Abstract
Social interactions with conspecifics markedly alter the neuroendocrine, behavioral and emotional responses to stressful events. Some of these effects involve observational learning and result in lasting changes of fear-motivated behavior. While most evidence reveals increased fearfulness after observation of fearful demonstrators (models) in a number of species, a few reports from human and non-human primates indicate that observational learning can also attenuate some forms of fear. In the present study, we set out to determine the effects of social modeling and observational learning on fear conditioning in the mouse. Observers were pre-exposed to a novel context in the presence of fearful (F group) or non-fearful (NF group) demonstrators. Mice of the F group acquired control levels of conditioned fear. On the other hand, mice of the NF group exhibited profound and persistent reduction of fear. The decrease of fear in NF observers was most likely due to context-specific impairments of fear conditioning, as revealed by selective effects on long- but not short-term contextual fear memory, and normal fear conditioning in response to a novel context or cue. The effect was lasting, but constrained by the shock intensity. Attenuation of fear conditioning resulting from interactions with non-fearful conspecifics was largely, but not entirely, mediated by vicarious learning. These findings identify an important social buffering process serving to prevent a lasting induction of fear in response to isolated, moderately intense stressful events.
Keywords: social modeling, contextual fear conditioning, observational (vicarious) learning, social buffering, mice, anxiety, autism
1. Introduction
Fear conditioning is essential for entraining coping strategies in various stressful situations. Nevertheless, if fear responses become excessive and persistent they significantly contribute to the development of anxiety disorders [39]. Animal models have been successfully employed to elucidate the neurobiology of fear. Exposure of rodents to distinctive environments followed by a footshock typically generates context-specific conditioned fear on subsequent re-exposures. Such responses are triggered by fear memories and persist [12] or even gain intensity over time [16] unless regulatory mechanisms bring about the cessation of fear. Research focusing on fear regulation has identified some of the key processes, such as extinction [31, 36], serving to retroactively alleviate conditioned fear. However, the proactive regulation of fear conditioning remains less understood.
Emotional responses, including aversively-motivated behaviors, can be modeled by social factors. In the presence of non-fearful conspecifics, observers exhibit a significant decrease (social buffering) of fear-motivated behavior, as seen in cats [17, 26], rats [3, 7] and pigeons [14, 15]. On the other hand, in the presence of littermates experiencing pain, observers exhibit pain hypersensitivity [24]. Although reporting profound behavioral changes, most of these studies did not explore whether the effects of social interactions are limited to the presence of the demonstrator or involve learning processes causing lasting alterations of emotional behavior.
Notably, behavior can also be modeled proactively by observational (vicarious) learning [2]. Many species, including invertebrates, acquire emotionally neutral behaviors by observational learning [5, 10]. This process can also contribute to the acquisition of aversively-motivated behaviors [1, 19, 30, 35, 38], although not in all paradigms [6, 42]. Interestingly, unlike social modulation of pain hypersensitivity [24], observational learning is not limited to known conspecifics, and may be even intensified by observations of individuals of different strains [40].
Most studies involving fear-motivated paradigms have established that observation of fearful conspecifics induces similar behavior in otherwise non-fearful subjects [29, 30, 35]. The possibility of an opposite outcome of social interaction, i.e. attenuation of fear, has not been investigated in detail. Primate and human studies have provided supporting evidence for fear prevention by observational learning. Pre-exposure to monkeys behaving non-fearfully with snakes blocks subsequent fear learning [28]. Furthermore, observing positive maternal reactions can over-ride childrens’ fearful responses to relevant stimuli [8]. Due to the limited number of subjects and experimental manipulations possible for these types of studies, the key parameters constraining or enhancing social buffering of fear remain unknown.
The proactive effects of social interactions on fear conditioning have not been studied in detail with rodent models. Here we provide evidence for a marked, lasting and context-specific attenuation of conditioned fear induced by pre-exposure of observer mice to non-fearful models and report the role of observational learning in this process.
2. Materials and Methods
2.1 Animals and housing conditions
Nine-week old C57Bl/6N male mice were obtained from Harlan Laboratories. Mice were individually housed in a satellite facility adjacent to the behavioral equipment. The satellite facility was maintained at 20 ± 2°C and 40%–50% humidity under a 12/12 dark light cycle (7am–7pm). All studies were approved by the Animal Care and Use Committee at Northwestern University and are in compliance with the National Institute of Health standards.
