Abstract
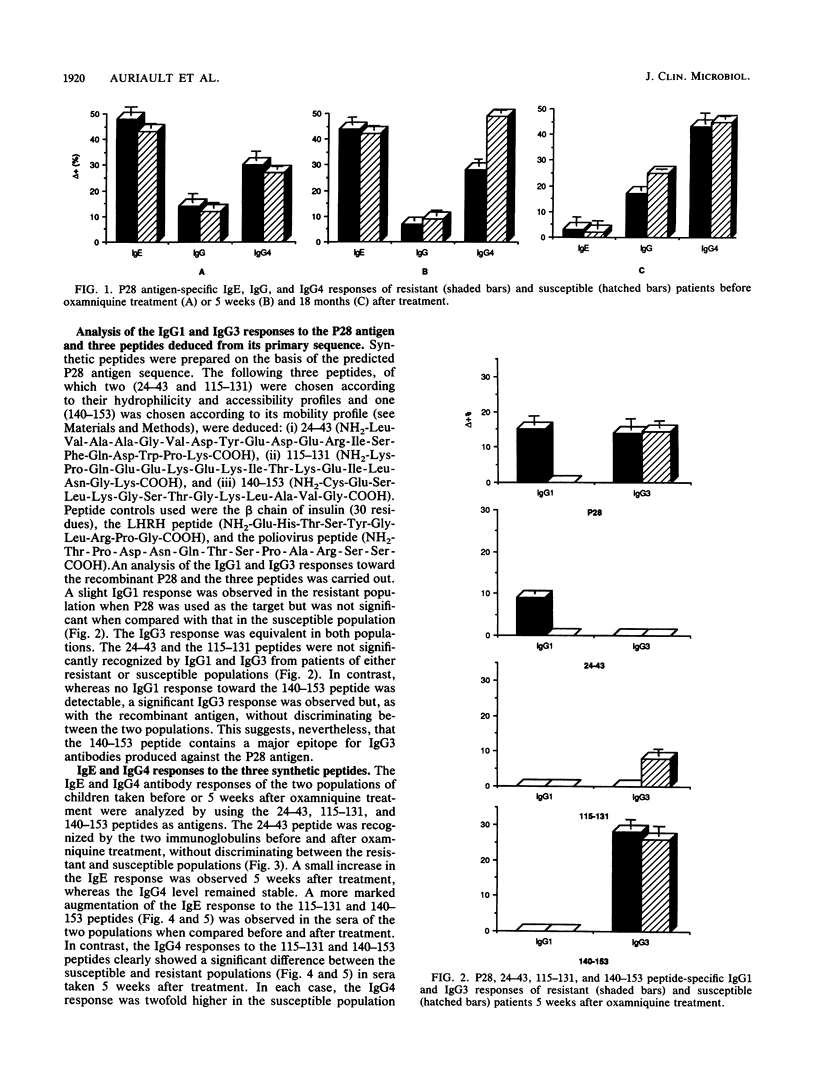
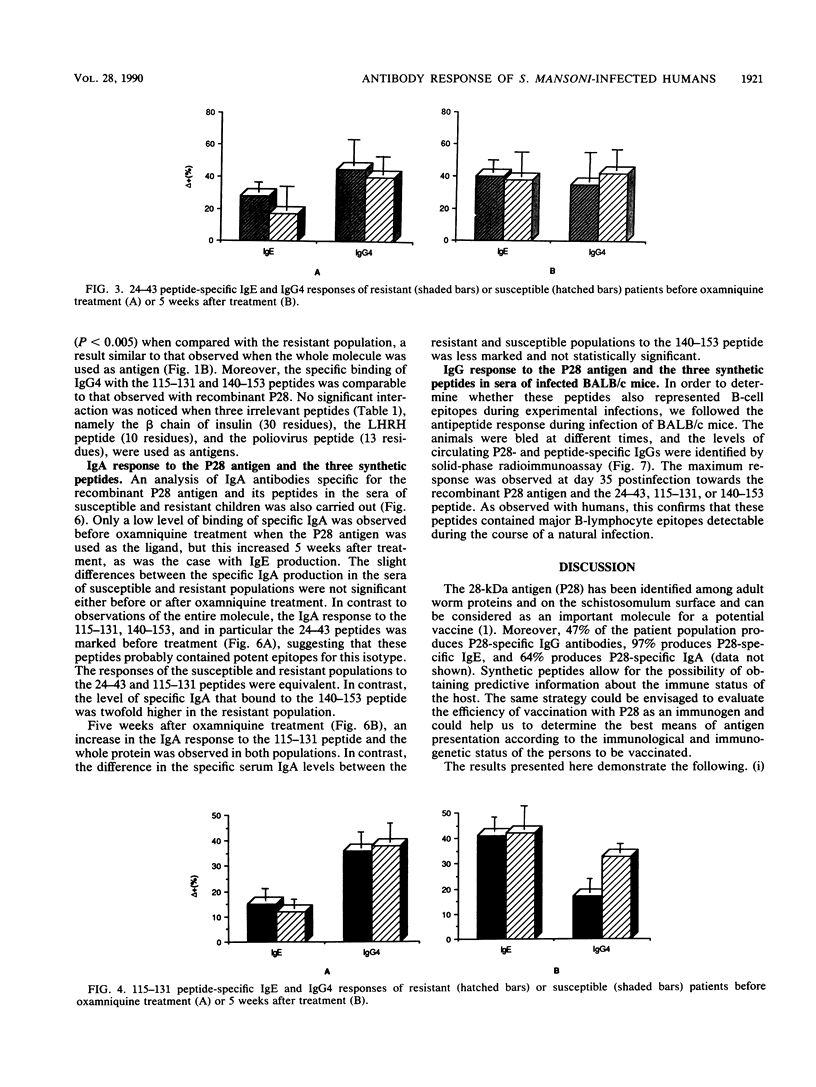
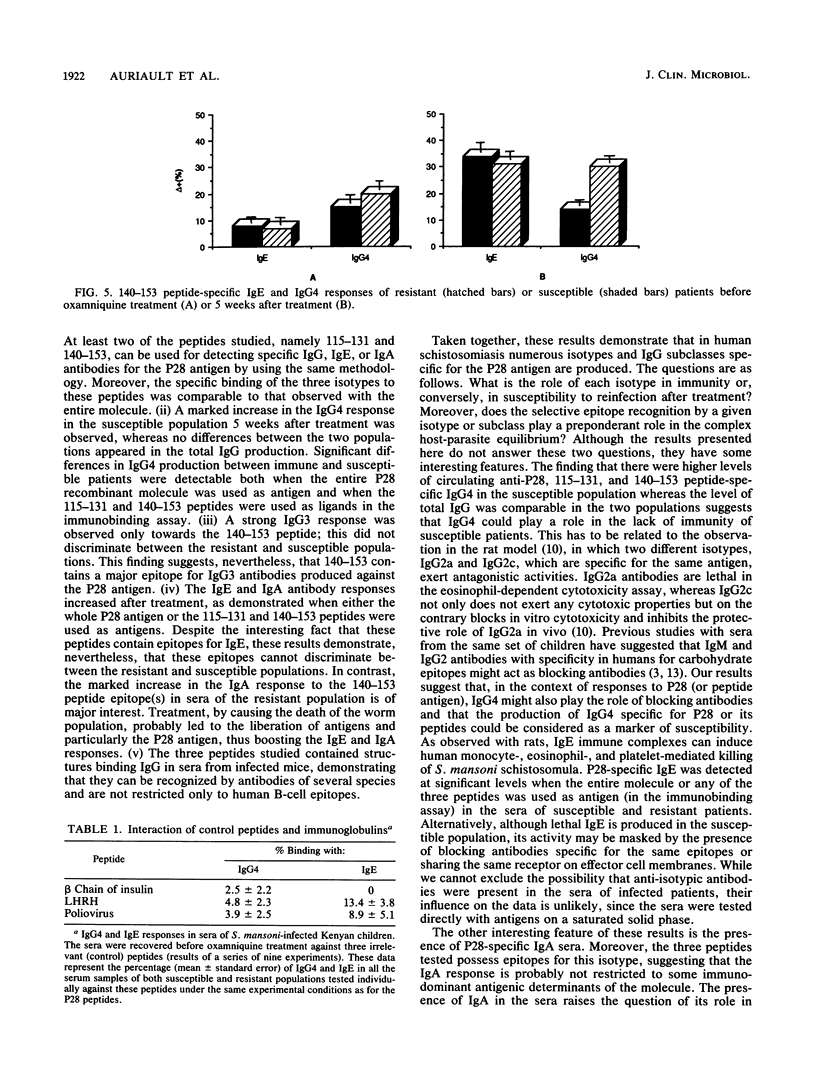
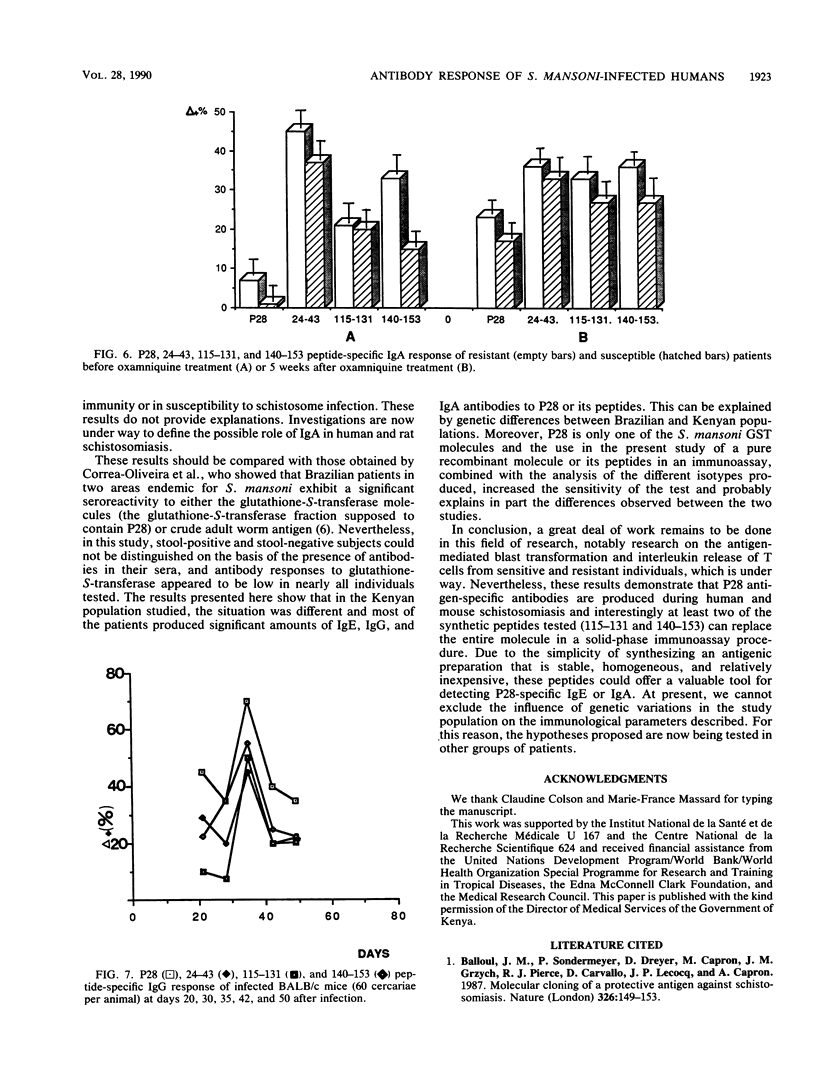
The 28-kilodalton antigen of Schistosoma mansoni has been previously described as having importance as the basis for a potential vaccine. The P28 recombinant molecule and three peptides derived from its primary sequence, namely the 24-43, 115-131, and 140-153 peptides, have been tested to evaluate the humoral responses of Kenyan school children previously classified as susceptible or resistant to reinfection after chemotherapy. We report here that the P28 molecule and two of the peptides studied (peptides 115-131 and 140-153) can be used for detecting specific immunoglobulin G (IgG), IgE, and IgA antibodies. Moreover, the IgG4 response of the susceptible population was significantly greater than that of the resistant group, whereas no differences between the two populations were noticed in total IgG anti-P28 antibodies. This suggested that IgG4 could play a role in the lack of immunity of susceptible patients. A strong IgG3 response restricted to the 140-153 peptide was observed but did not discriminate between the resistant and susceptible populations. In