Abstract
Both the retrosplenial cortex (RSC) and the hippocampus are important for spatial learning across species. Although hippocampal acetylcholine (ACh) release has been associated with learning on a number of spatial tasks, relatively little is understood about the functional role of ACh release in the RSC. In the present study, spatial exploration was assessed in rats using a plus-maze spontaneous alternation task. ACh efflux was assessed simultaneously in the hippocampus and two sub-regions of the RSC (area 29ab and area 30) before, during and after maze exploration. Results demonstrated that there was a significant rise in ACh efflux in RSC area 29ab and the hippocampus during maze traversal. The rise in ACh efflux across these two regions was correlated. There were no significant behaviorally driven changes in ACh efflux in RSC area 30. While both the hippocampal sectors and area 29ab displayed increases in ACh efflux during maze exploration, the percent ACh rise in area 29ab was higher than that observed in the hippocampus and persisted into the post-baseline period. Joint efflux analyses demonstrated a key functional role for ACh release in area 29ab during spatial processing.
Keywords: rat, microdialysis, cortex, spontaneous alternation
1. Introduction
The retrosplenial cortex (RSC) is important for spatial processing and navigation through space [14, 61, 72, 80]. The neuroanatomical connectivity of the RSC includes extensive reciprocal connections with areas integral for spatial cognition—including the hippocampal formation, anterior nuclei of the thalamus, and various neocortical sites mediating perceptual processes that assist in spatial coding and accurate navigation [57, 67, 79]. As such, the RSC may serve as a crucial junction of information flow that holds the capacity to not only mediate the transmission of signals between the hippocampal formation and neocortical sites, but to also participate in the functional integration of varying types of spatial information including visual and self motion cues.
Electrophysiological investigations of cell activity within the RSC have revealed the presence of cells displaying strong spatial and movement-related correlates [10, 11, 12]. There are head direction cells and place cells within the RSC, as well as other neurons that demonstrate response patterns to a mixture of place, angular velocity and running speed [12]. Furthermore, Cooper and Mizumori [13] found that temporary inactivation of the RSC lead to transient shifts in hippocampal place cell responsivity that resulted in place cells firing outside of their preferred location during a radial arm maze. This demonstrates a direct functional interrelationship between signals derived from the RSC and localization processes in the hippocampus. Therefore, the RSC provides key information to the hippocampal complex about distal sensory information and self motion cues that are necessary for certain mnemonically guided navigational strategies, such as path integration and allocentric memory processes [13, 14, 41, 61, 72].
The RSC is composed of four cytoarchitectonical areas: granular areas 29 a–c and dysgranular area 30 [79]. Furthermore, these areas display unique patterns of connectivity including dense and reciprocal connections with the visual cortex that may underlie different facets of cognitive function [57, 67, 70, 74, 79, 83]. Spatial memory performance was found to be differentially affected as a function of selective lesions to either retrosplenial granular b (area 29b) or retrosplenial granular a (area 29a). Damage confined to area 29b, but not 29a, resulted in select spatial deficits on an open field water maze task, although concomitant destruction of both regions tends to result in greater impairment [71]. This finding concurs with anatomical studies demonstrating higher connectivity between area 29a and structures integral for spatial coding including the parietal cortex, dorsal subiculum, and various thalamic nuclei [82, 83]. Additionally, ablation of area 30 increased reliance upon egocentric cues during a radial arm maze task, suggesting impaired utilization of distal visual cues and allocentric memory processes [72]. Thus, the organization of the RSC may reflect a diversity of function, with each area uniquely contributing to spatial cognition.
The RSC, like the hippocampus, receives its primary cholinergic input from the basal forebrain. Cholinergic cells in the rat forebrain are organized into 4 subdivisions (Ch1–4) that have different innervation patterns: Ch1 (medial septal area [MS]) and Ch2 (diagonal band [DB]) innervate the hippocampus and surrounding cortical areas; Ch3 innervates the olfactory bulb, whereas Ch4 innervates the neocortex and amygdala [16]. Amaral and Kurz [3] further noted three distinct pathways within the MS/DB cholinergic projection system (dorsal, ventral, and intermediate). The dorsal group innervates the ventral hippocampus, whereas the ventral group innervates the dorsal hippocampus. In contrast, the intermediate group innervates the cingulate cortex—without providing hippocampal connections. Recent evidence suggests that the RSC receives greater innervation from Ch2 over Ch1 [27]. In addition, the RSC receives abundant cholinergic innervations from the nucleus basalis of Meynert [5, 17, 24, 46, 56]
To date the evidence for the functional involvement of ACh in the RSC comes from lesion and pharmacological studies. These studies found that disruption of the septo-retrosplenial cholinergic pathway, via transection of the fornix and cingulate pathway, led to a nearly complete loss of choline acetyltransferase (ChAT) positive nerve terminals and major reductions of high affinity choline uptake levels (HACU) in the RSC that corresponded with significant impairments on several spatial memory related tasks [37, 38, 39]. Subsequent transplantations of fetal septal neurons into the RSC attenuated the spatial deficits that initially followed retrosplenial deafferentation [39]. Furthermore, it has been demonstrated that intracerebral infusions of scopolamine, a muscarinic ACh antagonist, into the RSC before behavioral training impaired spatial learning during a water maze task [51]. These observations suggest that cholinergic activity in the RSC is critical for spatial memory function and navigational skills.
Despite growing evidence for a functional role of ACh in the RSC, surprisingly little is known about dynamic fluctuations in ACh release in the RSC when spatial information is being processed. In contrast, numerous studies have demonstrated that changes in hippocampal ACh efflux are related to spatial learning and navigation [19, 20, 25, 54]. In vivo ACh efflux has proven to be a neurochemical marker of regional activation and is associated with attention and memory-related processes [25, 53, 54, 55]. Thus, if ACh is critical for processing spatial information within the RSC circuit, changes as a function of spatial demands should be observed.