2.2 Experimental Design
2.2.1 Experimental Apparatus
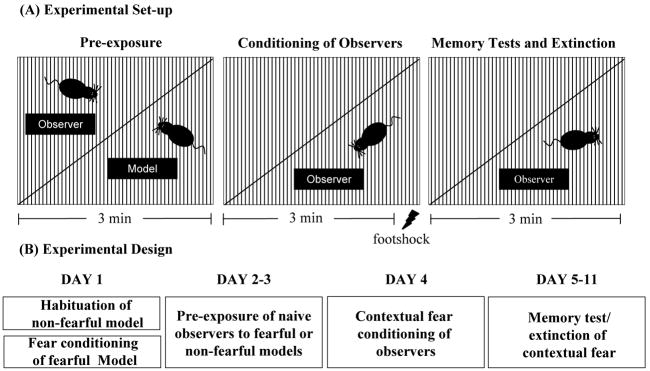
A conditioning chamber (35 cm long × 20 cm wide × 20 cm high) was bisected into a 350 cm2 area with a transparent or opaque Plexiglas partition (Fig. 1A). Animals were placed on either side of the box during pre-exposures. The Plexiglas wall prevented physical interactions but allowed for normal transmission of visual, olfactory and acoustic stimulus. The floor of the conditioning chamber consisted of removable shock grid made of stainless steel rods (diameter 4 mm; bars spaced 0.9 cm apart). The conditioning chamber was cleaned between each animal with 1% acetic acid. A second context (Context 2) consisted of an undivided Plexiglas chamber that was cleaned with 70% ethanol.
Figure 1.
Experimental set-up and design. (A) The conditioning chamber was divided in half by a transparent Plexiglas wall as illustrated above. During pre-exposure sessions naïve observer animals were placed in the left side of the chamber while a model animal occupied the right side of the chamber. Animals were left undisturbed during the 3 min of the pre-exposure session (left). Twenty four hrs after the last pre-exposure session observer animals were placed on the side of the chamber originally occupied by the model and fear conditioning was performed by a 3min exposure to the context followed by a single 2-s 0.7 mA footshock (middle). Short-term memory and long-term memory were examined 30 min or 24 hrs after contextual fear conditioning (right). (B) General time layout of the experimental design.
2.2.2 Experimental models
Model animals were housed as described above. There were two types of model animals: non-fearful and fearful (freezing). Fearful models were trained in contextual fear conditioning in the divided conditioning chamber 24 hrs before pre-exposures sessions. The contextual fear conditioning protocol consisted of a 3-min exposure to the context followed by a 2-s 0.7 mA footshock with constant current as described previously [27, 37]. Non-fearful models were habituated to the divided conditioning chamber for 90 min, 24 hrs prior to pre-exposure sessions. The purpose of habituating the models to the box was to induce non-fearful behavior and reduce arousal. During habituation of the non-fearful model, the conditioning chamber was cleaned every three minutes, in order to accustom the animals to the upcoming procedures.
2.2.3 Pre-exposure to model mice
Groups consisted of non pre-exposed controls (No-PE) or naïve observers pre-exposed to a fearful model (F group), non-fearful model (NF group), toy mouse (TM group), or box only (Box group). Pre-exposures were performed for two days and consisted of placing the observer animals and the model animals in the divided conditioning chamber for 3 min (Fig. 1A,B). After each pre-exposure the grid floor in the observer area and the underneath tray were cleaned with 1% acetic acid. A single animal was used as a non-fearful model for the NF group to minimize variability of individual interactions. Only comparisons between non-fearful and fearful models employed different animals for each pre-exposure (because a fearful mouse would extinguish fear if used repeatedly) [41]. Animals in the TM or Box groups were pre-exposed to a toy mouse or to an empty context, respectively. The non-observational group was separated from the non-fearful models by an opaque partition. Description of all groups is summarized in Table 1.
Table 1.
Summary of the pre-exposure conditions for the different groups
| Group | Pre-exposure* | Pre-exposure | Conditioning | Test |
|---|---|---|---|---|
| No-PE | - | - | Context 1 | Context 1 (24 hrs) |
| NF | non-fearful | non-fearful | “ | “ |
| F | fearful | fearful | “ | “ |
| TM | toy | toy | “ | “ |
| Box | box | box | “ | “ |
| NF non-observational | non-fearful** | non-fearful** | “ | “ |
All pre-exposures were performed in Context 1.
Pre-exposure was done with an opaque wall in order to avoid observation of demonstrator
2.2.4 Contextual Fear Conditioning Training and Extinction for Observers
Contextual fear conditioning was performed with an automated system (TSE Inc.) 24 hrs after the last pre-exposure session. During fear conditioning observer animals were placed on the side of the box originally occupied by the model and trained as described above for F models (Fig. 1A). The fear response was determined by scoring freezing behavior (defined as lack of movement except that needed for respiration) every 10th second for 3 min by a trained observer and expressed as a percentage of total number of observations. Short-and long-term memory tests were performed 30 min and 24 hrs after training, respectively. Extinction was carried out daily by exposing the mice to the context for 3 min without the footshock. Once animals reached extinction criteria (freezing significantly lower than on extinction day 1) they were exposed to a shock reminder and subsequently tested for reinstatement of fear. Twenty-four hours after the last extinction day animals received a footshock reminder (2-s 0.7 mA) immediately after placement in a novel context and were removed 10 s later. The effect of the footshock reminder was assayed after 24 hrs. The immediate shock paradigm was used to test for reinstatement of fear [11] because it serves as a shock reminder but fails to induce additional fear conditioning [9].
