Abstract
Interleukin-10 deficient (IL-10-/-) mice develop chronic T cell-mediated colitis when colonized with normal commensal bacteria, but germ-free (GF) IL-10-/- mice remain disease-free. Antigen presenting cells (APC) secrete regulatory cytokines that help determine T lymphocyte activation or tolerance. CD4+ T cells from the mesenteric lymph nodes of inflamed IL-10-/- mice secrete more IFNγ and IL-17 when cultured with cecal bacterial lysate-pulsed splenic APC from IL-10-/- mice than when cultured with normal control APC. GF IL-10-/- APC induce similar IFNγ and IL-17 responses; therefore, the functional difference between normal and IL-10 deficient APC is inherent to the lack of IL-10 and not secondary to inflammation. Bacterial lysate-pulsed normal APC cultured with CD4+ cells from colitic IL-10-/- mice or with exogenous IFNγ secrete higher amounts of IL-10 compared to the same APC cultured with naïve T cells. APC enriched for CD11c+ cells are potent activators of IFNγ and IL-17 production by CD4+ cells from IL-10-/- mice. These APC also produce IL-12/IL-23 p40 and IL-10. Recombinant IL-10 suppressed and anti-IL-10 receptor antibody increased IFNγ, IL-17 and IL-12/IL-23 p40 production in bacterial lysate-pulsed APC and plus CD4+ T cell co-cultures. Taken together, our results show that endogenous IL-10 produced by APC inhibits responses to commensal bacteria and influences the ability of APC to stimulate IFNγ-producing effector lymphocytes, which reciprocally, induce IL-10 production by APC. Cytokines produced by APC are an important determinant of pathogenic versus protective mucosal immune responses to colonic bacterial stimulation.
Keywords: Cytokine regulation, antigen presenting cells, IL-10 deficient mice
1. Introduction
Inflammatory bowel diseases (IBD) are a constellation of disorders (Crohn’s disease and ulcerative colitis) characterized by chronic, immune-mediated inflammation that affects the digestive tract. Although these are idiopathic conditions, dysregulated cell-mediated immune responses to nonpathogenic commensal enteric bacteria play a role in the pathogenesis of these disorders [1, 2]. The mucosal immune system maintains a delicate balance; it must be able to defend against a pathogenic microbial threat yet remain unresponsive to resident bacterial and dietary antigens and adjuvants. Specifically in Crohn’s disease, loss of tolerance to normal luminal bacteria is documented by increased antibody production and T lymphocyte responses to antigens of intestinal bacteria [3-5]. Patients with Crohn’s disease exhibit high mucosal levels of certain cytokines including IL-23, IL-12, IL-18, IFNγ, IL-17 and TNF [6-12] and respond to anti-TNF and anti-IL-12/IL-23p40 monoclonal antibody treatment [13,14]. Further evidence of a role for bacteria in the pathogenesis is the attenuation of symptoms after antibiotic treatment [15,16].
Rodent models of intestinal inflammation, although not able to fully recapitulate human disease, provide important insights into the pathogenesis of IBD. First, chronic intestinal inflammation is T cell dependent; B cells are not required and in certain situations are protective [17-21]. The profile of cytokines in most models of chronic intestinal inflammation is Th1/Th17 [17,20,22,23]. IL-23, a heterodimeric cytokine composed of one unique component (p19) that associates with IL-12p40, has recently emerged as a key inducer of IL-17-mediated inflammation [23]. However, recent work by Izcue et. al. shows that IL-17 is not required for development of colitis in all model systems [24]. Second, in most, but not all models, germ-free (GF), sterile rodents do not develop chronic colitis and continuous exposure to luminal bacteria is necessary to maintain disease [25-27]. Furthermore, T cells from mice with chronic intestinal inflammation respond to components of commensal enteric bacteria [17,20,26,28-31]. Therefore, endogenous nonpathogenic colonic bacteria provide the antigenic and adjuvant stimulation necessary to maintain intestinal inflammation. This loss of tolerance in genetically susceptible hosts can be due either to an overly aggressive effector cell activity, to defective regulatory cell function, or to inadequate response to regulatory signals. Regulatory cells producing TGFβ and IL-10 mediate tolerance in normal hosts [32].
IL-10 deficient mice spontaneously develop progressive colitis in specific pathogen-free (SPF) conditions [33], while GF mice develop neither histologic evidence of disease nor immune activation [25]. GF IL-10 deficient mice develop progressively more aggressive colitis one to four weeks after colonization with SPF fecal bacteria; however wild-type controls colonized with the same bacteria do not exhibit colitis or immune activation [25,34]. This clearly identifies the role of IL-10 in maintaining immunologic tolerance to the normal enteric microorganisms.
IL-10 is a key immunosuppressive cytokine that acts directly on antigen presenting cells (APC) to inhibit IL-12 secretion and down regulate the expression of MHC Class II and costimulatory molecules such as CD80 and CD86 [35]. This direct action on APC indirectly inhibits T cell activation. IL-10 is produced by multiple cell types, including T cells, dendritic cells (DC), macrophages, and B cells [35-37]. Regulatory T cells are a well-documented source of IL-10 and have been shown to prevent onset of colitis in several murine models. After transfer of CD45RBhigh cells, syngeneic scid recipients develop colitis. Cotransfer of CD45RBlo cells, however, prevents disease [38]. If the CD45RBlo population is derived from IL-10 deficient mice, this population cannot prevent development of colitis [39]. Furthermore, transfer of IL-10 secreting enteric bacterial-responsive regulatory T cell lines can prevent disease in the C3H/HeJBir cotransfer model [40]. However, IL-10 regulatory cell function has been described for other cell populations as well, including DC and B lymphocytes in models of pulmonary or intestinal inflammation [19, 41,42].
Despite the proven importance of IL-10 as an immunosuppressive agent both in vivo and in vitro, the relative roles of T-cell derived and non-T cell derived IL-10 remain unclear. The purpose of this investigation is to explore the ability of intrinsic IL-10 produced by APC versus CD4+ T lymphocytes to regulate bacterial antigen-induced IFNγ, IL-17, and IL-12/IL-23 p40 responses. We demonstrate that APC-derived IL-10 is an important regulator of T cell responses to physiologic colonic bacterial stimulation.
2. Materials and Methods
2.1 Mice
IL-10 deficient (IL-10-/-) mice on a 129S6/SvEv background and normal and 129S6/SvEv (129 wild type) mice were maintained GF in Trexler isolators in the Gnotobiotic Core of the Center for Gastrointestinal Biology and Disease at the College of Veterinary Medicine, North Carolina State University, Raleigh, NC. Sterility was confirmed by aerobic and anaerobic fecal cultures every two weeks. A SPF colony of 129S6/SvEv mice was established from mice obtained from Taconic Laboratories, Germantown, NY and known to be free of Helicobacter species. GF→SPF mice used for the source of CD4+ MLN cells were transferred from GF isolators to the SPF facility at 8-14 weeks of age and euthanized 8 weeks after being colonized with the fecal contents from the SPF 129S6/SvEv mice described above. The North Carolina State University Institutional Animal Care and Use Committee (IACUC) approved all animal protocols.
2.2 Cecal bacterial lysate
Cecal bacterial lysate (CBL) was prepared directly from the cecal contents of 129 wild type SPF mice according to the protocol of Cong [28]. Briefly, the cecum was isolated, placed in 1 ml of sterile RPMI, and vortexed thoroughly. After removal of the cecal tissue and the addition of 0.25 ml of MD solution (0.1 mg/ml DNase I, 0.02 mg/ml MgCl2), this mixture was disrupted by 0.1 mm glass beads in a Mini-bead beater (Biospec Products, Bartlesville OK) for 3 minutes. After centrifugation, the supernatant was filter-sterilized (0.45 μM filter) and the protein concentration was measured using a standard assay (Biorad Laboratories, Hercules, CA). Cecal bacterial lysate was either used immediately after isolation or was aliquoted and frozen at −80°C.
