Abstract
Agonists for TLR7, TLR8, and TLR9 have been shown to enhance vaccine immunogenicity. We evaluated the impact of TLR activation on RSV disease in a murine model by administering TLR7/8 and TLR9 agonists during FI-RSV immunization or RSV infection. CpG administered during immunization reduced disease following challenge as evidenced by decreased lung pathology, illness, and cytokines. In marked contrast, TLR7/8 agonist had little impact. To evaluate potential therapeutic use, TLR agonists were administered during primary infection. Although type 2 cytokine responses decreased and type 1 cytokines and MIP1-α/β increased, both TLR7/8 and TLR9 agonists increased clinical symptoms and pulmonary inflammation when administered during primary infection. Thus, TLR9-induced signaling during FI-RSV immunization reduced vaccine-enhanced disease whereas immunostimulatory properties of TLR agonists enhanced disease severity when used during RSV infection. Immunomodulation elicited by TLR9 agonist confirms the adjuvant potential of TLR agonists during RSV immunization. However, in contrast to work done HIV-1 vaccines, the inability of TLR7/8 agonist to boost type 1 vaccine-induced RSV immunity demonstrates pathogen-TLR specificity. These data reveal that the timing of administration of immunomodulatory agents is critical. Furthermore, these data underscore that amplification of anti-viral immune responses may result in immunopathology rather than immune-mediated protection.
Keywords: RSV, Toll-like receptors, eosinophil, adjuvant, pathogenesis, immunization, pathology
INTRODUCTION
Respiratory syncytial virus (RSV) is the leading cause of childhood hospitalizations due to viral infections and causes significant disease in the elderly and in bone marrow transplant recipients. Despite extensive efforts, no safe and effective vaccine or antiviral therapy is currently licensed for RSV. Passive prophylactic administration of the RSV-specific neutralizing antibody Synagis® to premature infants is the only approved intervention for RSV [1]. Development of RSV vaccines has been hampered by failed vaccine trials in which infants immunized with formalin-inactivated alum-precipitated RSV (FI-RSV) were not protected against subsequent RSV infection, but experienced more severe disease, often requiring hospitalization and resulting in two deaths. Despite use of molecular cloned RSV immunogens it has been difficult to arrive at the right degree of attenuation for live candidate RSV vaccines that have sufficient immunogenicity and acceptable reactogenicity in all infants [2].
Toll-like receptors (TLRs) are a group of pattern recognition receptors (PRR) serving as sentinel molecules for detection of pathogens expressing pathogen-associated pattern molecules (PAMPs) [3]. Several TLRs are of particular importance to induction of antiviral immunity and include TLR3, TLR7 and TLR8, and TLR9 [4]. While traditionally thought of as dendritic cell proteins, PRRs are also expressed on many cells of immune and non-immune function. Distribution of these TLRs and their impact on antiviral immunity has been recently reviewed [5;6]. TLR-PAMP interactions at mucosal surfaces elicit cytokine production, recruiting dendritic cells to and initiating immune responses at sites of inflammation and infection. Thus, selective activation of individual TLRs through agonist binding has been hypothesized as a means to modulate and enhance immunity [7].
While severe RSV disease, induced by immunization, allergen sensitization in animal models, or primary infection in humans, has often been associated with the induction of type 2 T cell responses and eosinophilia, the complexity of RSV disease suggests there are multiple factors, of both viral and host origin, contributing to immunopathology [8-11]. Interference with cytokine function and the use of adjuvants does not completely alleviate disease, but has modest effects or modulates only certain aspects of disease [12-17], illustrating the intricacies of RSV pathogenesis. The multi-factorial nature of severe RSV disease, led us to hypothesize that “targeted” activation of specific innate immune response pathways might more effectively induce protective and disease-sparing immunity than interference with a single component of adaptive immunity. We therefore investigated TLR agonists as adjuvants or immunotherapy for RSV. TLR7/8 and TLR9 agonists were used to modulate the immune responses induced by FI-RSV immunization either during the initial induction phase (at priming) or during the recall effector phase (at challenge), hypothesizing that administration of TLR agonists would boost the induction of type 1 primary immune responses to minimize generation of Th2 responses during immunization and to counterbalance Th2 memory responses during challenge. In particular, we hypothesized that augmenting relevant TLR7/8 signaling pathways may provide an immune advantage to the host since TLR7 and TLR8 recognize ssRNA, creating responses well-suited to control ssRNA viruses such as RSV. Natural RSV infection does not induce long-term protective immunity [18;19]. We therefore evaluated the potential therapeutic use of TLR agonists to enhance RSV-specific immune responses during primary infection, hypothesizing that TLR-mediated signals might overcome the ability of RSV to inhibit type I IFN production [20;21] and result in sustained immunity.