contrast, a marked increase in the IgA response to the 140-153 peptide epitope(s) in sera of the resistant population was noticed. Taken together, these results suggest that the P28 antigen and two of the three peptides selected could give predictive information about the development of the disease or the efficiency of vaccination with P28 as the immunogen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balloul J. M., Sondermeyer P., Dreyer D., Capron M., Grzych J. M., Pierce R. J., Carvallo D., Lecocq J. P., Capron A. Molecular cloning of a protective antigen of schistosomes. Nature. 1987 Mar 12;326(6109):149–153. doi: 10.1038/326149a0. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Bensted-Smith R., Capron A., Capron M., Dalton P. R., Dunne D. W., Grzych J. M., Kariuki H. C., Khalife J., Koech D. Immunity in human schistosomiasis mansoni: prevention by blocking antibodies of the expression of immunity in young children. Parasitology. 1987 Apr;94(Pt 2):281–300. doi: 10.1017/s0031182000053956. [DOI] [PubMed] [Google Scholar]
- Butterworth A., Dunne D., Fulford A., Capron M., Khalife J., Capron A., Koech D., Ouma J., Sturrock R. Immunity in human schistosomiasis mansoni: cross-reactive IgM and IgG2 anti-carbohydrate antibodies block the expression of immunity. Biochimie. 