2.2.5 Statistical Analysis
One-way ANOVA was used for group comparisons. Repeated measure ANOVA (Test) with Group as an additional factor was used for analysis of fear extinction. Fisher’s LSD test was employed for post-hoc comparisons. When required, a two-way ANOVA was used in order to analyze interaction between variables. Statistically significant differences are marked as *p < 0.05 **p < 0.01 and ***p < 0.001.
3. Results
3.1 Modeling of fear conditioning by pre-exposure to fearful or non-fearful conspecifics
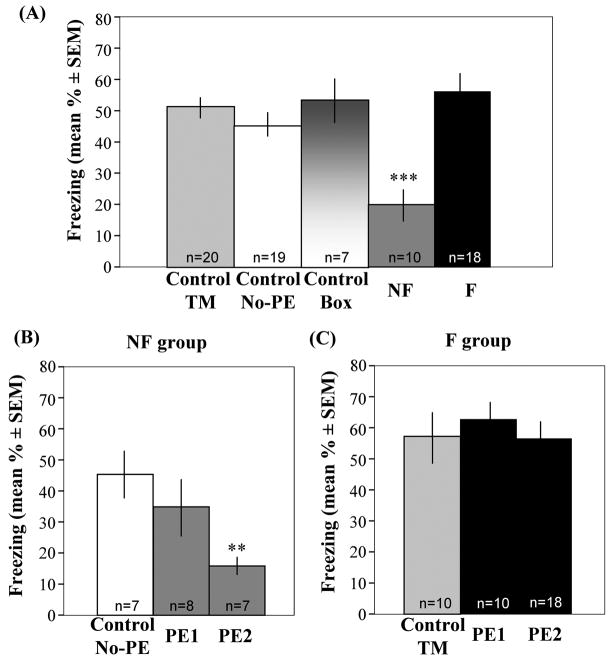
Pilot experiments revealed no significant differences between the control groups (No-PE, TM, and Box; Fig. 2A). Therefore, one of these groups was randomly employed as a control in subsequent experiments. None of the experimental groups in this study showed freezing prior to contextual fear conditioning (supplementary Fig. 1), therefore only data obtained after training are presented throughout the report. In the first experiment, the effect of social interactions on contextual fear conditioning was examined. There were no significant differences between the F and control groups (Fig. 2A, 2C), suggesting that pre-exposure to a fearful model did not enhance fear responses to the context. NF observers, on the other hand, showed a significant decrease of freezing when compared to F and control groups [Fig. 2A; F(4,91) = 6.24, p < 0.001]. This robust decrease of freezing behavior was not due to latent inhibition given that the Box and TM groups showed similar levels of freezing as the No-PE group (Fig. 2A). Furthermore, this social buffering effect was modality-specific as revealed by the lack of an effect in animals trained with tone-dependent fear conditioning (supplementary Fig. 2).
Figure 2.
Proactive modulation of fear conditioning after exposures to fearful and non-fearful models. (A) The NF group exhibited a significant reduction of fear when compared to all other groups. This was not due to latent inhibition as revealed by similar freezing levels among the No-PE, Box and TM groups. Statistically significant differences: ***p < 0.001, NF vs F, No-PE, Box and TM groups. (B) Two brief trials of social interactions were required for modulation of fear in the NF but not (C) F groups of mice when compared to TM controls.
A minimum of two pre-exposures was needed in order to observe a consistent protective effect in the NF group [F(2,21) = 5.56, p = 0.013]. Although some subjects showed this protective effect with only one trial, the within-group variability was rather large (Fig. 2B).
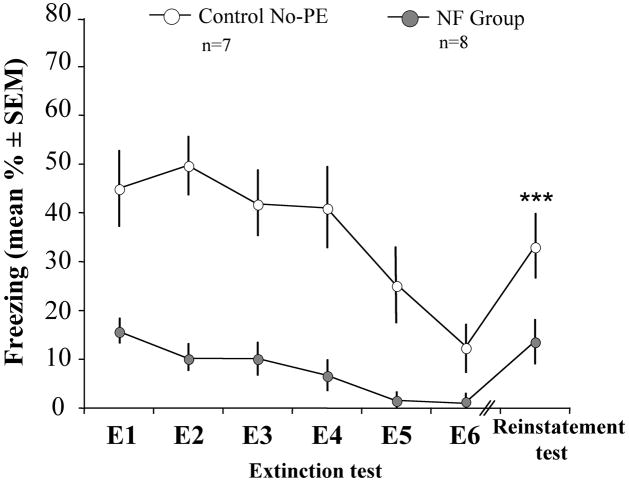
To examine whether mice in the NF group possibly experienced a temporary retrieval deficit, mice underwent extinction training until extinction criterion was reached [Fig. 3; F(5,65) = 22.98, p < 0.001], and subjected to a reminder shock 24 hrs later. Both groups showed a significant increase of freezing on a subsequent memory test when compared to the last extinction training [Fig. 3; F(1,13) = 35.08, p < 0.001], however there were no significant differences between reinstatement and the first memory test (E1). The NF group froze significantly less when compared to No-PE controls [F(1,14) = 7.25, p = 0.018] revealing an impairment of contextual fear memory formation.