The goal of the present study was to assess and compare and relate the profiles of ACh efflux in both area 29 ab and area 30 to hippocampal ACh efflux during a spontaneous alternation task. Performance on the spontaneous alternation is not a direct measure of spatial memory per se; however, spontaneous alternation performance is related to spatial memory ability [34, 35]. Appropriate alternation requires a rat to discriminate between arms on the basis of which arms were most recently visited. Such alternation behavior has been described to be a memory-dependent response to the environment driven by a natural tendency toward novel locations [34]. Furthermore, spontaneous alternation is affected by brain manipulations that alter memory performance on other tasks (lesions of the hippocampus, medial septum, cholinergic drugs, etc.—see 34, 35) and by environmental manipulations of cues within and external to the maze [36]. Given that the RSC has multiple sources of cholinergic innervation and a greater connectivity with areas processing visual and perceptual spatial information, we expected that the RSC—in particular area 29ab—would display greater changes in ACh efflux relative to the hippocampus during spatial exploration. Such data would support the idea the RSC plays a significant role in spatial navigation.
2. Methods
2.1 Subjects
Male Sprague-Dawley rats (3–4 months; 250–300 g) were used as subjects in this study. They were housed one per cage with unlimited access to water and Purina rodent chow in a colony room with a 12-hour/12-hour light-dark cycle (onset at 7:00 am). Each subject was implanted with two plastic cannulae (CMA/11 mm, Carnegie Medicine Associates, Chelmsford, MA) aimed at the hippocampus or the RSC via a stereotaxic apparatus (David Kopf Instruments, USA). Half the rats had a cortical cannulae aimed to transect area 29ab and the other half had cortical cannulae aimed at area 30. Prior to surgery, animals were anesthetized with an i.p. injection (0.1 ml/kg) of a ketamine (83 mg/kg)/Xylazine (17 mg/kg) mixture. Hemispheric placement (L, R) of cannulae was counterbalanced across subjects. One cannula (CMA/11) was lowered into either the left or right hippocampus (5.0 mm posterior to Bregma, 5.0 mm lateral to the midline, and 4.2 mm DV) and another guide cannula was lowered into either area 29ab (6.3 mm AP, 1.0 mm lateral to the midline, and 1.25 mm DV) or area 30 (6.7 mm AP, 1.2 mm lateral to the midline, and 1.20 mm DV) of the retrosplenial cortex in the left or right RSC according to the atlas of Paxinos and Watson [40]. After surgery animals were allowed a four day recovery period followed by five days of handling (5 min/day) prior to behavioral testing. All rats were fasted the night before behavioral testing.
2.2 In vivo microdialysis and behavioral testing
Nine days following surgery two microdialysis probes (CMA/11, 3 mm for the hippocampus, CMA/11, 2 mm for area 29ab of the RSC or CMA/11, 1 mm for area 30 of the retrosplenial cortex) were inserted into the guide cannulae. The depth of the structure determined the length of the probe used in the region. The probes were connected to plastic tubing and driven by a microinfusion system (CMA/100 pump). The dialysis probes were perfused continuously at a rate of 2.0 μl/min with artificial CSF (in mM: 128 NaCL, 2.5 KCL, 1.3 CaCL, 2.1 MgCL, NaHPO, and 1.0 glucose, brought to a pH of 7.4), which contained the acetylcholinesterase inhibitor neostigmine (500nM). Prior to maze testing, the microdialysis probes were inserted into the hippocampal and retrosplenial cannulae (either area 29ab or area 30) and the animal was placed into the holding cage (41 cm × 30 cm × 35 cm) located in the testing room. After 60 min of stabilization, dialysis samples (sample volume 12 μl) were collected every 6 min for a period of 18 min in the holding cage to determine basal levels of ACh in awake rats. During this initial baseline phase, the animal was free to move about the holding cage. After initial exploration, most animals sat in a corner and occasionally groomed. After 3 baseline samples were collected, the rat was gently picked up and placed on the center of the maze. The plus maze used for behavioral testing was made of wood with clear Plexiglas sidewalls (12 cm high) and a painted black floor with the four arms of equal distance (55 cm) was used for training. It was elevated 80 cm from the floor. The rat was allowed to transverse the maze freely for an 18 min period, the number and sequence of arms entered were recorded to determine alternation scores.
An alternation score was determined by recording the number of arms (minimum = 1, maximum =4) entered during a 4-choice sequence, dividing the number of different arms entered by 4 and multiplying that fraction by 100. The averaged probability of making certain behavioral choices is listed in Table 1. The maze testing room contained various extramaze cues (posters, doors, tables, etc). Upon completion of 18 min of maze testing, rats were transferred back to the holding cage for an additional 18 min and dialysate samples were collected throughout this period (post baseline).
Table 1.
The random probability of making certain alternation patterns in a four-choice sequence
| Response Pattern | Number of Permutations | Probability |
|---|---|---|
| 1111 | 24 | 0.093 |
| 0211 | 144 | 0.56 |
| 0022 | 72 | 0.28 |
| 0031 | 12 | 0.46 |
| 0004 | 4 | 0.015 |
The number 0 in the response pattern column denotes that the rat did not an arm.
Dialysate samples were assayed for ACh using High Performance Liquid Chromatography (HPLC) with electrochemical detection (Bio Analytic Systems, West Lafayette, IN). The system included an ion-exchange microbore analytical column, a microbore ACh/Ch immobilized enzyme reactor containing acetylcholinesterase and choline oxidase, and perioxidase wired working electrode. The detection limit of this system is 10 fentomols. ACh peaks were quantified by comparison to peak heights of standard solutions (ACh [Sigma-Aldrich, St Louis, MO] at 100 and 20nM) and corrected for in vitro recovery of the probe.
2.3 Histology
After completion of the behavioral testing, animals were anesthetized with 0.5 mg/kg, i.p. injection of Sleep-Away (26% sodium pentobarbital in 7.8% isopropyl alcohol and 20.7% propylene glycol solution; Fort Dodge, Iowa). Animals’ brains were removed, post fixed in a 10% formalin solution for at least 72 h and then transferred to a 30% sucrose solution. Coronal sections from the brains were cut (60 μm thick) on a sliding microtome (Sm2000r; Lecia Instruments, Germany) and later stained with cresyl violet for assessment of probe location.