2.3 Antigen presenting cell (APC) preparation
APC were prepared as previously described [26]. Briefly, spleens were isolated from 129 wild type or IL-10-/- mice. T cells were depleted by rabbit complement-mediated lysis using anti-Thy1.2 monoclonal antibody. The resulting population contained less than 6% CD4+ and 1% CD8+ cells. In select experiments, B220+ and CD11c+ cells were enriched by magnetic activated cell sorting (MACS). Briefly, T cell depleted splenocytes were incubated with magnetic beads coupled to antibodies and then passed through the magnetic column (Miltenyi, Auburn, CA). B220+ cells were negatively selected using anti-CD11c and anti-CD11b magnetically labeled antibodies and passed through an LD column. CD11c+ cells were enriched by the following two methods: 1) positive selection using anti-CD11c magnetically labeled antibodies and passed through an LS column. These cells were pulsed overnight with an unrelated antigen, keyhole limpet hemocyanin (KLH: Pierce, Rockford, IL), cecal bacterial lysate at 50 μg/ml, or cultured without antigen in complete medium (RPMI 1640 plus 5% heat inactivated fetal calf serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 μM 2-mecaptoethanol, and 50 μg/ml gentamicin). 2) cell sorting of spleen cells after incubation with FITC-labeled anti-mouse CD11c (BD Biosciences, San Diego, CA) using a DakoCytomation MoFlo High-Speed Cell Sorter (DakoCytomation, Fort Collins, Co). The 129 wild type and IL-10-/- MoFlo sorted CD11c+ cells were 92.4% and 94.8% CD11c+, respectively. Due to the low number of CD11c+ APC obtained after MoFlo sorting, the cells were not pulsed overnight. Instead, 1 × 104 sorted cells were added directly to 96 well plates for co-culture as described in the following section.
2.4 CD4+ T cell isolation and stimulation
CD4+ cells were isolated and stimulated as previously described [26]. GF IL-10-/- mice were moved to SPF housing conditions and colonized with commensal microorganisms from 129 wild type mice that were free of Helicobacter species. After 8-14 weeks, the mesenteric lymph nodes (MLN) were isolated and single cell suspensions were prepared. The CD4+ T cells were separated by negative selection using MACS as follows: MLN cells were incubated with magnetic beads coupled to anti-CD8 and anti-B220 antibodies (Miltenyi), and passed through and LD column allowing CD4+ cells to be enriched. The enriched cell population was greater than 95% CD4+. APC were collected after overnight pulsing and washed twice to remove soluble antigens and other bacterial products. APC were cultured at 3 × 105 cells per well with CD4+ cells at 2 × 105 cells per well in flat bottom 96 well plates (Costar 3595). The T cell number was selected based on our previously published results showing maximal responses using 2 × 105 CD4+ cells per well in 200 μl [43]. In preliminary experiments for the current studies, in which we titrated APC numbers, we obtained higher amounts of IFN-γ and IL-17 produced by CD4+ T cells co-cultured with 3 × 105 compared to 1 × 105 APC per well (200 μl), and therefore we used 3 × 105 cells per well for co-culture (data not shown). For co-culture with MoFlo sorted CD11c+ APC, cecal bacterial lysate (50 μg/ml) was added directly to wells containing 1 × 104 CD11c+ cells plus 2 × 105 highly enriched CD4+ T cells obtained by negative selection with magnetic beads coated with anti-CD8, anti-B220, anti-CD11b, and anti-CD11c. The resulting cell population was greater than 98% CD4+ and did not produce IFN-γ in cultures containing cecal bacterial lysate without APC (data not shown). In selected experiments recombinant murine IFNγ (R&D Systems, Minneapolis, MN), recombinant murine IL-10 (PeproTech, Rocky Hill, NJ), anti-IL-10 receptor antagonistic antibody [44, clone 1B1.3a, BD Biosciences], anti-mouse IFN-γ (clone R4-6A2, BD Biosciences), or rat IgG1 isotype control (clone R3-34, BD Biosciences) was added during the APC pulse or during the APC/CD4+ lymphocyte cocultures. Triplicate supernatants were collected at 72 hours.
2.5 Flow cytometry
Cells were incubated for 30 minutes at 4°C with fluorochrome-conjugated antibodies, including anti-CD4, CD8, B220 (Caltag, Burlingame, CA), CD11b, CD11c, CD40, CD45RB, MHC Class II (I-Ab), CD80, and CD86, (BD Biosciences). Cells were washed and then analyzed on a FACScan (Becton Dickinson, San Jose, CA).
2.6 Cytokine measurement
Commercially available monoclonal capture and detection antibodies specific for mouse IFNγ and IL-12/IL-23 p40 were used in ELISA protocols as described [26]. To measure IL-17, we used clone TC11-18H10 for capture and biotin-labeled clone TC11-8H4.1 for detection (both from BD Biosciences). For the IL-23 ELISA, we used anti-IL-23 p19, clone G23-8 (eBioscience, San Diego, CA) as capture antibody and biotin-labeled anti-IL-12/IL-23 p40, clone C17.8 (BD Biosciences) for detection. To measure IL-12 p70 heterodimer, we used clone 9-A5 as capture antibody and biotin labeled anti-IL-12/IL-23 p40 clone C17.8 for detection (both from BD Biosciences). For the IL-10 ELISA, we used JES5-2A5 as the capture antibody and biotin-labeled SXC-1 as the detection antibody (both from BD Biosciences). The background of the IL-10 ELISA ranges from 10 to 100 pg/ml. Backgrounds are negligible for all other ELISAs. Limits of detection are approximately 5 pg/ml for each cytokine.
2.7 Statistical analysis
Data were analyzed using the Student’s t test and p values of less than 0.05 were considered significant.
3. Results
3.1 Cecal bacterial lysate-pulsed APC from IL-10-/- mice stimulate secretion of higher concentrations of IFNγ and IL-17 by CD4+ MLN T lymphocytes than do APC from wild type mice
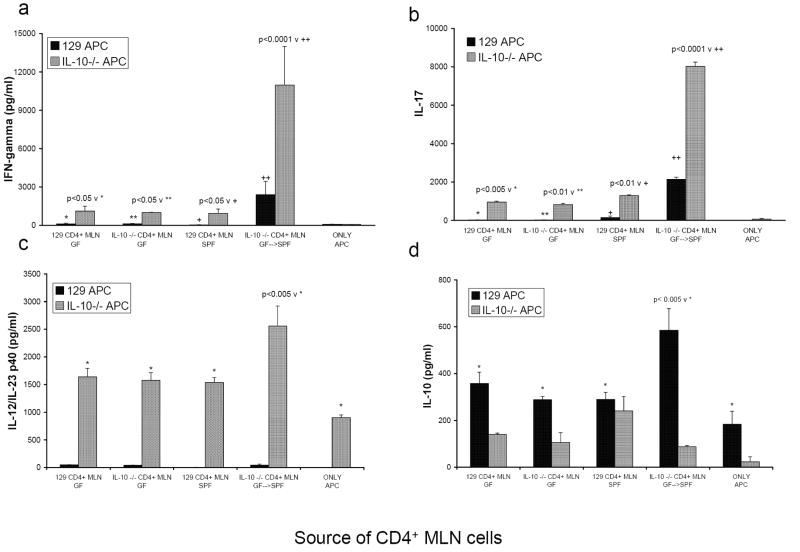
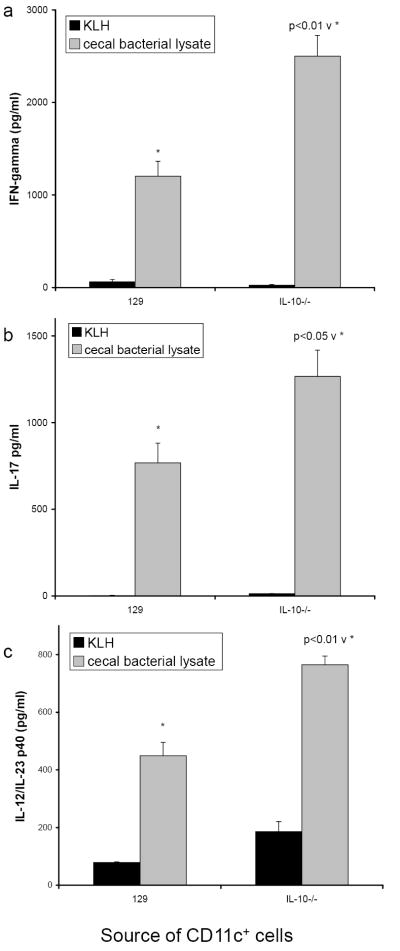
IL-10-/- mice, born in GF housing conditions and then moved to SPF housing as adults (GF→SPF), consistently developed severe colitis (blinded histology score of cecum is 3.8 ± 0.2 on a 0-4 scale). The MLN from the inflamed GF→SPF IL-10-/- mice were grossly enlarged, containing 4-10 times the number of total cells compared to MLN from normal mice (89.1 × 106 versus 8.4 × 106 cells per mouse, 3 mice per group). CD4+ lymphocytes from the MLN of GF or SPF wild type controls and from GF or GF→SPF IL-10-/- mice were isolated for stimulation in vitro with cecal bacterial lysate-pulsed splenic APC from SPF IL-10-/- or normal 129 wild type mice. Because GF IL-10-/- mice do not develop colitis (blinded histology score of cecum was 0.12 ± 0.05 on a 0-4 scale), the MLN were not grossly enlarged and the number of cells was no different from GF 129 wild type mice (18.6 × 106 for GF IL-10-/- versus 16.7 × 106 cells per mouse for GF 129 wild type, 4 mice per group). When cecal bacterial lysate-pulsed APC from SPF IL-10-/- mice were used to stimulate GF→SPF IL-10-/- CD4+ lymphocytes, the amounts of detectable IFNγ and IL-17 were 3-5 fold higher than IFNγ and IL-17 in cultures of CD4 cells stimulated with bacterial lysate-pulsed APC from 129 wild type mice (Fig. 1a, 1b). In addition, CBL-pulsed APC from IL-10-/- mice induced low but detectable IFNγ and IL-17 responses in both SPF and GF 129 CD4+ lymphocytes and in GF naïve IL-10-/- CD4+ cells. For each source of CD4+ T cells, APC from IL-10-/- mice induced significantly greater IFNγ and IL-17 than did wild type APC. This ability of IL-10-/- APC to stimulate IFNγ and IL-17 secretion supports the hypothesis that IL-10-/- APC have the capacity to induce pathogenic CD4+ lymphocyte responses to commensal cecal bacteria and to skew T cell responses. In contrast, bacterial lysate-pulsed APC from IL-10 replete mice did not stimulate IFNγ or IL-17 secretion by CD4+ lymphocytes from the SPF wild type mice or from the naïve GF wild type or IL-10-/- mice.