Fig 3.
Pre-exposure to non-fearful models impaired the formation of contextual fear memory. The NF group showed persistent reduction of fear after training, extinction and a shock reminder. On the reinstatement test there was a significant increase of freezing when compared to the sixth extinction trial (E6 ; ***p < 0.001), but not to the first extinction test (E1). In spite of the increase of freezing after the shock reminder, the NF group still showed a significantly lower freezing when compared to the No-PE group (*p = 0.018).
3.2 Effects of social buffering on the formation of short and long-term contextual fear memory
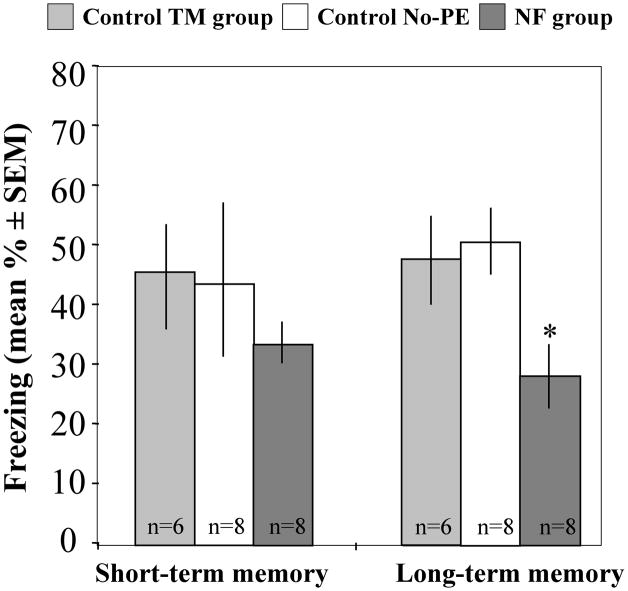
In order to examine whether pre-exposure to a non-fearful model affects the general emotionality or involves a learning process, we next compared the behavior of the NF group in short- and long-term memory tests. Mice were pre-exposed and trained as described above. Memory tests were performed 30 min and 24 hrs after fear conditioning. One-Way ANOVA analysis revealed a significant reduction of freezing 24 hrs [Fig. 4; F(2,21) = 4.53, p = 0.025] but not 30 min after fear conditioning [Fig. 4; F(2,21) = 0.64, p = 0.535]. However, repeated measures ANOVA did not show a significant Group × Time interaction [F(2,19) = 0.70, p = 0.508], suggesting that lack of effect at the 30 min memory test might have been due to the high variance of the control group. The consistent effects on long-term memory suggested that cognitive rather than emotional factors mainly contributed to the behavioral effects seen in the NF group.
Fig 4.
Interactions with non-fearful mice consistently impair long-term memory. The NF group tested 30 min after training did not show significant reduction of freezing when compared to the TM or No-PE groups (p = 0.535). When the same NF observers where tested 24 hrs later, freezing levels were significantly lower than in the TM and No-PE groups (*p = 0.025).
3.3 Contextual specificity and duration of the social buffering effect
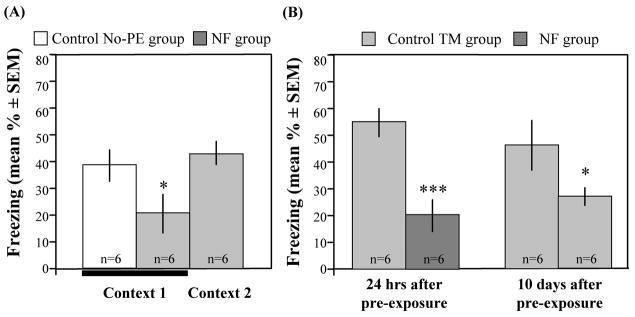
To provide evidence for the specificity of the social buffering effect, we used two NF groups of mice. One group was trained and tested in the pre-exposure context (Context 1) whereas another group was trained and tested in a different context (Context 2). Consistent with the idea that social modeling of fear is based on a learning process, the NF group exhibited significantly lower freezing in Context 1 [F(2,19) = 5.01, p = 0.019], but normal freezing levels in Context 2 when compared to their controls (Fig 5A).
Fig 5.
Specificity and duration of social buffering of fear. (A) The NF group showed freezing deficits after training and testing in the pre-exposure context (Context 1) when compared to the No-PE group or NF group trained and tested in a novel context (Context 2; *p = 0.019). (B) The significant reduction in freezing seen after pre-exposure to a non-fearful model occurred in animals trained 24 hrs (***p = 0.001) or 10 days (*p = 0.046) after the social interactions.
