Abstract
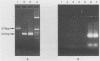
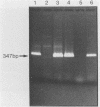
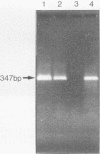
By using a set of four nested oligonucleotide primers, a two-step polymerase chain reaction assay for the detection and identification of Mycobacterium leprae that does not require the use of radioactivity labeled hybridization probes was developed. The nested-primer procedure amplified a 347-base-pair product from M. leprae genomic DNA. No amplification products were produced from DNAs of 19 other Mycobacterium species, 19 non-Mycobacterium species, mouse cells, or human cells. Minor amplification products were observed with three additional Mycobacterium species, i.e., "M. lufu", M. simiae, and M. smegmatis. These products were easily distinguished from the M. leprae product by size and restriction enzyme cleavage patterns. The assay could amplify the 347-base-pair product from samples containing as little as 3 fg of M. leprae genomic DNA--the amount of DNA in a single bacillus. The assay also amplified target sequences in crude lysates of M. leprae bacilli isolated from tissue biopsy specimens from infected animals and humans. The entire assay, from sample preparation to data analysis, can be completed in less than 8 h.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brisson-Noël A., Gicquel B., Lecossier D., Lévy-Frébault V., Nassif X., Hance A. J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989 Nov 4;2(8671):1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- Clark-Curtiss J. E. Benefits of recombinant DNA technology for the study of Mycobacterium leprae. Curr Top Microbiol Immunol. 1988;138:61–79. [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Docherty M. A. A species-specific repetitive sequence in Mycobacterium leprae DNA. J Infect Dis. 1989 Jan;159(1):7–15. doi: 10.1093/infdis/159.1.7. [DOI] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Jacobs W. R., Docherty M. A., Ritchie L. R., Curtiss R., 3rd Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J Bacteriol. 1985 Mar;161(3):1093–1102. doi: 10.1128/jb.161.3.1093-1102.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Walsh G. P. Conservation of genomic sequences among isolates of Mycobacterium leprae. J Bacteriol. 1989 Sep;171(9):4844–4851. doi: 10.1128/jb.171.9.4844-4851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayet O., Ziegelhoffer T., Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989 Mar;171(3):1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Grandchamp B., Lévy-Frébault V., Lecossier D., Rauzier J., Bocart D., Gicquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989 Jul;3(7):843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Hartskeerl R. A., de Wit M. Y., Klatser P. R. Polymerase chain reaction for the detection of Mycobacterium leprae. J Gen Microbiol. 1989 Sep;135(9):2357–2364. doi: 10.1099/00221287-135-9-2357. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Seydel J. K., Wempe E. G. Bacterial growth kinetics of "M. lufu" in the presence and absence of various drugs alone and in combination. A model for the development of combined chemotherapy against M. leprae? Int J Lepr Other Mycobact Dis. 1982 Mar;50(1):20–30. [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Shepard C. C., van Landingham R., Walker L. L. Searches among mycobacterial cultures for antileprosy vaccines. Infect Immun. 1980 Sep;29(3):1034–1039. doi: 10.1128/iai.29.3.1034-1039.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. B., Lowe A. C., Rees R. J., Colston M. J. Vaccination of mice against Mycobacterium leprae infection. Infect Immun. 1989 Feb;57(2):653–655. doi: 10.1128/iai.57.2.653-655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Isolation of deoxyribonucleic acid from mycobacteria. J Bacteriol. 1968 Apr;95(4):1481–1482. doi: 10.1128/jb.95.4.1481-1482.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. A., Cole S. T. A rapid method for the detection of potentially viable Mycobacterium leprae in human biopsies: a novel application of PCR. FEMS Microbiol Lett. 1989 Dec;53(3):305–309. doi: 10.1016/0378-1097(89)90235-8. [DOI] [PubMed] [Google Scholar]