FIGURE 1.
Cytokine production from co-cultures of CD4+ T lymphocytes isolated from MLN of either noncolitic mice (GF 129, GF IL-10-/-, SPF 129) or colitic mice (GF→SPF IL-10-/-) stimulated by cecal bacterial lysate-pulsed APC from either wild type 129 (black bars) or IL-10-/- mice (grey bars). Supernatants were harvested at day 3 and assayed by ELISA for (a) IFNγ, (b) IL-17, (c) IL-12/IL-23 p40, and (d) IL-10. The amounts of IL-10 measured in supernatants of IL-10-/- cells are equivalent to the assay background. Results shown are mean ± SD of cytokines in triplicate cultures and are representative of three independent experiments.
3.2 IL-12/IL-23 p40 is produced by cecal bacterial lysate-pulsed IL-10-/- APC
Cecal bacterial lysate-pulsed APC from wild type mice did not secrete detectable IL-12/IL-23 p40 under any of our culture conditions (Fig. 1c). However, bacterial lysate-pulsed APC from IL-10-/- mice secrete this molecule even when cultured without T cells (905 ± 47 pg/ml). IL-12/IL-23 p40 secretion by APC from IL-10-/- mice was not spontaneous because none was detected in supernatants of APC from IL-10-/- mice pulsed with KLH (data not shown). IL-10-/- APC cultured with CD4+ lymphocytes from GF→ SPF IL-10-/- colitic mice produced significantly more IL-12/IL-23 p40 than did APC in cultures containing CD4+ T cells from GF mice and SPF 129 wild type mice. The ability of cecal bacterial lysate to stimulate IL-12/IL-23 p40 production by APC from IL-10-/- mice indicates that antigens and adjuvants normally present in the mouse cecum activate APC from IL-10-/- mice but not APC from IL-10 replete mice. Neither the IL-12 p70 nor the IL-23p19/p40 heterodimer were detectable in these culture supernatants.
3.3 IL-10 production by cecal bacterial lysate-stimulated wild type APC is enhanced by culture with IL-10-/- CD4+ T cells from colitic mice
Because both APC and CD4+ lymphocytes can produce IL-10, using APC and CD4+ lymphocytes from 129 wild type and IL-10-/- mice allowed us to determine the source of IL-10 produced in the mixed co-cultures. Cecal bacterial lysate-pulsed APC from wild type mice secreted IL-10 even when cultured without T cells (Fig. 1d). The addition of CD4+ lymphocytes from GF→ SPF IL-10-/- colitic mice significantly increased the amount of IL-10 produced by bacterial lysate-pulsed 129 wild type APC compared to IL-10 in cultures containing wild type APC plus naïve T cells. CD4+ lymphocytes from SPF wild type mice also produced IL-10 and their contribution could be seen in cultures that contain IL-10-/- APC (Fig. 1d). The ability of wild type APC to produce IL-10 in response to CD4+ T cells from an inflamed mouse supports the hypothesis that APC from normal hosts have the capacity to regulate inflammation.
3.4 APC from GF and from SPF IL-10-/- mice stimulate similar levels of IFNγ and IL-17
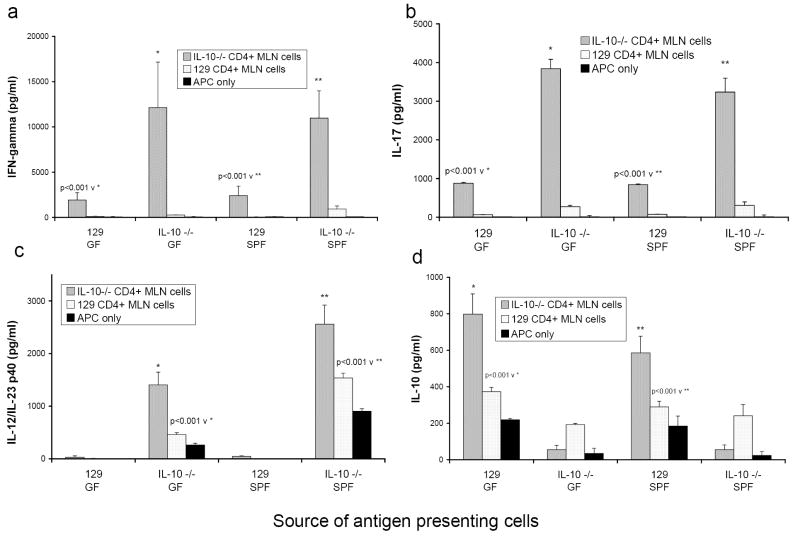
In our in vitro culture system, GF IL-10-/- CD4+ lymphocytes produced low amounts of IFNγ and IL-17 that were comparable to both GF and SPF IL-10 replete CD4+ lymphocytes (Fig 1a, 1b). All three sources of CD4+ cells induced a similar amount of IL-12/IL-23 p40 production by APC (Fig 1c). Therefore, the high levels of IFNγ and IL-17 produced by GF→SPF IL-10-/- CD4+ MLN lymphocytes and their ability to induce elevated IL-12/IL-23 p40 are not directly caused by their IL-10 deficiency but instead this response is either secondary to their activation by in vivo exposure to enteric bacteria or secondary to chronic inflammation. We therefore designed experiments to determine whether or not differences in cytokine production by the IL-10-/- APC were also secondary to inflammation or in vivo bacterial activation. For this analysis, we compared 129 wild type and IL-10-/- APC isolated from GF and from SPF mice. GF and SPF IL-10-/- APC, pulsed with cecal bacterial lysate, induced production of equivalent amounts of IFNγ and IL-17 by CD4+ lymphocytes from GF→SPF IL-10-/- mice (Fig. 2a, 2b), showing that in vivo activation was not necessary for enhanced stimulation of either cytokine by in vitro bacterial lysate-activated IL-10-/- APC. Consistent with previous results, bacterial lysate-pulsed 129 wild type APC stimulated production of significantly less IFNγ and IL-17 by activated IL-10-/- MLN CD4+ T cells than did IL-10-/- APC (Fig 2a, 2b).
FIGURE 2.