Furthermore the impairments of contextual fear were seen when social interactions and training were spaced by 10 days [Fig. 5B; F(1,15) = 4.82, p = 0.046]. These results revealed a lasting protective effect of social interactions that might involve association of non-fearful models with the environmental context where these interactions have taken place.
3.4 Social buffering effects are constrained by the footshock intensity
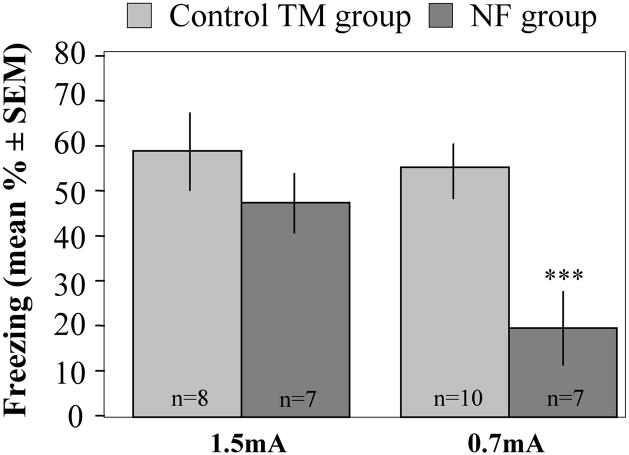
In order to further characterize fear conditioning in the NF group, mice were trained with 0.7 or 1.5 mA footshocks. Two-way ANOVA revealed a significant Group × Intensity interaction [F(1, 32), p = 0.032]. Post-hoc analysis revealed a significant decrease of freezing in the NF group trained with 0.7 mA footshock [F(1,16) = 16.8, p = 0.001], but not with 1.5 mA footshock resulting in similar freezing levels between the NF and TM groups (Fig. 6). Thus, stressors of high intensity may over-ride social buffering of conditioned fear.
Fig 6.
Effect of shock intensity on social buffering of fear. The NF group trained with a footshock stimulus of 1.5 mA did not show reduced freezing when compared to No-PE animals trained with the same current intensity. NF observers that received a 0.7 mA footshock showed a significant reduction of freezing (***p = 0.001) versus their TM controls.
3.5 A role for observational learning in social modeling of fear
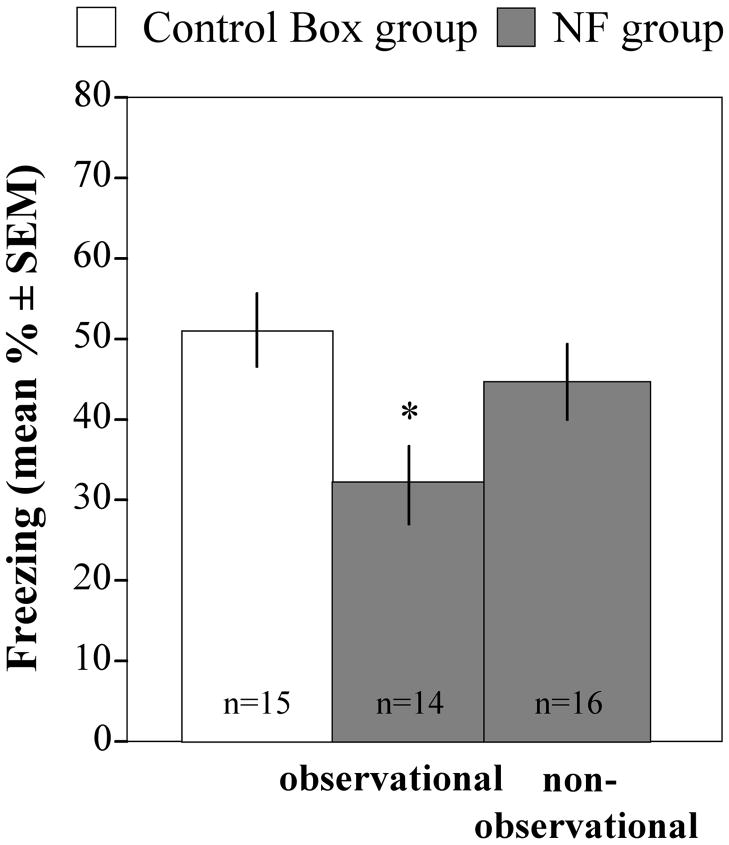
Social modeling of fear conditioning can occur via a variety of sensory modalities, which include but are not limited to observation, ultrasonic vocalizations or odors. To isolate the role of observation, we introduced an additional NF group (non-observational) that was pre-exposed to a non-fearful model in the presence of an opaque partition. One-way ANOVA revealed a significant group difference between Box, NF observational and the NF non-Observational Groups [Fig. 7; F(2,44) = 3.99, p = 0.026]. Post-hoc tests showed that freezing in the NF observational group was significantly lower from the Box group (p = 0.008). The NF non-observational group, on the other hand, exhibited similar freezing as the Box group (Fig. 7; p = 0.327) suggesting that observation was an important sensory modality mediating social buffering of fear. However, other sensory stimuli could also contribute given that freezing of the NF non-observational and NF groups did not differ significantly from one another (Fig. 7; p = 0.071).