3. Results
3.1 Cannulae Placements
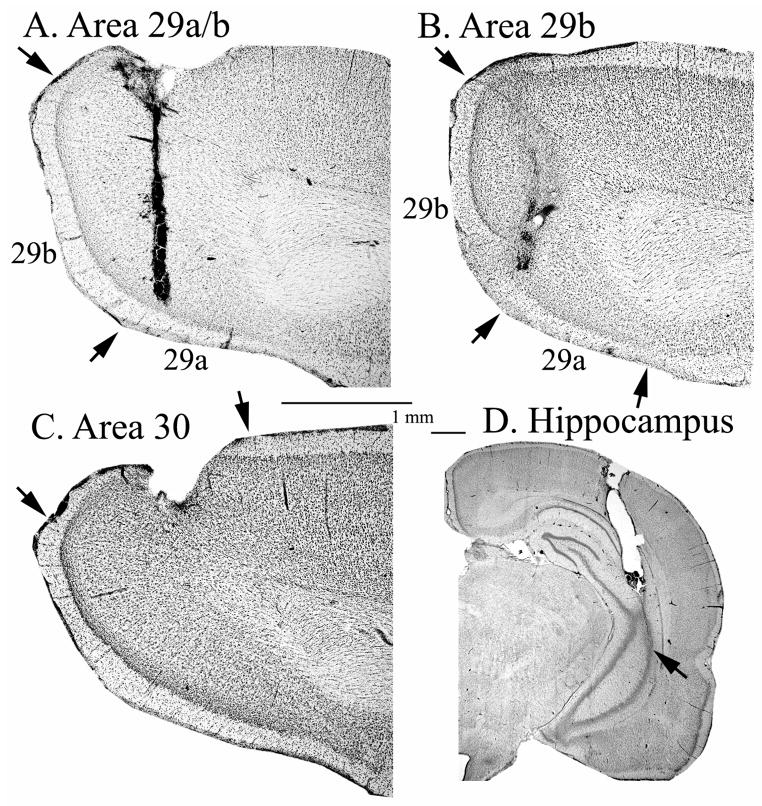
Histological analysis showed that cannula tips and sampling sites were localized in two cortical sites: areas 29a/b and area 30. One subject (RS24) was not used because the cannula was too medial where cortical folding is particularly acute posteriorally and the sampling site likely included a higher proportion of CSF than in other penetrations. Another subject (RS16) was not used because the cannula was placed in area 18, which is lateral to the site of interest. Two additional subjects (RS 32, RS 34) had cannulae that did not penetrate the CA regions of the hippocampus and were not included in any analyses. The remaining 13 rats (n=8 with placement in RSC 29 a/b and hippocampus; n=5 with placement in RSC 30 and hippocampus) had accurate placement of cannulae. Figure 1 displays representative cannulae placement in the hippocampus and areas 29ab and 30 of RSC.
Figure 1.
Cresyl violet stained sections showing an acceptable placement of microdialysis cannulae in the (A) retrosplenial cortex area 29a/b; (B) retrosplenial cortex area 29b; (C) retrosplenial cortex area 30; and (D) hippocampus.
3.2 Behavior
No significant differences in alternation scores were observed as a function of retrosplenial cannula placement in the two groups (RSC 29ab vs. RSC 30; [F [1,11]<1]). There was a nonsignificant trend for the group with RSC 30 cannulae placements to have a greater number arm entries than the groups with RSC 29 cannulae placements (38 vs 26; F [1,11]= 4.10, p=.07), but the mean number of arms traversed was 32.7 ± 4.25. The average percent alternation score for animals in this study was 77.2% ± 1.01 (SEM). This means that the rats entered on average 3.1 of the four arms in a 4 choice sequence. The chance probability of rats achieving a score of 3 out of four arms or better is 65.625%. The behavioral alternation pattern observed is significantly greater than the chance score (t[12]=10.50, p<.01). The behavioral values are within the range of alternation behavior for normal rats implanted with cannulae.
3.3 Acetylcholine Efflux
The neurochemical microdialysis/HPLC data were analyzed two ways: First the two subregions of the RSC were compared with a mixed model design of one between-subjects variable (area 29ab vs. area 30) and two within-subjects variables (Phase [baseline, maze, post-baseline], Sample Time [one, two, three]) ANOVA. Second, given that each animal had one probe in the RSC and another in the hippocampus, differences in ACh efflux between each of the RSC sub-regions (areas 29ab or 30) and the hippocampus were analyzed with a design that included three within-subjects variables ANOVA (Area [RSC vs Hipp], Phase [baseline, maze, post-baseline], Sample Time [one, two, three]).
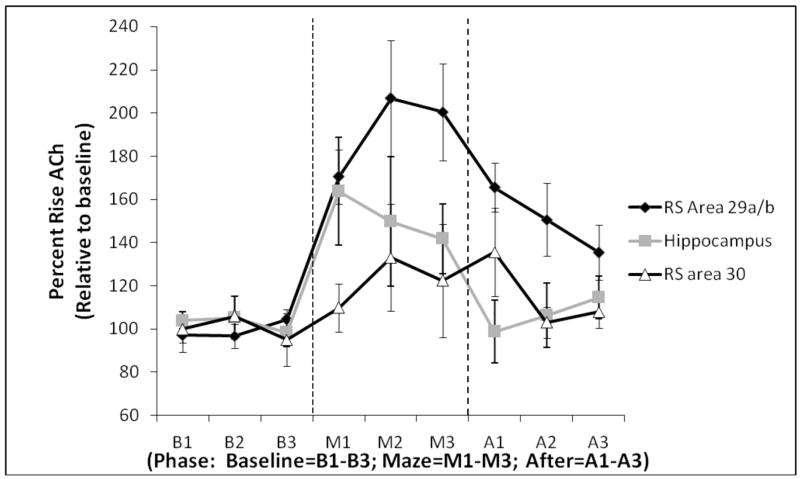
As seen in Figure 2, there was a significant difference between the ACh profiles between areas 29ab and 30 during and after maze exploration (Main effect of Area: F [1,11] = 6.65, p<.05), despite no differences in baseline ACh (fentomole) between the two sub-regions (Area 29ab mean: 80.72± 26.37; area 30 mean: 100.62 ± 32.63 [F (1,11) <1]). Furthermore, there was a significant Area × Phase interaction (F [2, 22] = 4.85, p<.05): Higher levels of ACh efflux were observed in area 29ab, relative to area 30, during both maze transversal (F [1, 11] = 5.88, p<.05) and post baseline measurements (F [1, 11] = 5.35, p<.05). Post-hoc analyses revealed that the level of ACh efflux in area 29ab significantly increased as a function of phase (Main effect of phase (F [2, 14] = 17.36, p<.01); whereas area 30 of the RSC displayed no such changes (F [2,8] = 1.24, p>.1). Specifically, ACh efflux in area 29ab significantly rose above baseline during both behavioral testing (F [1, 7] = 21.46, p<.01) and post baseline measures (F [1, 7] = 20.73, p<.01). In summary, area 29ab demonstrated significantly higher levels of ACh efflux compared to area 30 during both maze traversal and post-baseline measurements.