Cytokine production from co-cultures of CD4+ T lymphocytes isolated from MLN of either colitic mice (GF→SPF IL-10-/- CD4+ MLN cells, grey bars) or noncolitic mice (SPF 129 wild type CD4+ MLN cells, white bars) stimulated by cecal bacterial lysate-pulsed APC (APC only, black bars). APC were obtained from either noncolitic mice (GF 129, GF IL-10-/-, SPF 129) or from colitic mice (SPF IL-10-/-). Triplicate supernatants were harvested at day 3 and assayed by ELISA for (a) IFNγ, (b) IL-17, (c) IL-12/IL-23 p40, and (d) IL-10. Results shown are mean ± SD of cytokines in triplicate cultures and are representative of four independent experiments.
3.5 APC from GF mice produce low levels of IL-12/IL-23 p40 and high levels of IL-10
Both GF and SPF IL-10-/- bacterial lysate-pulsed APC produced detectable levels of IL-12/IL-23 p40 (Fig. 2c). However, significantly more IL-12/IL-23 p40 was detected in cultures of IL-10-/- CD4+ lymphocytes stimulated with SPF IL-10-/- APC compared to cultures stimulated with GF IL-10-/- APC (2559 ± 361 v 1405 ± 245, p<0.001). These results indicate that in vivo exposure to enteric bacteria or the in vivo inflammatory milieu enhances the ability of cecal bacterial lysate-pulsed APC to produce IL-12/IL-23 p40 when co-cultured with IL-10-/- CD4+ from colitic mice. Nonetheless, while GF IL-10-/- APC secrete less IL-12/IL-23 p40 than do SPF IL-10-/- APC, both induce equivalent amounts of IFNγ and IL-17 production by IL-10-/- CD4+ lymphocytes from mice with colonic inflammation (Fig. 2a, 2b). Thus, the difference between the ability of 129 wild type and IL-10-/- APC to induce both of these cytokines is inherent to the lack of IL-10 and not secondary to chronic inflammation. APC obtained from GF and SPF 129 wild type mice produce equivalent amounts of IL-10 in response to cecal bacterial lysate (Fig. 2d). IL-10 production by GF and SPF APC is significantly upregulated when co-cultured with CD4+ lymphocytes from inflamed GF→SPF IL-10-/- mice. As noted in Fig. 1d, 129 wild type CD4+ MLN T cells secreted small amounts of IL-10 when cultured with bacterial lysate-pulsed IL-10-/- APC. These results demonstrate that components of cecal contents stimulate IL-10 production by APC and that activated CD4+ MLN cells can augment IL-10 production by APC from normal hosts.
3.6 IFNγ addition to cecal bacterial lysate-pulsed APC augments IL-10 production by 129 wild type APC but not IL-12/IL-23 p40 production by IL-10-/- APC
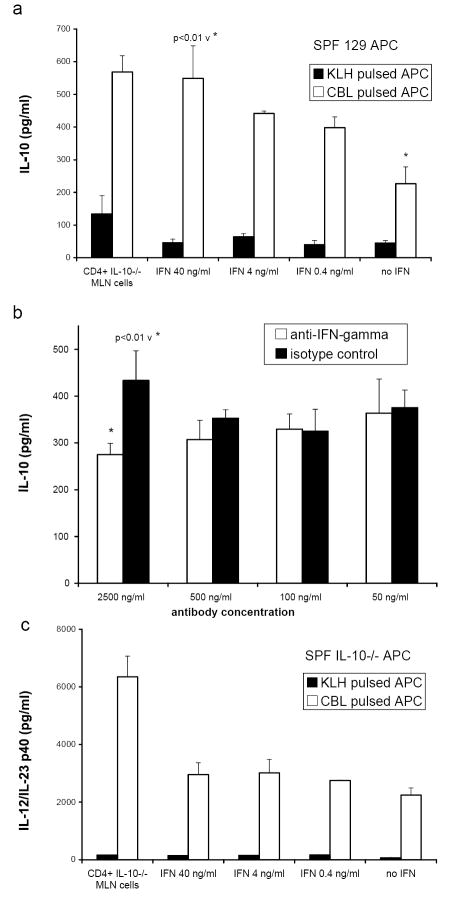
We then sought to determine whether IFNγ was responsible for the increased IL-10 secretion induced in 129 wild type APC. APC were pulsed overnight with either bacterial lysate or KLH and then cultured either with CD4+ lymphocytes from the MLN of inflamed IL-10-/- mice or with varying concentrations of recombinant murine IFNγ. The addition of IFNγ in concentrations equivalent to those produced by bacterial lysate-pulsed APC/CD4+ MLN lymphocyte cultures, induced IL-10 production by bacterial lysate-pulsed but not by KLH-pulsed 129 wild type APC (Fig. 3a). Recombinant IFNγ (40 ng/ml) induced APC to secrete a comparable amount of IL-10 as found in co-cultures with activated CD4+ MLN cells from IL-10-/- mice. Therefore, activation by cecal bacterial lysate stimulation and IFNγ are both necessary for the induction of IL-10 by APC while signals provided by contact with CD4+ lymphocytes are not required. APC cultured without exogenous antigen functioned similarly to KLH-pulsed APC in all experiments (data not shown).
FIGURE 3.
Cytokine production by APC pulsed with either control protein KLH (black bars) or cecal bacterial lysate (CBL, white bars) and co-cultured with CD4+ T lymphocytes from colitic mice (GF→SPF IL-10-/-) or recombinant murine IFNγ (0.4, 4, or 40 ng/ml) (a) and (c) or anti-IFN-γ antibody (white bars) or isotype control antibody (black bars) at 50, 100, 500, or 2500 ng/ml (b). Triplicate supernatants were harvested at day 3 and assayed by ELISA for (a) IL-10 secretion by 129 wild type APC co-cultured with IL-10-/- CD4+ cells or recombinant IFN-γ, (b) IL-10 secretion by 129 wild type APC co-cultured with IL-10-/- CD4+ T cells in the presence of either anti-IFN-γ antibody or isotype control antibody, and (c) IL-12/IL-23 p40 secretion by IL-10-/- APC co-cultured with IL-10-/- CD4+ cells or recombinant IFN-γ. Results shown are mean ± SD of cytokines in triplicate cultures and are representative of three independent experiments.
Further evidence to support a role for IFNγ in the induction of IL-10 in APC-T cell co-cultures was obtained in experiments using anti-IFNγ antibody. Although the effect is modest, we observed a significant reduction in the amount of IL-10 detected in supernatants of cecal bacterial lysate pulsed APC co-cultured with SPF IL-10-/- CD4+ T cells in the presence of 2500 ng/ml anti-IFNγ antibody compared to an equivalent amount of isotype control antibody (Fig. 3b).
Bacterial lysate-pulsed IL-10-/- APC produced dramatically more IL-12/IL-23 p40 than did KLH-pulsed IL-10-/- APC (Fig 3c). However, in contrast to IL-10 production, the addition of recombinant IFNγ to bacterial lysate-pulsed IL-10-/- APC did not significantly increase IL-12/IL-23 p40 production over this baseline, yet bacterial lysate-pulsed IL-10-/- APC produced even higher amounts of IL-12/IL-23 p40 when cultured with CD4+ MLN lymphocytes from GF→SPF IL-10-/- mice. Therefore, cecal bacterial lysate stimulation and IFNγ do not provide the necessary signals for the full induction of IL-12/IL-23 p40 by APC. It is likely that either contact with activated CD4+ lymphocytes or molecules other than IFNγ enhanced IL-12/IL-23 p40 production by bacterial lysate-pulsed IL-10-/- APC.