Fig 7.
Role of observational learning in social buffering of fear. Mice of the NF group were pre-exposed to non-fearful models in the presence of a transparent (observational) or opaque (non-obsearvational) partition. One-way ANOVA revealed a significant decrease of freezing in the NF group pre-exposed with a transparent (*p = 0.026) but not an opaque partition when compared to the control Box group.
4. Discussion
In the present study we demonstrated significant social modeling of fear in observer mice pre-exposed to non-fearful demonstrators. About 84% of all animals of the NF group exhibited markedly lower freezing levels when compared to the control groups. Whereas primate experiments reveal similarly strong modeling effects by both fearful and non-fearful demonstrators [28, 29], this mouse study uncovered a more profound impact of interactions with non-fearful conspecifics. Possibly, the effectiveness of fearful models may be limited to familiar conspecifics [24].
It is important to note that adequate non-fearful demonstrators were only those mice that were explicitly habituated to the context prior to pre-exposure with the observers. Non-habituated mice, despite showing high exploratory activity and absence of freezing, did not attenuate observers’ fear conditioning (supplementary Fig. 3). This suggests that learned safety, rather than the mere lack of fear, represented the main modeling stimulus for observer mice. Additionally, the effects were more consistent when observers were exposed to a single habituated demonstrator, possibly because variability of individual social interactions was minimized. In line with observational learning studies [19, 28, 38], but unlike pain-related behaviors [24], buffering of fear was not limited to known conspecifics.
The robust decrease of freezing behavior was consistently found with two pre-exposures to the non-fearful model in the training context. One pre-exposure may be insufficient due to counteracting effects of novelty and social interactions. Alternatively, stable encoding of social buffering signals could require multiple reinforcements [28].
The presented findings suggested that learning processes contributed to the main effect in the NF group. Along with the predominant attenuation of long-versus short-term fear, the impairment of fear conditioning showed high specificity for the context where social interactions had previously taken place, but not to contexts or cues that have not been associated with safety signals. Moreover, this effect lasted for at least 10 days. Thus, contrary to the acute or short-term effects on neuroendocrine mechanisms, such as activity of the hypothalamo-pituitary-adrenal axis or behaviors occurring during social interactions [21, 23], social modeling exhibited a relatively lasting effect on fear conditioning. Importantly, pre-exposure to non-fearful demonstrators was ineffective under conditions employing a footshock of double intensity, suggesting that the fear responses can be acquired normally during strong stressful experiences. The stress intensity and contextual specificity were identified as important constraints of social buffering that may be essential for the development of adequate coping strategies in response to different environmental stressors.
A number of acoustic, olfactory or visual stimuli [13, 18, 20, 34, 43, 44] may contribute to learning process underlying the effect in the NF group. By introducing a non-transparent partition between observers and demonstrators, we showed that vicarious learning was the most likely process mediating resistance to fear conditioning in the present paradigm. However, the freezing response of the non-observational group was not fully restored to control freezing levels, suggesting that other forms of social communication may additionally take place. A role for olfactory stimuli is unclear at this time, given that pheromone-mediated social buffering was recently shown to be transmitted by fearful rather than non-fearful demonstrators [4].
Taken together the presented data demonstrate that observational learning from experienced non-fearful conspecifics markedly buffers the conditioning of fear. This process may confer an evolutionary advantage in coping with stressors by preventing robust fear conditioning in response to isolated and moderately stressful events. The findings have important implications for the regulation of normal fear responses, as well as the development of pathological fear and anxiety. For example, molecular mechanisms and brain areas thought to be specific for social learning [25, 32], are likely to interact with memory systems underlying contextual fear conditioning [22, 33]. Therefore, deficits of social buffering may be an important contributing factor to excessive fear and anxiety seen in a number of psychiatric disorders, ranging from post-traumatic stress disorder to autism.
Acknowledgments
This research was supported by the National Institute of Mental Health (MH-078064 and Dunbar Funds to JR) and by The CLIMB Program (NIGMS# R25 GM079300-02 to YG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yomayra F. Guzman, Department of Psychiatry and Behavioral Sciences, Northwestern University, Feinberg School of Medicine, 303 E Chicago Ave, Ward 9-268, Chicago, IL 60611, E-mail: yguzman@u.northwestern.edu
Natalie C. Tronson, Department of Psychiatry and Behavioral Sciences, Northwestern University, Feinberg School of Medicine, 303 E Chicago Ave, Ward 9-268, Chicago, IL 60611, E-mail: n-tronson@northwestern.edu
Anita Guedea, Department of Psychiatry and Behavioral Sciences, Northwestern University, Feinberg School of Medicine, 303 E Chicago Ave, Ward 9-268, Chicago, IL 60611, E-mail: a-guedea@northwestern.edu.