Figure 2.
Profiles of ACh efflux (Mean ± SEM) from the retrosplenial cortex (area 29a/b, area 30) and hippocampus measured in 6-min time bins (1–3) during baseline (B), maze testing (M) and post-baseline (A).
The ACh profile of both RSC areas differed from the pattern of ACh efflux in the hippocampus—but in opposite directions. First, comparing ACh efflux patterns between area 29ab and the hippocampus revealed overall higher level of ACh release in area 29ab compared to the hippocampus across all phases (Main effect of Area: F [1,7] = 6.02, p<.05). There was also a significant Phase effect (F [2,14] = 12.55, p<.01). Additionally, an Area × Phase interaction revealed that post-baseline ACh levels in area 29ab were significantly higher than hippocampal post-baseline levels (F [2,14] = 4.16, p<.04). Furthermore, there was a significant three way interaction between Area × Phase × Time (F [4,28] = 4.50, p<.01). Follow-up analyses revealed that this interaction was due to significantly higher ACh efflux in area 29ab during the last time bin during maze transversal (M3, F [1,7] = 6.13, p<.05 ), higher ACh efflux during the first time bin of the post-baseline period (A1, F [1,7] = 16.77, p<.01) and higher ACh during the second time bin of the post baseline period (F [1,7] = 6.70, p<.05). Thus, when looking at the regional patterns of ACh efflux, the hippocampal ACh levels were greatest during the initial and middle time segment (M1 and M2) of maze exploration and then underwent a decrease during the last portion of maze traversal and returned to pre-baseline levels in the post-baseline period (p>.6). In contrast, ACh levels increased in area 29ab during the maze and, although the ACh levels decreased during post-baseline, it did not return to baseline levels. Thus, area 29ab demonstrated slightly higher levels of ACh efflux compared to the hippocampus while also demonstrating a prolonged duration of ACh release in response to behavioral challenge.
The three within-subjects variables ANOVA revealed significant differences between the hippocampus and area 30 (Area × Phase interaction: F [2,8] = 5.99, p<.05). Post hoc analyses showed a higher behaviorally evoked release of ACh during maze testing in the hippocampus when compared to area 30 (F [1,4] = 6.78, p=.05). ACh efflux in area 30 was blunted during both maze transversal and did not show any rise relative to baseline (F [1,4] = 1.37, p>.1). However, there was a trend for the post-baseline measurements to be higher than the original baseline measures (F [1,4] = 5.49, p=.08).
NOTE: There were no differences in either amount [F (1,11) <1]) or level of ACh efflux in the hippocampus in the two groups of rats (Area 29ab and area 30; both (F [1, 11] <1). Thus, the data for the hippocampus is represented as a composite of both groups in Figure 2.
3.4 Correlations Between ACh efflux across regions and behavior
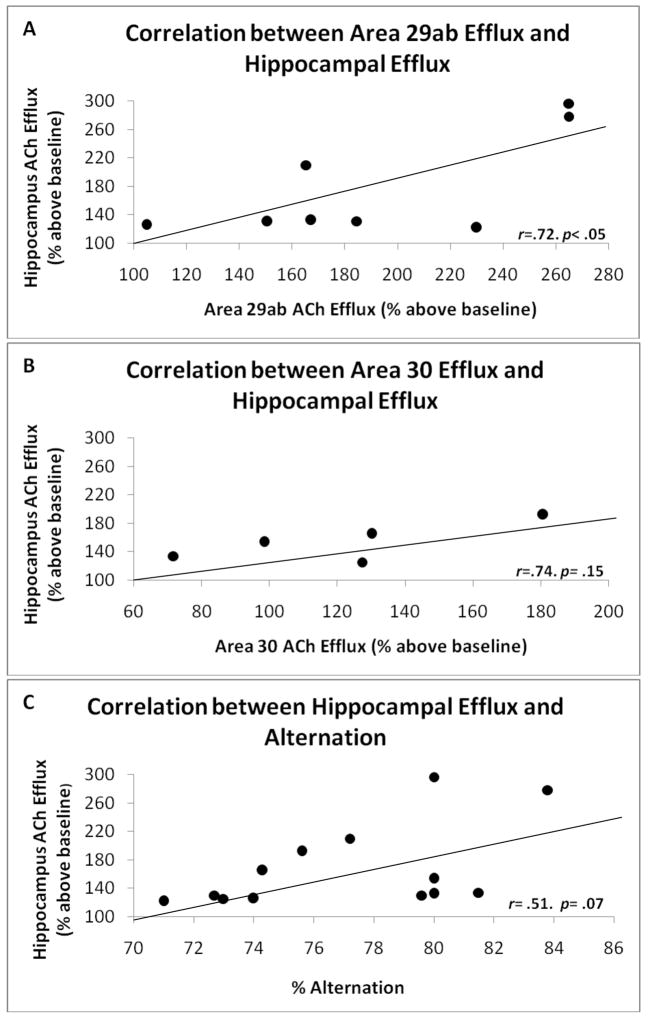
Simple regression analysis revealed that ACh efflux across the hippocampus and RS 29a/b was positively correlated (r=.71, p<.05). Although hippocampus ACh efflux was also correlated with ACh efflux in RS 30 that correlation did not reach significance but due to low sample size (n=5; r=.74, p=.14). Furthermore, there was a trend for a positive correlation between hippocampal ACh efflux and alternation performance (r=.51, p=.07). Figure 3 shows the positive correlations between ACh efflux across structures and behavior. The correlation between RSC area 29a/b ACh efflux and alternation was positive, but not significant (r=.35, p>.1). ACh efflux in any region was not significantly correlated with the number of arms entered (r’s<.15, p’s>.2).
Figure 3.
Correlative relationships between ACh efflux in (A) area 29a/b and hippocampus, (B) area 30 and hippocampus, and (C) hippocampal ACh efflux and spontaneous alternation behavior.
4. Discussion
The results of this study reveal two important aspects about cholinergic functioning in the RSC. First, in area 29ab there are significant changes in the ACh release profile as a function of maze exploration. In addition, the pattern of ACh efflux in area 29ab is different, in peak and temporal profile, from that observed in the hippocampus. This data suggest that these regions play separate-- but complementary-- roles in spatial navigation. Second, area 30, unlike area 29ab, displayed no significant variation in ACh efflux as a function of maze exploration. Thus, ACh efflux does not seem to be critical for spatial processing in area 30.