3.7 Elevated proportion of CD11c+ cells in the spleens of IL-10-/- mice
We next analyzed the phenotype of the spleen cells from SPF 129 wild type mice and from SPF colitic IL-10-/- mice that were used as the source of APC after the depletion of T lymphocytes. The T cell depleted splenocytes from 129 wild type mice contained higher proportions of B220+ cells (129 wild type v IL-10-/-; 89.2 ± 2.8% v 82.7 ± 3.5%, p < 0.05), while the IL-10-/- spleens contained a reciprocally higher percentage of CD11c+ cells (129 wild type v IL-10-/-; 4.8 ± 0.6% v 7.8 ± 1.5%, p < 0.01). Likewise, IL-10-/- T cell depleted spleens contained more CD11b single positive cells (129 wild type v IL-10-/-; 1.6 ± 0.4% v 6.6 ± 0.9% p < 0.02) and CD11b/CD11c double positive cells (129 wild type v IL-10-/-; 2.0 ± 0.3% v 3.6 ± 0.6%, p < 0.1). In addition, we evaluated the expression of the costimulatory molecules on wild type and IL-10-/- APC after pulsing overnight with cecal bacterial lysate. A higher percentage of bacterial lysate-pulsed IL-10-/- APC expressed CD86 compared to bacterial lysate-pulsed wild type APC. The difference in CD86 expression was consistently observed in four separate experiments (71.4 ± 4.1% v 56.7 ± 4.6%, p < 0.005). No reproducible differences in CD40, CD80 and MHC Class II expression were noted between wild type and IL-10-/- APC, and no significant differences were seen between wild type and IL-10-/- APC pulsed with the control protein KLH (data not shown).
A small percentage of CD4+ cells remained after T cell depletion (<5%), although this population was greatly diminished after overnight pulsing. Therefore, the composition of the two sources of APC is phenotypically distinct.
3.8 Percentage of CD11c cells in APC correlates with increased IFNγ, IL-17, IL-12/IL-23 p40, and IL-10 production
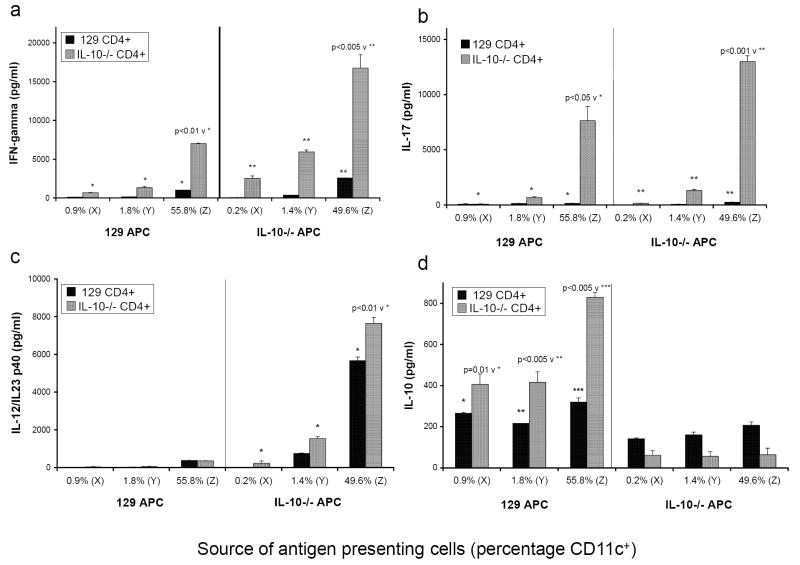
To determine which cell type was primarily responsible for APC activity, the APC preparations were enriched for B220+ cells or for CD11c+ cells as described in Materials and Methods. The percentage of CD11c+ cells, determined by flow cytometry after overnight pulsing, is indicated (Fig. 4). As the percentage of CD11c+ cells increased in the culture, the induction of IFNγ and IL-17 production by IL-10-/- CD4+ lymphocytes was enhanced for both 129 wild type and IL-10-/- APC, although IL-10-/- APC were consistently more efficient (Fig. 4a, 4b). As shown in Figures 1b and 2b, unseparated 129 wild type APC did not produce detectable IL-12/IL-23 p40 under any culture conditions. Although 129 wild type APC did produce small amounts of IL-12/IL-23 p40 when enriched for CD11c+ cells, the amount was the same when the APC were cultured with either 129 wild type CD4+ T lymphocytes or with activated IL-10-/- CD4+ T lymphocytes (Fig 4c, 383 ± 5 v 355 ± 11 pg/ml). For IL-10-/- APC, as the percentage of CD11c+ cells increased, the production of IL-12/IL-23 p40 significantly increased and production of this molecule was enhanced further by the presence of activated IL-10-/- CD4+ T lymphocytes (Fig. 4c). Conversely, the depletion of CD11c+ cells and the enrichment of B220+ cells eliminated most of the IL-12/IL-23 p40 from the IL-10-/- APC cultures (Fig. 4c). The production of IL-10 in co-cultures of IL-10-/- APC with 129 wild type CD4+ lymphocytes remained fairly constant as the percentage of CD11c+ cells increased, reflecting IL-10 production by the wild type 129 CD4+ T lymphocytes (Fig. 4d). IL-10 detected in cultures of 129 APC with 129 CD4+ lymphocytes also remained constant as the percentage of CD11c+ cells increased, although the source of the IL-10 could not be identified. However, the production of IL-10 in cultures of wild type 129 APC with IL-10-/- CD4+ lymphocytes increased significantly as the percentage of CD11c+ cells increased in the APC population. Because only the wild type 129 APC in these cultures can produce IL-10, the APC can be identified as the source of the IL-10. As in the cultures containing unseparated APC, 129 APC cocultured with IL-10-/- CD4+ T lymphocytes from colitic mice secreted significantly more IL-10 than did 129 APC cultured with 129 CD4+ T lymphocytes. These results indicate that CD11c+ cells provide the primary source for IL-12/IL-23 p40 and IL-10 secretion following cecal bacterial lysate stimulation and are the most active APC governing CD4+ cell stimulation. It should be noted that neither IL-12 p70 nor IL-23 p19/p40 were detectable in these culture supernatants. Either these two molecules are not secreted, or they are produced in very low amounts, or utilized during the 3 day incubation period.
FIGURE 4.
T cell depleted SPF 129 and IL-10-/- APC were either (X) enriched for B220+ cells by negative selection of CD11b and CD11c cells using MACS, (Y) unfractionated after T cell depletion, or (Z) enriched for CD11c+ by positive selection using MACS. The percentage of CD11c+ cells was determined by flow cytometry after overnight pulsing with cecal bacterial lysate and is indicated in the figure below each set of bars for each population. B220+ enriched cells (X), T cell depleted (Y), and CD11c+ enriched (Z) APC from both SPF 129 mice and SPF IL-10-/- mice were co-cultured with CD4+ cells from noncolitic SPF 129 mice (black bars) or colitic GF→SPF IL-10-/- mice (grey bars). Triplicate supernatants were harvested at day 3 and assayed by ELISA for (a) IFNγ, (b) IL-17, (c) IL-12/IL-23 p40, and (c) IL-10. Results shown are mean ± SD of cytokines in triplicate cultures and are representative of four independent experiments.
For a more direct analysis of capacity of CD11c+ cells to function as APC, we used high speed cell sorting to obtain purified CD11c+ cells from the spleens of SPF 129 and IL-10-/- mice. Due to low yield of this cell type, especially from spleens of wild type mice, we did not pulse the cells overnight. Instead, we co-cultured CD11c+ cells (1 × 104 per well) together with cecal bacterial lysate and IL-10-/- MLN cells highly enriched for CD4+ cells by elimination of cells expressing CD11b, CD11c, B220 and CD8 as described in Material and Methods. This population of enriched CD4+ cells did not respond to bacterial lysate in the absence of APC (data not shown). Even though we detected much lower levels of IFNγ and IL-17 compared to amounts in supernatants of co-cultures shown in Figure 4, probably as a result of using 30-fold lower numbers of APC, the results presented in Figure 5 confirm that CD11c+ cells are potent APC that are capable of producing IL-12/IL-23 p40 when cultured with cecal bacterial lysate. IL-10 was undetectable in these supernatants, most likely due to the low number of CD11c+ APC. It should be noted that the differences in the ability of APC from IL-10-/- vs wild type mice to induce of CD4+ T cell responses are less dramatic when CD11c+ cells are enriched (Fig 4a, Fig 4b, Fig 5a, and Fig 5b) compared to APC after T cell depletion only (Fig. 2a, Fig. 2b).
FIGURE 5.
SPF 129 or IL-10-/- spleen cells were enriched for CD11c+ cells by cell sorting and co-cultured with CD4+ cells from colitic GF→SPF IL-10-/- mice in the presence of KLH (black bars) or cecal bacterial lysate (grey bars). Supernatants were harvested at day 3 and assayed by ELISA for (a) IFNγ, (b) IL-17, and (c) IL-12/IL-23 p40. Results shown are mean ± SD of cytokines in triplicate cultures and are representative of two independent experiments.