Kyu Hwan Huh, Department of Psychiatry and Behavioral Sciences, Northwestern University, Feinberg School of Medicine, 303 E Chicago Ave, Ward 9-268, Chicago, IL 60611, E-mail: kyuhuh@u.northwestern.edu.
Can Gao, Department of Psychiatry and Behavioral Sciences, Northwestern University, Feinberg School of Medicine, 303 E Chicago Ave, Ward 9-268, Chicago, IL 60611, E-mail: can-gao@northwestern.edu
Jelena Radulovic, Department of Psychiatry and Behavioral Sciences, Northwestern University, Feinberg School of Medicine, 303 E Chicago Ave, Ward 9-221, Chicago IL, 60611, Phone: (312) 503-4627, Fax: (312) 503-0466, E-mail: j-radulovic@northwestern.edu
Bibliography
- 1.Arai T, Tominaga O, Seikai T, Masuda R. Observational learning improves predator avoidance in hatchery-reared Japanese flounder Paralichthys olivaceus juveniles. J Sea Res. 2007;58:59–64. [Google Scholar]
- 2.Bandura A. Vicarious and self-reinforcement processes. New York Holt: Rinehart & Winston; 1969. [Google Scholar]
- 3.Baum M. Extinction of an avoidance response motivated by intense fear: Social facilitation of the action of response prevention (flooding) in rats. Behaviour Research and Therapy. 1969;7:57–62. doi: 10.1016/0005-7967(69)90049-7. [DOI] [PubMed] [Google Scholar]
- 4.Bredy TW, Barad M. Social modulation of associative fear learning by pheromone communication. Learn Mem. 2009;16:12–18. doi: 10.1101/lm.1226009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlier P, Jamon M. Observational learning in C57BL/6j mice. Behavioural Brain Research. 2006;174:125–31. doi: 10.1016/j.bbr.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Church RM. Emotional reactions of rats to the pain of others. Journal of Comparative and Physiological Psychology. 1959;52:132–34. doi: 10.1037/h0043531. [DOI] [PubMed] [Google Scholar]
- 7.Davitz JR, Mason DJ. Socially Facilitated Reduction of a Fear Response in Rats. Journal of Comparative and Physiological Psychology. 1955;48:149–51. doi: 10.1037/h0046411. [DOI] [PubMed] [Google Scholar]
- 8.Egliston K-A, Rapee RM. Inhibition of fear acquisition in toddlers following positive modelling by their mothers. Behaviour Research and Therapy. 2007;45:1871–82. doi: 10.1016/j.brat.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Fanselow MS. Associative vs Topographical Accounts of the Immediate Shock-Freezing Deficit in Rats: Implications for the Response Selection Rules Governing Species-Specific Defensive Reactions. Learning and Motivation. 1986;17:16–39. [Google Scholar]
- 10.Fiorito G, Scotto P. Observational Learning in Octupus Vulgaris. Science. 1992;256:545–47. doi: 10.1126/science.256.5056.545. [DOI] [PubMed] [Google Scholar]
- 11.Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct Roles of Hippocampal De Novo Protein Synthesis and Actin Rearrangement in Extinction of Contextual Fear. J Neurosci. 2004;24:1962–66. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–15. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez-Garcia AG, Contreras CM, Mendoza-Lopez MR, Garcia-Barradas O, Cruz-Sanchez JS. Urine from stressed rats increases immobility in receptor rats forced to swim: role of 2-heptanone. Physiol Behav. 2007;91:166–72. doi: 10.1016/j.physbeh.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Hake DF, Laws DR. Social facilitation of responses during a stimulus paired with electric shock. J Exp Anal Behav. 1967;10:387–92. doi: 10.1901/jeab.1967.10-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hake DF, Powell J, Olsen R. Conditioned Suppression as a Sensitive Baseline for Social Facilitation. Journal of the Experimental Analysis of Behavior. 1969;12:807–16. doi: 10.1901/jeab.1969.12-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of Age on the Generalization and Incubation of Memory in the F344 Rat. Learning and Memory. 1999;6:111–19. [PMC free article] [PubMed] [Google Scholar]
- 17.John ER, Chesler P, Bartlett F, Victor I. Observation Learning in Cats. Science. 1968;159:1489–91. doi: 10.1126/science.159.3822.1489. [DOI] [PubMed] [Google Scholar]
- 18.Kavaliers M, Choleris E, Agmo A, Pfaff DW. Olfactory-mediated parasite recognition and avoidance: linking genes to behavior. Hormones and Behavior. 2004;46:272–83. doi: 10.1016/j.yhbeh.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Kavaliers M, Choleris E, Colwell DD. Learning from others to cope with biting flies: social learning of fear-induced conditioned analgesia and active avoidance. Behavioral Neuroscience. 2001;115:661–74. [PubMed] [Google Scholar]
- 20.Kikusui T, Takigami S, Takeuchi Y, Mori Y. Alarm pheromone enhances stress-induced hyperthermia in rats. Physiol Behav. 2001;72:45–50. doi: 10.1016/s0031-9384(00)00370-x. [DOI] [PubMed] [Google Scholar]
