02). The statistical significance of control versus combination treated groups was P=0.003 (Fig. 1).
Figure 1. Dual treatment of SU11657 and doxorubicin increases survival of PR-FLT3 mice.
Cohorts of 8 FVBN mice per group were sublethally irradiated and injected with PR leukemic cells (A) and PR-FLT3 leukemic cells (B) and followed for survival. Seven days after irradiation, treatment commenced. The treatment protocol included doxorubicin treatment (i.p.) three times a week (3 mg/kg/day) for the first week. SU11657 treatment (20 mg/kg/day) was conducted 3 days on, 4 days off for three weeks by oral gavage. Control group was treated with PBS and vehicle following the same protocol.
Recipients of PR leukemia were treated with the same protocol. Median survival of vehicle-treated mice was 48.5 days. The medians of survival for SU11657-treated mice (46 days), doxorubicin-treated mice (45 days) and combination-treated mice (53.5 days) were not significantly different (Fig. 1).
3.3. Acute treatment
Matched recipients of PR and PR-FLT3 leukemias were treated with vehicles only, SU11657 only, doxorubicin only or combination of both drugs, beginning once the mice developed disease as checked by CBC counts (day 18 for PR FLT3 and day 28 for PR mice). After three consecutive days of treatment, the mice were sacrificed. Complete pathology was performed on these animals; no major morphological differences were observed in treated PR mice (data not shown). As for PR-FLT3 mice, doxorubicin treatment had minimal effect on morphology (Fig. 2B, F, J). Livers of dual treated mice show substantial clearance of leukemic cells (Fig. 2D). Spleens of dual treated mice contain increased tangible body macrophages and apoptotic cells, as well as changes in morphology of the leukemic cells (chromatin condensation, decreased nuclear-cytoplasmic ratios; Fig. 2H). In dual treated bone marrow, these changes in cellular morphology are marked (Fig. 2L). These findings suggest that combination therapy causes increased cell death and partial differentiation. Spleens and bone marrows of mice treated with SU11657 alone show similar but less pronounced types of changes to those observed in dual treated animals (Fig. 2C, G, K).
Figure 2. Dual treatment changes morphology of spleen and liver of PR-FLT3 mice.
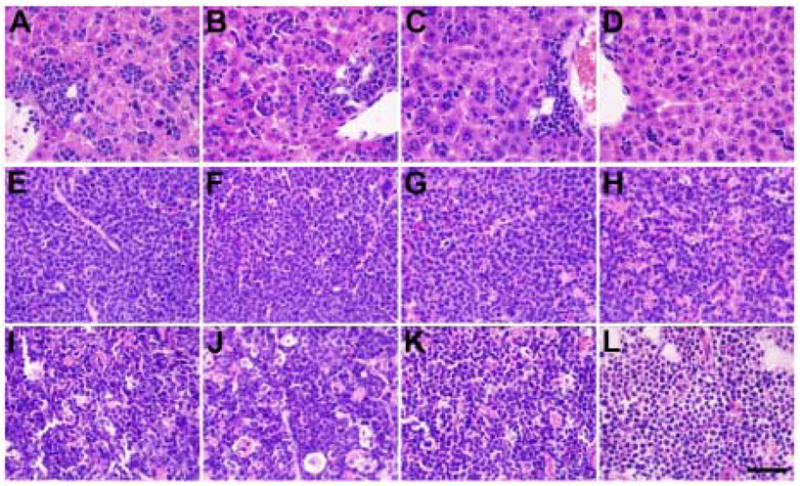
Liver (A–D), spleen (E–H) and sternum (I–L) cytology of PR-FLT3 mice are shown. Vehicle-treated mice show infiltration of leukemic cells in liver, spleen and sternum (A, E, I). While doxorubicin-treated (B, F, J) and SU11657-treated mice (C, G, K) show slight morphological differences in all organs, dual treatment (D, H, L) shows remarkable cytological differences in all organs shown, especially regression of blasts from liver and almost normal morphology of sternum. Black line - 50μm.
FLT3 kinase inhibitors have been shown to have some efficacy in mouse models of myeloid neoplasia [9]. In vitro studies have suggested that the effect of cytotoxic chemotherapy can be enhanced by FLT3 inhibition [6]. Our results extend these observations to demonstrate that a FLT3 kinase inhibitor can confer responsiveness to anthracycline chemotherapy in vivo. A closely related compound, SU11248 (sunitinib), has been FDA-approved for use in some patients with GIST and advanced renal cell carcinoma. Our results suggest that additional trials, for patients with AML, may be warranted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appelbaum FR, Gundacker H, Head DR, Slovak ML, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3488. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carow CR, Levenstein, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87:1089–1096. [PubMed] [Google Scholar]
- 3.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 4.Smith BD, Levis M, Beran M, et al. Single agent CEP 701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;104:3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 5.Fiedler W, Serve H, Dohner H, et al. A phase I study of SU11248 in the treatment of patients with refractory and resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 6.Yee KW, Schittenhelm M, O‘Farrel AM, et al. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood. 2004;104:4202–9. doi: 10.1182/blood-2003-10-3381. [DOI] [PubMed] [Google Scholar]
- 7.Sohal J, Phan VT, Chan PV, et al. A model of APL with FLT3 mutation is responsive to retinoic acid and a receptor tyrosine kinase inhibitor, SU11657. Blood. 2003;101:3188–3197. doi: 10.1182/blood-2002-06-1800. [DOI] [PubMed] [Google Scholar]
- 8.Kelly LM, Kutok JL, Williams IR, Boulton CL, et al. PML/RARalpha and FLT3ITD induce an APL-like disease in a mouse model. PNAS. 2002;99(12):8283–8288. doi: 10.1073/pnas.122233699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levis M, Allebach J, Tse KF, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–3891. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]