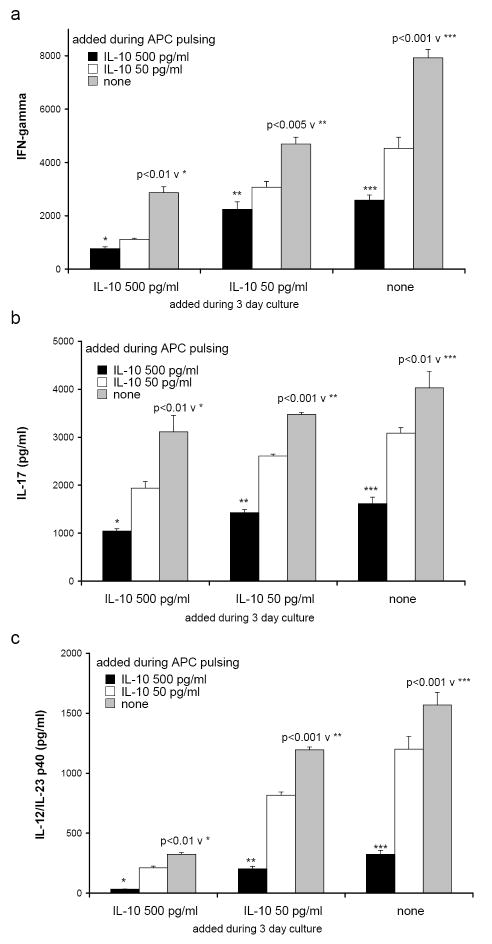
3.9 Recombinant murine IL-10 added to cultures containing IL-10-/- APC and IL-10-/- CD4+ lymphocytes reduced the production of IFNγ, IL-17, and IL-12/IL-23 p40
To confirm that IL-10 inhibits cytokine production in our model system, we next added exogenous recombinant murine IL-10 to IL-10-/- APC during the overnight pulse with cecal bacterial lysate. The APC were washed as usual to remove any excess bacterial components and then additional recombinant IL-10 was added directly to the co-cultures of IL-10-/- APC and IL-10-/- CD4+ T lymphocytes from colitic mice. We chose 500 and 50 pg/ml to approximate the amounts of IL-10 we measured in co-cultures of 129 wild type APC plus IL-10-/- CD4+ T lymphocytes. As the amount of IL-10 added (from 0 to 500 pg/ml) was increased during pulsing of APC with cecal bacterial lysate or independently during the APC plus CD4+ T cell co-cultures, the secretion of IFNγ by the CD4+ lymphocytes decreased (Fig. 6a). Likewise, addition of IL-10 inhibits IL-17 production (Fig. 6b). In parallel, as the amount of IL-10 was increased during the APC pulse and during co-culture, the production of IL-12/IL-23 p40 also decreased (Fig. 6c). Thus, the introduction of IL-10 to the in vitro cultures can alter the function of the IL-10-/- APC to approximate the function of the wild type APC. Inhibition is optimal when IL-10 is present both during the pulsing step with cecal bacterial lysate as well as the co-culture with CD4+ MLN cells, but significantly decreased IL-12/IL-23 p40, IFNγ, and IL-17 secretion are noted at either pulsing or co-culture.
FIGURE 6.
Recombinant murine IL-10 (500 pg/ml, black bars; 50 pg/ml, white bars; or medium alone, grey bars) was added to SPF IL-10-/- APC during the overnight pulse with cecal bacterial lysate. The APC were washed and then additional IL-10 (500 or 50 pg/ml or medium) was added for the duration of the 3 day co-culture with CD4+ T lymphocytes from GF→SPF IL-10-/- colitic mice. Triplicate supernatants were harvested at day 3 and assayed by ELISA for (a) IFNγ, (b) IL-17, and (c) IL-12/IL-23 p40. Results shown are mean ± SD of cytokines in triplicate cultures and are representative of two independent experiments.
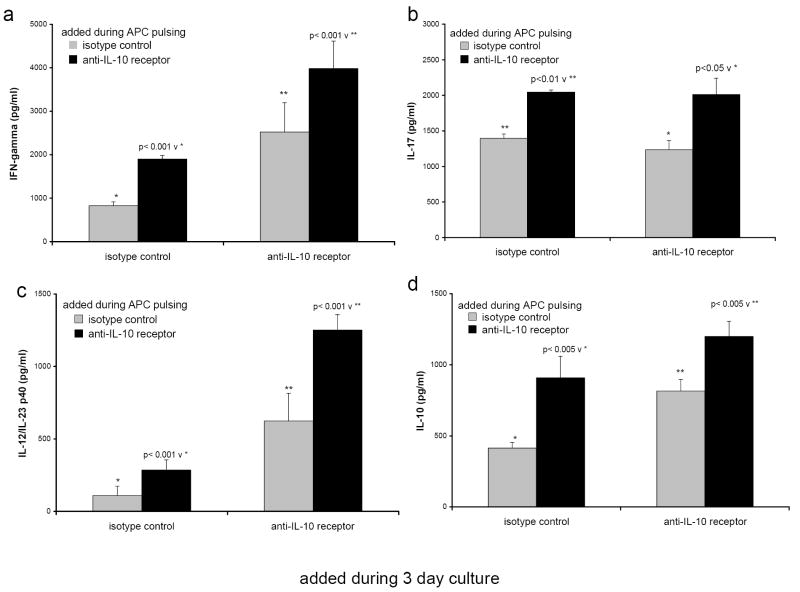
3.10 Anti-IL-10 receptor antibody added to wild type 129 APC plus IL-10-/- CD4+ lymphocyte cultures increased the production of IFNγ, IL-17, and IL-12/IL-23 p40
We next evaluated the effects of endogenous IL-10 by blocking its activity with anti-IL-10 receptor antibody (anti-IL-10R). This reagent or the isotype control was added to 129 wild type APC at 30 μg/ml during the overnight pulse with cecal bacterial lysate. The APC were washed as usual to remove excess bacterial components and then additional anti-IL-10R or isotype control antibody was added directly to the co-cultures of APC plus IL-10-/- CD4+ T lymphocytes from colitic mice. The anti-IL-10R used for this study acts as an antagonistic antibody blocking the binding and subsequent signaling by IL-10. Adding anti-IL-10R either during the pulse or the APC/CD4+ cell co-culture increased the production of IFNγ (Fig 7a) and IL-17 (Fig 7b) by the IL-10-/- CD4+ T lymphocytes. Likewise, adding anti-IL-10R either during the pulse or the co-culture increased the production of IL-12/IL-23 p40 by the wild type 129 APC (Fig 7c). When anti-IL-10R was added to both the pulse and the culture, the secretion of IL-12/IL-23 p40 by the 129 APC was elevated compared to 129 APC cultured with the isotype control. Blocking the IL-10 receptor resulted in an increase of IL-10 in the cultures (Fig 7d) presumably due to elevated IFNγ levels as show in Fig 7a. Thus, blocking IL-10 during the in vitro cultures can alter wild type APC function to approximate the function of the IL-10-/- APC. As with the recombinant IL-10 addition experiments, blocking IL-10 signaling with anti-IL-10R antibody was effective during APC pulsing with bacterial lysate and during APC/CD4+ lymphocyte co-culture, but was most effective when applied during both pulsing and co-culture.
FIGURE 7.
Anti-IL-10 receptor antibody (black bars) or the isotype control, purified rat IgG1 (grey bars), was added to SPF 129 APC at 30 μg/ml during the overnight pulse with cecal bacterial lysate. The APC were washed and then additional anti-IL-10R or rat IgG1 at 30 μg/ml was added for the duration of the 3 day co-culture of the 129 APC and IL-10-/- CD4+ T lymphocytes from colitic mice. Triplicate supernatants were harvested at day 3 and assayed by ELISA for (a) IFNγ, (b) IL-17, (c) IL-12/IL-23 p40, and (d) IL-10. Results shown are mean ± SD of cytokines in triplicate cultures and are representative of two independent experiments.