- 21.Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philosophical Transactions of the Royal Society. 2006;361:2215–28. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.KIm J, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–77. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 23.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner’s Stress Status Influences Social Buffering Effects in Rats. Behavioral Neuroscience. 2004;118:798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- 24.Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, et al. Social Modulation of Pain as Evidence for Empathy in Mice. Science. 2006;312:1967–70. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 25.Lee H-J, Caldwell HK, Macbeth AH, Tolu SG, 3rd, WSY A Conditional Knockout Mouse Line of the Oxytocin Receptor. Endocrinology. 2008;149:3256–63. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Measserman J. Behavior and Neurosis. 1943 [Google Scholar]
- 27.Milanovic S, Radulovic J, Laban O, Stiedl O, Henn F, Spiess J. Production of the Fos protein after contextual fear conditioning of C57BL/6N mice. Brain Research. 1998;784:37–47. doi: 10.1016/s0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- 28.Mineka S, Cook M. Immunization against the observational conditioning of snake fear in rhesus monkeys. J Abnorm Psychol. 1986;95:307–18. doi: 10.1037//0021-843x.95.4.307. [DOI] [PubMed] [Google Scholar]
- 29.Mineka S, Cook M. Mechanisms involved in the observational conditioning of fear. J Exp Psychol Gen. 1993;122:23–38. doi: 10.1037//0096-3445.122.1.23. [DOI] [PubMed] [Google Scholar]
- 30.Mineka S, Davidson M, Cook M, Keir R. Observational conditioning of snake fear in rhesus monkeys. J Abnorm Psychol. 1984;93:355–72. doi: 10.1037//0021-843x.93.4.355. [DOI] [PubMed] [Google Scholar]
- 31.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2006;12:120–50. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 32.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin Attenuates Affective Evaluations of Conditioned Faces and Amygdala Activity. The Journal of Neuroscience. 2008;28:6607–15. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips R, LeDoux J. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 34.Portfors CV. Types and Functions of Ultrasonic Vocalizations in Laboratory Rats and Mice. Journal of the American Association for Laboratory Animal Science. 2007;40:28–34. [PubMed] [Google Scholar]
- 35.Presley W, Riopelle A. Observational learning of an avoidance response. J Genet Psychol. 1959;95:251–54. doi: 10.1080/00221325.1959.10534265. [DOI] [PubMed] [Google Scholar]
- 36.Quirk GJ, Mueller D. Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology. 2007;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radulovic J, Kammermeier J, Spiess J. Relationship between Fos Production and Classical Fear Conditioning: Effects of Novelty, Latent Inhibition, and Unconditioned Stimulus Preexposure. The Journal of Neuroscience. 1998;18:7452–61. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riess D. Vicarious conditioned acceleration: Succesful observational learning of an aversive pavlovian stimulus contingency. Journal of the Experimental Analysis of Behavior. 1972;18:181–86. doi: 10.1901/jeab.1972.18-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothbaum BO, Davis M. Applying Learning Principles to the Treatment of Post-Trauma Reactions. Annals of the New York Academy of Sciences. 2003;1008:112–21. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 40.Saggerson AL, Honey RC. Observational learning of instrumental discriminations in the rat: the role of demonstrator type. Q J Exp Psychol (Colchester) 2006;59:1909–20. doi: 10.1080/17470210600705032. [DOI] [PubMed] [Google Scholar]
- 41.Waddell J, Dunnett C, Falls WA. C57BL/6J and DBA/2J mice differ in extinction and renewal of extinguished conditioned fear. Behavioural Brain Research. 2004;154:567–76. doi: 10.1016/j.bbr.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 42.White DJ, Jr, BGG Social influence on avoidance of dangerous stimuli by rats. Animal Learning and Behavior. 1998;26:433–38. [Google Scholar]
- 43.Williams WO, Riskin DK, Mott KM. Ultrasonic Sound as an Indicator of Acute Pain in Laboratory Mice. Journal of the American Association for Laboratory Animal Science. 2008;47:8–10. [PMC free article] [PubMed] [Google Scholar]
- 44.Zalaquett C, Thiessen D. The effects of odors from stressed mice on conspecific behavior. Physiol Behav. 1991;50:221–7. doi: 10.1016/0031-9384(91)90524-r. [DOI] [PubMed] [Google Scholar]