A number of studies have suggested that both the RSC and the hippocampus contribute to spatial cognition [14, 42, 82]. Our temporal data provide evidence that area 29a/b uniquely contributes to spatial navigation. Specifically, the hippocampus reaches peak ACh levels during early maze traversal which may reflect spatial encoding processes; whereas the later and sustained ACh rise observed in area 29ab may be indicative of ongoing integration of different spatial cues. Furthermore, the overall increase in ACh efflux as a function of spatial exploration in the RSC area 29a/b, relative to the hippocampus, may reflect greater cholinergic activity in the RSC as a function of spatial processing. Increases in ACh efflux in a given brain region has been taken as evidence for overall greater participation of a structure in a given behavioral challenge [9, 43, 49, 52, 54, 55]. However, there should be some caution in this interpretation, as the different probe lengths and variation in acetylcholinesterase activity across the regions may contribute to differences in extracellular concentrations of ACh.
The differences in ACh efflux observed in the RSC may be a function of direct connections from the basal forebrain cholinergic nuclei and indirect connections through the anterior thalamus (AT), via brain stem cholinergic nuclei. Whereas cholinergic projections from the basal forebrain hold the capacity to directly modulate RSC activity, cholinergic responses in the RSC could also be modulated indirectly by the thalamo-retrosplenial projection system. Specifically, brain stem cholinergic nuclei, such as the penduculopontine tegmental nucleus (PPT) and laterodorsal tegmental nucleus (LDT) effect RSC function through AT afferents [26]. Indeed, the PPT and LDT mainly project to the posterior subdivision of the posterior anteroventral thalamic nucleus (AVp) and less so to the medial anteroventral thalamic nucleus (AVm). Axons projecting from the AV terminate in layer Ia and IV of area 29 and provide an important source of excitation that is driven by muscarinic receptor activation [75, 77, 78]. This organization provides activation of area 29 neurons through presynaptic regulation of M2 receptors located on glutamatergic axon terminals that project to layers Ia and IV. As a result, cholinergic projections from the PPT and LDT indirectly modulate activity in the RSC by regulating glutamate release from thalamocortical axon terminals that innervate area 29, thus altering the excitatory profile of the RSC.
In regards to the differences between RSC sub-regions, granular area 29ab demonstrated behaviorally evoked ACh efflux whereas the dysgranular area 30 did not. One potential reason for this may be differences in ACh receptor distribution between the two regions. Neuropharmacological assays of both nicotinic and muscarinic receptor subtypes place a significant density within the granular layers of the retrosplenial cortex [59, 62]. Furthermore, various studies have placed a high concentration of muscarinic receptors in area 29, suggesting a substantial degree of cholinergic activity in this region [75, 77, 78]. Both areas 29a/b and 30 display similar baseline levels of ACh, as observed by fentomole measurements, but only area 29a/b underwent a significant behaviorally evoked rise in ACh efflux during maze traversal.
Although significant anatomical differences exist between species in retrosplenial structure and organization [40, 47], the neurobiological results obtained in rodents regarding RSC and hippocampal activation during spatial processing are similar to data obtained via neuroimaging in humans. Using fMRI, hippocampal activation was most prominent during the initial learning phase of a computer simulated visual navigation task and decayed after performance had approached ceiling level, whereas RSC activation peaked later in the learning curve [81]. Those authors conclude that during spatial navigation the RSC integrates spatial and motion cues throughout the navigational task, whereas the role of the hippocampus was to incorporate new information into an emerging spatial representation. In addition, a more recent fMRI study by Epstein et al. [18] showed a select elevation of hippocampal activity in response to scene perception, but RSC activation corresponded more with retrieval of long-term spatial information. These results suggest that the hippocampus mediated the encoding phase that was followed by later activation of the RSC. The later activation of the RSC was proposed to reflect the integration of spatial cues.
However, the functional distinctions –both behavioral, neuroimaging, and neurochemical-- between the hippocampus and RSC are still suggestive of a cooperative dynamic [2, 13, 30]. Our neurochemical correlative data support this conclusion. Electrophysiological data suggest that the anterior thalamus (AT) and postsubiculum (PoS), are involved in spatial navigation that could contribute to the integration of information across the RSC and hippocampus. Importantly, both the PoS and AT contain head direction (HD) cells and are important in the processing and propagation of the head direction signal.
Spatial information of the hippocampus may be conveyed to the AT via postcommisural fibers either directly or indirectly via projections from the mammillary bodies [63, 64]. The mammillary body projections to the AT are thought to transmit directional information to the HD cells and direct projections from the hippocampus may convey allocentric-based spatial information to the various nuclei of the AT [1, 68]. Furthermore, the directional signals from the AT are propagated forward to the RSC [57, 60, 66], where HD information converges with self motion cues from the AT and posterior parietal cortex [69, 83] and visual information stemming from visual areas 18b, 17, LDN via the dygranular cortex [67], geniculostriate and tectocortical visual pathways [66, 83].
It has been suggested that place cells use information from the HD system to establish and maintain place-field activity [44, 65]. A recent study by Calton et al. [8] showed that destruction of HD neurons in the ADN and PoS lead to place field alterations in hippocampal place cells, demonstrating a direct modulatory effect of HD signals on hippocampal spatial maps. This directional signal is then conveyed to the hippocampus via PoS projections to the superficial layers of the entorhinal cortex [58, 66]. Moreover, head direction signals, such as from the PoS and AT, may contribute to the generation and updating of entorhinal grid cells [7, 32]. These grid cells are thought to integrate various self motion (e.g. head direction, velocity) signals to produce a triangular matrix that remains stable even after the displacement or absence of sensory cues [29]. Grid cell activity and stability may be supported by path integration mechanisms [4, 29]. Information from the entorhinal grid cells is used to generate the site-specific place fields observed in hippocampal place cells [33]. One model suggests that the hippocampal place cells use grid cell information to produce short-term on demand spatial representations [33], whereas the RSC may be more involved in long-term integration of multiple spatial cues.