4. Discussion
IL-10 plays a critical role in maintaining the balance between immune activation and tolerance. Insufficient or absent IL-10 leads to overzealous immune activation and chronic inflammation while excess IL-10 leads to immune suppression and increased susceptibility to infection [35,36,45]. In this study we show that endogenous IL-10 from APC is a key regulator of both innate and acquired immune responses to physiologic enteric bacterial stimuli. CD4+ T cells from inflamed IL-10 deficient mice secreted more IFNγ and IL-17 when cultured with cecal bacterial lysate-pulsed IL-10 deficient APC than when cultured with 129 wild type APC. Moreover, IL-10 deficient APC secreted high levels of IL-12/IL-23 p40 when pulsed overnight with cecal bacterial lysate; this secretion was enhanced by co-culture with IFNγ producing CD4+ T cells from IL-10 deficient mice. APC from normal mice, however, did not secrete detectable levels of IL-12/IL-23 p40 after bacterial lysate pulsing. Instead, these APC responded to activated CD4+ T cells or to added recombinant IFNγ by secreting higher amounts of IL-10. Blockade of IL-10 signaling in normal APC or the addition of physiologic amounts of recombinant IL-10 to IL-10 deficient APC mimicked the function of IL-10 deficient or normal wild type APC, respectively.
Several important experiments highlight the role of IL-10 from regulatory T cell cells in the prevention of colitis. For example, the cotransfer of CD45RBlo cells from IL-10 deficient mice fails to prevent chronic colitis in the CD45RBhigh T cell transfer model [39]. In separate experiments, colitis induced by IFNγ-producing bacterial antigen specific CD4+ clones was prevented by co-transfer of IL-10-producing clones [40]. These and other studies clearly identify T cells as one source of IL-10 with the ability to regulate inflammation. However, our results demonstrate that IL-10 produced by bacterial antigen and adjuvant-pulsed APC regulates IFNγ and IL-17 production by bacterial antigen-responsive CD4+ T cells in vitro. We have extended these observations by evaluating intestinal inflammation in an adoptive CD4+ cell transfer system using Rag2-/-recipients crossed with either IL-10-/- or IL-10+/+ mice [46]. Our results clearly show that IL-10 production by antigen presenting cells but not by CD4 T cells plays a dominant role in preventing colitis. Also, recent studies published by Gu et al confirm that IL-10 is a potent inhibitor of IL-17 and of the transcription factor RORγt that is critical for the development of IL-17 producing cells [47].
IL-10 deficient mice develop chronic, immune mediated colitis in an SPF environment but inflammation does not develop under GF conditions [25]. CD4+ MLN T cells from GF→ SPF IL-10 deficient mice with colitis secrete IFNγ and IL-17 in response to endogenous enteric bacterial antigens processed and presented by APC from both IL-10 deficient and normal mice. CD4+ T cells isolated from GF mice are predominantly naïve and CD4+ T cells from GF IL-10 deficient mice did not secrete IFNγ when stimulated by cecal bacterial lysate-pulsed 129 wild type APC. Thus the naïve IL-10 deficient T cells from the MLN of GF mice are functionally distinct from IL-10 deficient T cells of SPF mice, which are activated in vivo, presumably by exposure to IL-10 deficient APC that have processed commensal enteric bacterial antigens. Once activated, these IL-10 deficient CD4+ MLN cells continued to produce IFNγ and IL-17 when exposed ex vivo to bacterial lysate-pulsed APC, even in the presence of IL-10 either secreted by wild type APC or added to IL-10 deficient APC. In contrast, APC isolated from either GF or SPF IL-10 deficient mice are functionally similar. Both cell populations, after overnight pulsing with cecal bacterial lysate, stimulated CD4+ IL-10 deficient T cells to secrete high amounts of IFNγ and IL-17, although GF APC secreted less IL-12/IL-23 p40 compared to SPF APC. Therefore, functional differences between IL-10 deficient APC and 129 APC are due to the presence or absence of endogenous IL-10 and are not dependent on in vivo activation by commensal bacteria or proinflammatory cytokines in the inflamed colon.
Normal wild type SPF mice maintain immune tolerance to their commensal microbiota. When MLN CD4+ T cells from GF or SPF wild type mice were cultured with bacterial lysate-pulsed wild type APC, IL-10 but not IFNγ, IL-17 or IL-12/IL-23 p40 was produced. The observed IL-10 was most likely secreted by both APC and CD4+ T cells. However, despite in vivo tolerance to enteric bacteria, CD4+ T cells from normal mice could be activated in vitro by bacterial lysate-pulsed IL-10 deficient APC to secrete IFNγ and IL-17, showing that lack of IL-10 production by APC can reverse tolerance. One possible explanation involves the increased expression of CD86 by IL-10 deficient APC [48]. Other cellular components that may also contribute to the potency of IL-10 deficient APC include the chemokines, cytokines, and additional signaling molecules identified in a proteomic analysis of Chlamydia antigen-activated dendritic cells from IL-10 deficient mice [49].
The importance of intrinsic IL-10 in suppressing pathologic mucosal immune responses to commensal enteric bacteria is confirmed by similar phenotypes of colitis with either IL-10 deletion or disruption of components of the IL-10 signaling pathway. Deleting class II cytokine receptor-4 (CRF2-4), a subunit of the IL-10 receptor, or the selective deletion of Stat3 in macrophages and neutrophils leads to colitis dominated by a Th1 profile of cytokines [50,51]. Similarly, in our in vitro culture system, the addition of neutralizing anti-IL-10R antibody both during the pulse and the co-culture of wild type 129 APC with CD4+ T cells significantly increased IFNγ and IL-17 secretion by IL-10-/- CD4+ T cells as well as the production of IL-12/IL-23 p40. Conversely, when physiologically relevant amounts of recombinant IL-10 were added both during the pulse and the co-culture with CD4+ T cells, the IL-10 deficient APC functioned similarly to 129 wild type APC: IL-12/IL-23 p40 secretion was eliminated while the induction of IFNγ and IL-17 in CD4+ MLN cells from IL-10 deficient mice with colitis was significantly reduced. Importantly, recombinant IL-10 added only during the overnight APC pulse also significantly reduced IL-12/IL-23 p40, IFNγ, and IL-17 production by co-cultured IL-10 deficient APC and CD4+ T cells, although not to levels observed in supernatants of co-cultures containing wild type 129 APC.
An important aspect of our study is the observation that activated IL-10-/- CD4+ MLN cells stimulated IL-10 secretion by wild type 129 APC. This upregulation of IL-10 appeared to be induced, at least in part, by IFNγ as demonstrated by the ability of relevant concentrations of recombinant IFNγ, in the absence of CD4+ MLN cells, to induce IL-10 secretion by bacterial lysate-pulsed APC. We also demonstrated a modest reduction in IL-10 secretion in supernatants of CD4+ IL-10-/- MLN cells stimulated with bacterial lysate-pulsed 129 APC in the presence of anti-IFNγ antibody. It is possible that the antibody does not effectively block IFN-γ mediated signaling or that IFN-γ is not the sole inducer of IL-10 in this system. Nevertheless, this response of normal APC to proinflammatory cytokines could provide an important mechanism of downregulating inflammatory responses to restore tolerance to commensal bacteria and to prevent chronic inflammation after a self-limited stimulus, such as clearance of a bacterial pathogen. In this context, it is of interest that IL-10 is capable of protecting epithelial cells from IFN-γ mediated damage [52].
Enrichment of B220+ cells (B lymphocytes) or CD11c+ cells (dendritic cells) indicated that CD11c+ cells were primarily responsible for bacterial lysate-stimulated APC-induced cytokine production. IL-10, IL-12/IL-23 p40 and induction of IFNγ and IL-17 increased proportionally with the percentage of CD11c+ cells. Splenic APC from colitic SPF IL-10 deficient mice contained approximately twice as many CD11c+ cells relative to spleen cells from 129 wild type mice., Although enrichment of CD11c+ cells reduced the difference between the amounts of cytokines produced in co-cultures containing IL-10 deficient APC compared to wild type APC, differential responses were not merely a consequence of different CD11c+ cell numbers, since different cytokine profiles were also observed in co-cultures containing equivalent proportions of wild type and IL-10 deficient CD11c+ APC.