1988 Aug;70(8):1053–1063. doi: 10.1016/0300-9084(88)90268-4. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P. Effector and regulatory mechanisms in immunity to schistosomes: a heuristic view. Annu Rev Immunol. 1985;3:455–476. doi: 10.1146/annurev.iy.03.040185.002323. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Correa-Oliveira R., Pearce E. J., Oliveira G. C., Golgher D. B., Katz N., Bahia L. G., Carvalho O. S., Gazzinelli G., Sher A. The human immune response to defined immunogens of Schistosoma mansoni: elevated antibody levels to paramyosin in stool-negative individuals from two endemic areas in Brazil. Trans R Soc Trop Med Hyg. 1989 Nov-Dec;83(6):798–804. doi: 10.1016/0035-9203(89)90334-9. [DOI] [PubMed] [Google Scholar]
- Dissous C., Prata A., Capron A. Human antibody response to Schistosoma mansoni surface antigens defined by protective monoclonal antibodies. J Infect Dis. 1984 Feb;149(2):227–233. doi: 10.1093/infdis/149.2.227. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The helical hydrophobic moment: a measure of the amphiphilicity of a helix. Nature. 1982 Sep 23;299(5881):371–374. doi: 10.1038/299371a0. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Grzych J. M., Capron M., Dissous C., Capron A. Blocking activity of rat monoclonal antibodies in experimental schistosomiasis. J Immunol. 1984 Aug;133(2):998–1004. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J. Surface and inside volumes in globular proteins. Nature. 1979 Feb 8;277(5696):491–492. doi: 10.1038/277491a0. [DOI] [PubMed] [Google Scholar]
- Khalife J., Dunne D. W., Richardson B. A., Mazza G., Thorne K. J., Capron A., Butterworth A. E. Functional role of human IgG subclasses in eosinophil-mediated killing of schistosomula of Schistosoma mansoni. J Immunol. 1989 Jun 15;142(12):4422–4427. [PubMed] [Google Scholar]
- Ponnuswamy P. K., Bhaskaran R. Differential equation model to study dynamic behaviour of globular proteins. Int J Pept Protein Res. 1984 Aug;24(2):168–179. doi: 10.1111/j.1399-3011.1984.tb00943.x. [DOI] [PubMed] [Google Scholar]
- Taylor J. B., Vidal A., Torpier G., Meyer D. J., Roitsch C., Balloul J. M., Southan C., Sondermeyer P., Pemble S., Lecocq J. P. The glutathione transferase activity and tissue distribution of a cloned Mr28K protective antigen of Schistosoma mansoni. EMBO J. 1988 Feb;7(2):465–472. doi: 10.1002/j.1460-2075.1988.tb02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