Thus, the PoS has an important role in the integration of spatial navigation information. PoS layers III and V are densely innervated by both the granular and dysgranular RSC and likely convey both directional information from HD cells localized in the RSC and visual information from dysgranular projections [74, 76]. Furthermore, HD cells of the AT and visual information stemming from the LDN heavily innervate PoS layer I while subicular axons project to layer V pyramidal cells [66]. Indeed, this region also receives dense connections from the hippocampus [21, 22, 23, 28, 45], thus making the PoS an important region of thalamo-cortico-hippocampal integration. These common overlapping neuroanatomical connections could explain why there is a correlation between ACh efflux in the hippocampus and area 29a/b.
In summary, this is the first study assessing in vivo ACh efflux simultaneously in different regions of the RSC and hippocampus during spatial navigation. ACh is known to have a modulatory influence on spatial navigation systems [50], as well as influence general activity within the limbic system [31]. There is evidence that muscarinic receptor activation in the hippocampus sharpens the location specific discharge of place cells during theta activity at a point when such sharpening is critical for the animal to determine and predict its location in space [6]. Although the modulation of ACh in other brain regions on specific navigational cell types is not known, our neurochemical data demonstrate that in area 29a/b, but not area 30, there is an evoked ACh response to exploration on a plus maze. Although a similar phenomenon occurs in the hippocampus, the difference in asymptote and time of the cholinergic response across the RSC and hippocampus is suggestive that these structures play different roles in processing spatial information. Specifically, the different temporal dynamics of ACh efflux between these regions suggest hippocampal involvement during the early phase of maze exploration, which may involve the processing and formation of various spatial cues, while the later ACh rise in area 29a/b may reflect retrieval operations or integration of spatial cues that continue into the post-baseline period. Thus, this study demonstrates not only differences in the presence of behaviorally evoked ACh efflux in cytoarchitecturally distinct areas of the RSC, but also demonstrates functional differences in ACh levels between the RSC and hippocampus that are likely reflective of differential involvement in processing spatial information.
Acknowledgments
This research was funded by grant NINDS RO1-054272 to LMS. The authors would like to thank Jess Blackwolf and Obeta Lavin for their assistance in histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal - anterior thalamic axis. Behav Brain Science. 1999;22:425–489. [PubMed] [Google Scholar]
- 2.Albasser MM, Poiriur GL, Warburton EC, Aggleton JP. Hippocampal lesions have-early gene protein counts in retrosplenial cortex: Distal dysfunction in spatial memory system. Eur J Neurosci. 2007;26:1254–1260. doi: 10.1111/j.1460-9568.2007.05753.x. [DOI] [PubMed] [Google Scholar]
- 3.Amaral DG, Kurz J. An analyses of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985;240:37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- 4.Biegler R. Possible uses of path integration in animal navigation. Anim Learn Behav. 2000;28:257–277. [Google Scholar]
- 5.Big IV, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Res. 1982;8:727–763. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- 6.Brazhnik ES, Muller RU, Fox SE. Muscarinic blockade slows and degrades the location-specific firing of hippocampal pyramidal cells. J Neurosci. 2003;23:611–621. doi: 10.1523/JNEUROSCI.23-02-00611.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess N, Barry C, O’Keefe An oscillatory interference model of grid cell firing. Hippocampus. 2007;17:801–12. doi: 10.1002/hipo.20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, Taube JS. Hippocampal place cell instability after lesions of the head direction cell network. J Neurosci. 2003;23:9719–9731. doi: 10.1523/JNEUROSCI.23-30-09719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Q, Gold PE. Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behav Brain Res. 2003;144:19–24. doi: 10.1016/s0166-4328(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex: I. Anatomical distribution and behavioral modulation. Exp Brain Res. 1994;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- 11.Chen LL, Lin LH, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. II. Contributions of visual and ideothetic information to the directional firing. Exp Brain Res. 1994b;101:24–34. doi: 10.1007/BF00243213. [DOI] [PubMed] [Google Scholar]
- 12.Cho J, Sharp PE. Head direction, place and movement correlates for cells in the rat retrosplenial rortex. Behav Neurosci. 2001;115:3–25. doi: 10.1037/0735-7044.115.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Cooper BG, Mizumori SJY. Retrosplenial cortex inactivation selectively impairs navigation in darkness. NeuroReport. 1999;10:625–630. doi: 10.1097/00001756-199902250-00033. [DOI] [PubMed] [Google Scholar]
- 14.Cooper BG, Manka TF, Mizumori SJY. Finding your way in the dark: The retrosplenial cortex contributes to spatial memory and navigation without visual cues. Behav Neurosci. 2001;115:1012–28. doi: 10.1037//0735-7044.115.5.1012. [DOI] [PubMed] [Google Scholar]
- 15.Cooper BG, Mizumori SJY. Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus. J Neurosci. 2001;21:3986–4001. doi: 10.1523/JNEUROSCI.21-11-03986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty KD, Turchin PI, Walsh TJ. Septocingulate and septohippocampal cholinergic pathways: involvement in working/episodic memory. Brain Res. 1991;810:59–71. doi: 10.1016/s0006-8993(98)00870-1. [DOI] [PubMed] [Google Scholar]
- 17.Eckenstein FP, Baughman RW, Quinn J. An anatomical study of cholinergic innervation in rat cerebral cortex. Neurosci. 1988;25:457–474. doi: 10.1016/0306-4522(88)90251-5. [DOI] [PubMed] [Google Scholar]
- 18.Epstein RA, Parker WE, Feiler AM. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J Neurosci. 2007;23:6141–6149. doi: 10.1523/JNEUROSCI.0799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadda F, Melis F, Stancampiano R. Increased hippocampal acetylcholine release during a working memory task. Eur J Pharmacol. 1996;307:R1–R2. doi: 10.1016/0014-2999(96)00289-0. [DOI] [PubMed] [Google Scholar]