Previous reports have shown IL-10 production by B lymphocytes, macrophages, and dendritic cells [19,31,41,45,49,53]. Recently, results published by Monteleone et al demonstrated IL-10 but not IL-12p70 production by TLR ligand-activated dendritic cells derived from the intestinal lamina propria [54]. These results are consistent with our previous observations that IL-10 deficient bone marrow-derived dendritic cells produce much higher concentrations of IL-12/IL-23 p40 in response to LPS than do dendritic cells from wild type mice [53]. In our current study, bacterial lysate pulsed APC enriched for CD11c+ cells produced 2 fold more IL-10 than did pulsed APC enriched for B220+ cells in co-cultures containing CD4+ IL-10 deficient cells. Similarly, Iwasaki and Kelsall demonstrated that a CD11c+CD11b+ enriched population of splenocytes upregulated IL-10 production after stimulation with Staph aureus, Cowan’s strain (SAC) plus recombinant IFNγ [55]. In contrast, IL-10 producing B cells are dominant in a model of arthritis that could be prevented by transfer of B cells, activated in vitro with antigen and anti-CD40 antibody, from normal but not IL-10 deficient mice [56]. In SPF HLAB27 transgenic rats that spontaneously develop colitis, B cells are the major source of IL-10 in in vitro cultures of MLN cells stimulated with cecal bacterial lysate [31]. In summary, our results strongly implicate endogenous IL-10 production by APC as a key regulator of tolerogenic innate and acquired mucosal immune responses to commensal intestinal bacteria. Moreover, induction of IL-10 secretion by APC, especially CD11c+ cells, in response to IFNγ could be an important determinant of the restoration of homeostasis after immune activation.
Acknowledgments
The authors thank Donna Kronstadt (Gnotobiotic Animal Core, Center for Gastrointestinal Biology and Disease (CGIBD), North Carolina State University, College of Veterinary Medicine, Raleigh) for maintaining the gnotobiotic mice; Desmond McDonnell, Robert Williams, and Lisa Wiltron for their technical assistance; and the CGIBD for providing administrative support and assistance with statistical analysis.
Grant support: NIH DK53347 (RBS and SLT), Center for Gastrointestinal Biology and Disease (NIH DK34987), Crohn’s and Colitis Foundation of America research grants (RBS)
Abbreviations
- APC
antigen presenting cells
- CBL
cecal bacterial lysate
- CRF2
class II cytokine receptor family
- DC
dendritic cells
- GF
germ-free
- IBD
inflammatory bowel diseases
- IL-10-/-
IL-10 deficient
- KLH
keyhole limpet hemocyanin
- MACS
magnetic activated cell sorting
- MLN
mesenteric lymph nodes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–94. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Duchmann R, May E, Heike M, Knolle P, Neurath M, Meyer zum Buschenfelde KH. T cell specificity and cross reactivity towards Enterobacteria, Bacteroides, Bifidobacterium, and antigens from resident intestinal flora in humans. Gut. 1999;44:812–18. doi: 10.1136/gut.44.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landers CJ, Cohavy O, Misra R, Yang H, Lin YC, Braun J, et al. Selected loss of tolerance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689–99. doi: 10.1053/gast.2002.35379. [DOI] [PubMed] [Google Scholar]
- 5.Elson CO. Genes, microbes, and T cells - New therapeutic targets in Crohn’s disease. N Engl J Med. 2002;346:614–16. doi: 10.1056/NEJM200202213460812. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, et al. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn’s disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, et al. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–78. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- 8.Monteleone G, Trapasso F, Parrello T, Biancone L, Stella A, Iuliano R, et al. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol. 1999;163:143–7. [PubMed] [Google Scholar]
- 9.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 10.Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, et al. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis. 2008;14:437–45. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]
- 11.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor α by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–9. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 14.Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004;351:2069–79. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 15.Colombel JF, Cortot A, Van Kruiningen HJ. Antibiotics in Crohn’s disease. Gut. 2001;48:647. doi: 10.1136/gut.48.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–33. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 18.Ma A, Datta M, Margosian E, Chen J, Horak I. T cells, but not B cells, are required for bowel inflammation in interleukin 2-deficient mice. J Exp Med. 1995;182:1567–72. doi: 10.1084/jem.182.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 20.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–76. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–10. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 22.Spencer DM, Veldman GM, Banerjee S, Willis J, Levine AD. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology. 2002;122:94–105. doi: 10.1053/gast.2002.30308. [DOI] [PubMed] [Google Scholar]
- 23.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izcue A, Hue S, Buonocore S, Arancibia-Cárcamo CV, Ahern PP, Iwakura Y, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–70. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sellon RK, Tonkonogy SL, Schultz M, Dieleman LA, Grenther W, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veltkamp C, Tonkonogy SL, de Jong YP, Albright C, Grenther WB, Balish E, et al. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tgε26 mice. Gastroenterology. 2001;120:900–13. doi: 10.1053/gast.2001.22547. [DOI] [PubMed] [Google Scholar]
- 27.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, et al. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: Increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–64. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirtz S, Finotto S, Kanzler S, Lohse AW, Blessing M, Lehr HA, et al. Cutting edge: chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transfer by TNF-plus IFN-γ-producing CD4+ T cells that respond to bacterial antigens. J Immunol. 1999;162:1884–8. [PubMed] [Google Scholar]
- 30.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieleman LA, Hoentjen F, Qian B-F, Sprengers D, Tjwa E, Torres MF, et al. Reduced ratio of protective versus proinflammatory cytokine responses to commensal bacteria in HLA-B27 transgenic rats. Clin Exp Immunol. 2004;136:30–9. doi: 10.1111/j.1365-2249.2004.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 34.Dieleman LA, Arends A, Tonkonogy SL, Goerres MS, Craft DW, Grenther W, et al. Helicobacter hepaticus does not induce or potentiate colitis in interleukin-10-deficient mice. Infect Immun. 2000;68:5107–13. doi: 10.1128/iai.68.9.5107-5113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 36.Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3–15. doi: 10.1189/jlb.0904484. [DOI] [PubMed] [Google Scholar]
- 37.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005;35:1831–40. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 38.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 39.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–19. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 41.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 42.Chen CC, Louie S, McCormick BA, Walker WA, Shi HN. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J Immunol. 2006;176:472–483. doi: 10.4049/jimmunol.176.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradley LM, Duncan DD, Tonkonogy S, Swain SL. Characterization of antigen-specific CD4+ effector T cells in vivo: immunization results in a transient population of MEL-14-, CD45RB- helper cells that secretes interleukin 2 (IL-2), IL-3, IL-4, and interferon gamma. J Exp Med. 1991;174:547–59. doi: 10.1084/jem.174.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips JM, Parish NM, Drage M, Cooke A. Interactions through the IL-10 receptor regulate autoimmune diabetes. J Immunol. 2001;167:6087–91. doi: 10.4049/jimmunol.167.11.6087. [DOI] [PubMed] [Google Scholar]
- 45.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 46.Liu B, Holt L, Sartor RB. APC regulate Th1/Th17-mediated colitis in IL-10 deficient mice. Gastroenterology. 2007;132:A33. [Google Scholar]
- 47.Gu Y, Yang J, Ouyang X, Liu W, Li H, Yang J, et al. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38:1807–13. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sansom DM, Manzotti CN, Zheng Y. What’s the difference between CD80 and CD86? Trends Immunol. 2003;24:314–9. doi: 10.1016/s1471-4906(03)00111-x. [DOI] [PubMed] [Google Scholar]
- 49.He Q, Moore TT, Eko FO, Lyn D, Ananaba GA, Martin A, et al. Molecular basis for the potency of IL-10-deficient dendritic cells as a highly efficient APC system for activating Th1 response. J Immunol. 2005;174:4860–9. doi: 10.4049/jimmunol.174.8.4860. [DOI] [PubMed] [Google Scholar]
- 50.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, et al. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187:571–78. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 52.Jarry A, Bossard C, Bou-Hanna C, Masson D, Espaze E, Denis MG, et al. Mucosal IL-10 and TGF-β play crucial roles in preventing LPS-driven, IFN-γ-mediated epithelial damage in human colon explants. J Clin Invest. 2008;118:1132–42. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-κB recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105:689–96. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]
- 54.Monteleone I, Platt AM, Jaensson E, Agace WW, Mowat AM. IL-10-dependent partial refractoriness to Toll-like receptor stimulation modulates gut mucosal dendritic cell function. Eur J Immunol. 2008;38:1533–47. doi: 10.1002/eji.200737909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8α+, and double-negative Peyer’s patch dendritic cells. J Immun. 2001;166:4884–90. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 56.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]