- 20.Fadda F, Cocco S, Stancampiano R. Hippocampal acetylcholine release correlates with spatial learning performance in freely moving rats. Neuroreport. 2000;10:2265–2269. doi: 10.1097/00001756-200007140-00040. [DOI] [PubMed] [Google Scholar]
- 21.Finch DM, Babb TL. Demonstration of caudally directed hippocampal efferents in the rat by intracellular injection of horseradish perioxidase. Brain Res. 1981;214:405–410. doi: 10.1016/0006-8993(81)91203-8. [DOI] [PubMed] [Google Scholar]
- 22.Finch DM, Nowlin NL, Babb TL. Demonstration of axonal projections of neurons in the rat hippocampus and subiculum by intracellular injection of HRP. Brain Res. 1983;271:201–216. doi: 10.1016/0006-8993(83)90283-4. [DOI] [PubMed] [Google Scholar]
- 23.Finch DM, Derian EL, Babb TL. Afferent fibers to rat cingulate cortex. Exp Neurol. 1984;83:468–485. doi: 10.1016/0014-4886(84)90116-x. [DOI] [PubMed] [Google Scholar]
- 24.Gage SL, Keim SR, Simon JR, Low WC. Cholinergic innervations of the retrosplenial cortex via the fornix pathway as determined by high affinity choline uptake, choline acetyltransferase activity, and muscarinic receptor binding in the rat. Neurochem Res. 1994;19:1379–1386. doi: 10.1007/BF00972466. [DOI] [PubMed] [Google Scholar]
- 25.Gold PE. Coordination of multiple memory systems. Neurobiol Learn Mem. 2004;82:230–42. doi: 10.1016/j.nlm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalo-Ruiz A, Sanz-Anquela MJ, Lieberman AR. Cholinergic projections to the anterior thalamic nuclei in the rat: a combined retrograde tracing and choline acetyl -transferase imunohistochemical study. Anat Embryol. 1995;192:335–349. doi: 10.1007/BF00710103. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalo-Ruiz A, Morte L. Localization of amino acids, neuropeptides and cholinergic markers in neurons of the septum-diagonal band complex projecting to the retrosplenial granular cortex of the rat. Brain Res Bull. 2000;52:499–510. doi: 10.1016/s0361-9230(00)00287-2. [DOI] [PubMed] [Google Scholar]
- 28.Groenewegen HJ, Vermeulen-Van der Zee E, Te Kortschoot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport to phaseolus vulgaris leucoagglutin. Neurosci. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 29.Hafting T, Fyhn M, Molden S, Moser MB, Moser E. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 30.Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasselmo ME. The role of acetylcholine in learning and memory. Curr opin Neurobiol. 2006;16:1–6. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasselmo ME, Giocomo LM, Zilli EA. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus. 2007;17:1252–1271. doi: 10.1002/hipo.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasselmo ME. Temporally structured replay of neural activity in a model of entorhinal cortex, hippocampus and postsubiculum. Eur J Neurosci. 2008;28:1301–1315. doi: 10.1111/j.1460-9568.2008.06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:494–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 36.Lennartz RC. The role of extramaze cues in spontaneous alternation in a plus maze. Learn Mem. 2008;36:138–14. doi: 10.3758/lb.36.2.138. [DOI] [PubMed] [Google Scholar]
- 37.Li YJ, Low WC. Intra-retrosplenial cortical transplants of cholinergic neurons derived from the septal nucleus of fetal rats: pattern of innervation. J Neural Transplant Plast. 1993;3:190–191. [Google Scholar]
- 38.Li YJ, Low WC. Intra-retrosplenial grafts of fetal cholinergic neurons and the restoration of spatial memory function. Cell Transplant. 1996;6:85–93. doi: 10.1177/096368979700600113. [DOI] [PubMed] [Google Scholar]
- 39.Li YJ, Low WC. Intra-retrosplenial cortical grafts of cholinergic neurons: Functional incorporation and restoration of High Affinity Choline Uptake. Neurochem Res. 1997;22:589–595. doi: 10.1023/a:1022422103674. [DOI] [PubMed] [Google Scholar]
- 40.Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scan J Psychol. 2001;42:225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- 41.Markowska AL, Olton DS, Murray EA, Gaffan D. A comparative analysis of the role of fornix and cingulate cortex in memory. Exp Brain Res. 1989;74:187–201. doi: 10.1007/BF00248292. [DOI] [PubMed] [Google Scholar]
- 42.Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- 43.McIntyre CK, Marriott LK, Gold PE. Cooperation between memory systems: Acetylcholine release in the amygdala correlates positively with performance on a hippocampus-dependent task. Behav Neurosci. 2003;117:320–326. doi: 10.1037/0735-7044.117.2.320. [DOI] [PubMed] [Google Scholar]
- 44.McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- 45.Meibach RC, Siegal A. Efferent connections of the hippocampal formation in the rat. Brain Res. 1977;124:197–224. doi: 10.1016/0006-8993(77)90880-0. [DOI] [PubMed] [Google Scholar]
- 46.Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- 47.Morris R, Paxinos G, Petrides M. Architectonic analysis of the human retrosplenial cortex. J Comp Neurol. 2000;421:14–28. doi: 10.1002/(sici)1096-9861(20000522)421:1<14::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- 49.Pych JC, Chang Q, Colon-Rivera C, Gold PE. Acetylcholine release in hippocampus and striatum during testing on a rewarded spontaneous alternation task. Neurobiol Learn Mem. 2005;2:93–101. doi: 10.1016/j.nlm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Redish AD, Touretzhy DS. Cognitive maps beyond the hippocampus. Hippocampus. 1997;7:15–35. doi: 10.1002/(SICI)1098-1063(1997)7:1<15::AID-HIPO3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Riekkinen P, Kuitunen J, Riekkinen M. Effects of scopolamine infusions into the anterior and posterior cingulate on passive avoidance and water maze navigation. Brain Res. 1995;685:46–54. doi: 10.1016/0006-8993(95)00422-m. [DOI] [PubMed] [Google Scholar]
- 52.Roland JJ, Savage LM. Blunted hippocampal, but not striatal, acetylcholine efflux parallels learning impairment in diencephalic-lesioned rats. Neurobiol Learn Mem. 2007;87:123–132. doi: 10.1016/j.nlm.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Savage LM, Chang Q, Gold PE. Diencephalic damage decreases hippocampal acetylcholine release during spontaneous alternation testing. Learn Mem. 2003;10:242–246. doi: 10.1101/lm.60003. [DOI] [PubMed] [Google Scholar]
- 55.Savage LM, Roland J, Klintsova A. Selective hippocampal- but not forebrain amygdalar- cholinergic dysfunction in diencephalic amnesia. Brain Res. 2007;1139:210–219. doi: 10.1016/j.brainres.2006.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senut MC, Menetrey D, Lamour Y. Cholinergic and Peptidergic projections from the medial septum and the nucleus of the diagonal band of Broca to dorsal hippocampus, cingulate cortex and olfactory bulb: a combined wheat germ agglutinin-apohorseradish perioxidase-gold immunohistochemical study. Neuroscience. 1989;30:385–403. doi: 10.1016/0306-4522(89)90260-1. [DOI] [PubMed] [Google Scholar]
- 57.Shibata H. Organization of projections of rat retrosplenial cortex to the anterior thalamic nuclei. Eur J Neurosci. 1998;10:3210–3219. doi: 10.1046/j.1460-9568.1998.00328.x. [DOI] [PubMed] [Google Scholar]
- 58.Shipley MT. The topographical and laminar organization of the presubiculum’s projection to the ipsi- and contralateral entorhinal cortex in the guinea pig. J Comp Neurol. 1975;160:127–145. doi: 10.1002/cne.901600108. [DOI] [PubMed] [Google Scholar]
- 59.Son JH, Winzer-Serhanu H. Postnatal expression of alpha2 nicotinic acetylcholine receptor subunit mRNA in developing cortex and hippocampus. J Chem Neuroanat. 2006;32:179–90. doi: 10.1016/j.jchemneu.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sripanidkulchai K, Wyss JM. The laminar organization of efferent neuronal cell bodies in the retrosplenial granular cortex. Brain Res. 1987;406:255–269. doi: 10.1016/0006-8993(87)90790-6. [DOI] [PubMed] [Google Scholar]
- 61.Sutherland RJ, Whishaw IQ, Kolb B. Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci. 1988;8:1863–1872. doi: 10.1523/JNEUROSCI.08-06-01863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Svedberg MM, Bednar I, Nordberg A. Effect of subchronic galantamine treatment on neuronal nicotinic and muscarinic receptor subtypes in transgenic mice overexpressing human acetylcholinesterase. Neuropharmacology. 2004;47:558–71. doi: 10.1016/j.neuropharm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Swanson LW, Kohler C, Bjorkland A. Handbook of Chemical Neuroanatomy. Amsterdam: Elsevier; 1987. The limbic region. I: The septohippocampal system; pp. 1334–1360. [Google Scholar]
- 64.Taube JS, Muller RU, Ranck JB., Jr Head direction cells recorded from the postsubiculum in freely moving rats: I. Description and quantitative analyses. J Neurosci. 1990;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Touretzky DS, Redish D. Theory of rodent navigation based on interacting representations of space. Hippocampus. 1996;6:247–270. doi: 10.1002/(SICI)1098-1063(1996)6:3<247::AID-HIPO4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 66.Van Groen T, Wyss JM. Connections of the Retrosplenial Granular A cortex in the Rat. J Comp Neurol. 1990;300:593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- 67.Van Groen T, Wyss JM. Connections of the Retrosplenial dysgranular cortex in the rat. J Comp Neurol. 1992;315:200–216. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- 68.Van Groen T, Vogt BA, Wyss JM. Interconnections between the thalamus and retrosplenial cortex in rodent brain. In: Vogt BA, Gabriel M, editors. The Neurobiology of the Cingulate Cortex and Limbic Thalamus. Boston: Birkauser; 1993. pp. 123–150. [Google Scholar]
- 69.Van Groen T, Wyss JM. Projections from the anterodorsal and anteroventral nucleus of the thalamus to the limbic cortex in the rat. J Comp Neurol. 1995;358:584–604. doi: 10.1002/cne.903580411. [DOI] [PubMed] [Google Scholar]
- 70.Van Groen T, Wyss JM. Connections of the Retrosplenial Granular B cortex in the Rat. J Comp Neurol. 2003;463:249–63. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- 71.Van Groen T, Kadish I, Wyss JM. Retrosplenial cortex lesions of area Rgb (but not of area Rga) impair spatial learning and memory in the rat. Behav Brain Res. 2004;154:483–491. doi: 10.1016/j.bbr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 72.Vann SD, Aggleton JP. Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav Neurosci. 2002;116:85–94. [PubMed] [Google Scholar]
- 73.Vann SD, Aggleton JP. Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behav Neurosci. 2005;119:1682–1686. doi: 10.1037/0735-7044.119.6.1682. [DOI] [PubMed] [Google Scholar]
- 74.Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular corticies. J Comp Neurol. 1983;216:192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- 75.Vogt BA. Afferent specific localization of muscarinic acetycholine receptors in cingulate cortex. J Neurosci. 1984;4:2191–2199. doi: 10.1523/JNEUROSCI.04-09-02191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vogt BA, Sikes RW, Swadlow HA, Weyand TG. Rabbit cingulate cortex: cytoarchitecture, physiological border with visual cortex, and afferent cortical connections of visual, motor, postsubicular, and intracingulate origin. J Comp Neurol. 1986;248:74–94. doi: 10.1002/cne.902480106. [DOI] [PubMed] [Google Scholar]
- 77.Vogt BA, Burns DL. Experimental localization of muscarinic receptor subtypes to cingulate cortical afferents and neurons. J Neurosci. 1988;8:643–652. doi: 10.1523/JNEUROSCI.08-02-00643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vogt BA, Crino PB, Jensen EL. Multiple heteroreceptors on limbic thalamic axons: M2 acetylcholine, serotonin 1B, beta 2-adrenoreceptors, mu opioid, and neurotensin. Synapse. 1992;10:44–53. doi: 10.1002/syn.890100107. [DOI] [PubMed] [Google Scholar]
- 79.Vogt BA, Vogt L, Farber NB. Cingulate cortex and disease models. In: Paxinos G, editor. The rat nervous system. 3. New York: Elsevier Academic Press; 2004. pp. 705–27. [Google Scholar]
- 80.Whishaw IQ, Maaswinkel H, Gonzalez CL, Kolb B. Deficits in allothetic and ideothetic spatial behavior in rats with posterior cingulate cortex lesions. Behav Brain Res. 2001;118:67–76. doi: 10.1016/s0166-4328(00)00312-0. [DOI] [PubMed] [Google Scholar]
- 81.Wolbers T, Buchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J Neurosci. 2005;25:3333–3340. doi: 10.1523/JNEUROSCI.4705-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wyss JM, Van Groen T. Connections between the retrosplenial cortex and hippocampal formation in the rat: A review. Hippocampus. 1992;2:1–11. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]
- 83.Ziles K, Wree A. Cortex: areal and laminar structure. In: Paxinos G, editor. The rat nervous system. 2. San Diego: Academic press; 1995. pp. 649–685. [Google Scholar]