In the course of these studies, reports were published examining CpG effects on immune responses induced during immunization with purified RSV F or G [22-24] or with killed bovine RSV [25;26] or during allergen sensitization [27]. Collectively, these studies show the presence of CpG during induction of immunity results in more Th1-associated immune responses and increases protection against RSV challenge. We confirm these studies using formalin-inactivated human RSV and expand upon them evaluating airway mucus and a broad array of cytokines and chemokines. Furthermore, we test the efficacy of TLR7/8 agonists as an adjuvant, examine the ability of CpG and TLR7/8 agonists given at challenge to modulate Th2 memory responses generated by FI-RSV, and evaluate the effects of these agonists when given during primary RSV infection. While FI-RSV will not be used as a vaccine in humans, it is a well-defined complex, multi-component immunogen that induces type 2 memory immune responses and provides a system for evaluating immunomodulatory strategies. These data support the potential for using TLR agonists during RSV immunization, even in the context of a more complex whole virus immunogen. However, the importance of timing and the specific TLR agonist used in a particular host will have to be cautiously evaluated due to the potential for TLR agonists to induce immunopathology rather than immunoprotection.
MATERIALS AND METHODS
Viruses and TLR Agonists
HEp-2 and Vero cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD) and maintained in Eagle’s minimal essential media supplemented with 10% fetal calf serum, glutamine, and antibiotics (10% EMEM). A challenge stock of RSV A2 strain was prepared as previously described [28]. A formalin-inactivated alum-precipitated stock RSV (FI-RSV) was prepared in Vero cells as described [29]. CpG 1826 oligodeoxynucleotides (Class B) were purchased from Coley Pharmaceutical Corp (Ottawa, Canada). TLR7/8 agonists R848 and the structural analog 3M-012 were provided by 3M Pharmaceuticals (St. Paul, MN).
Mouse Immunization and Challenge
Wild-type BALB/c mice were purchased from Charles Rivers Laboratories (Indianapolis, IN). Mice were immunized intramuscularly with 0.1 ml FI-RSV diluted 1:10 in 1X PBS. For the mice receiving TLR agonist at challenge, 25 μg/ml CpG, 20 μg/ml TLR7/8 agonist (R848 in experiment 1 and 3M-012 in experiment 2), or indicated concentrations of each agonist was added to the FI-RSV. Doses of CpG and TLR7/8 were used as this is an established model of viral vaccine immunization at the Vaccine Research Center [30]. This dose of CpG is also in the range of doses which have been used in murine and cotton rat studies of RSV vaccines [22;24;25]. Mice were challenged with live RSV (107 pfu in 0.1ml) six weeks later as previously described [31]. To evaluate the effects of TLR agonist at challenge of FI-RSV-immunized mice or during primary infection, mice were anesthetized, and CpG, TLR7/8 agonist, or both (in 0.1 ml PBS) were administered intranasally at day 2 post-infection. DC trafficking through regional lymph nodes peaks by 16-24 hours after pulmonary infection [32] and DC recruitment to the lung peaks by day 4 after RSV infection [33], Therefore, TLR agonists were administered at day 2 post-RSV infection to insure maximal effects of TLR agonist treatment on RSV pathogenesis might be observed. Control mice were anesthetized and administered 0.1 ml PBS. The mice were weighed daily following RSV challenge.
RSV Titers
Mice were euthanized at day 4 and day 7 post-challenge, and lungs were removed and quick-frozen. Plaque-forming units (pfu) of virus were measured by plaque assay as previously described [31]. Data are expressed as log10(pfu/gram lung tissue).
Cytokine Levels
IL-4, IL-5, IL-10, IL-12, IL-13, IFN-γ, MIP-α, MIP-1β, eotaxin and TNF-α protein levels in lung supernatants from day 4 and day 7 plaque assays were quantitated by ELISA using cytokine-specific kits (R & D Systems, Minneapolis, MN).
Peripheral and Bronchoalveolar Lavage (BAL) Eosinophils
To examine the effect of TLR agonist administration on the primary activation of eosinophils during immunization, mice were bled retro-orbitally at day seven post-immunization. Ten microliters of blood were spun onto slides, stained with Diff-Quik, and differential cell counts were made. To examine the eosinophilic response during the memory phase, BAL was performed seven days after RSV challenge as previously described [34].
Statistical Analysis
Illness, weight loss, and viral titers data were maintained in a Paradox database and analyzed with SAS statistical software as described [31]. Data for cytokine levels and BAL eosinophil were analyzed by analysis of variance comparing multiple groups using SigmaStat 3.0.1 software (SPSS Inc., Chicago, IL). P values less than 0.05 were considered statistically significant.
RESULTS
Reduction in vaccine-enhanced RSV disease is dependent on TLR specificity and timing of administration
Mice were immunized with FI-RSV containing CpG, TLR7/8 agonist, or both Statistically significant improvements in weight loss were observed when CpG was present during FI-RSV immunization, with either CpG alone (Figure 1A, p<0.05 relative to FI-RSV only controls on days 7-10 post-infection) or in combination with TLR7/8 agonist (days 5-10 post-infection). In contrast, TLR7/8 agonist alone had minimal impact. Administration of TLR agonists during RSV challenge of FI-RSV-immunized mice increased weight loss (Figure 1B, p<0.05 CpG or TLR7/8 agonist alone relative to PBS-treated FI-RSV-immunized mice at days 3 and 4).
Figure 1. Weight Loss Following RSV Challenge in FI-RSV-Immunized Mice Treated with TLR Agonists.
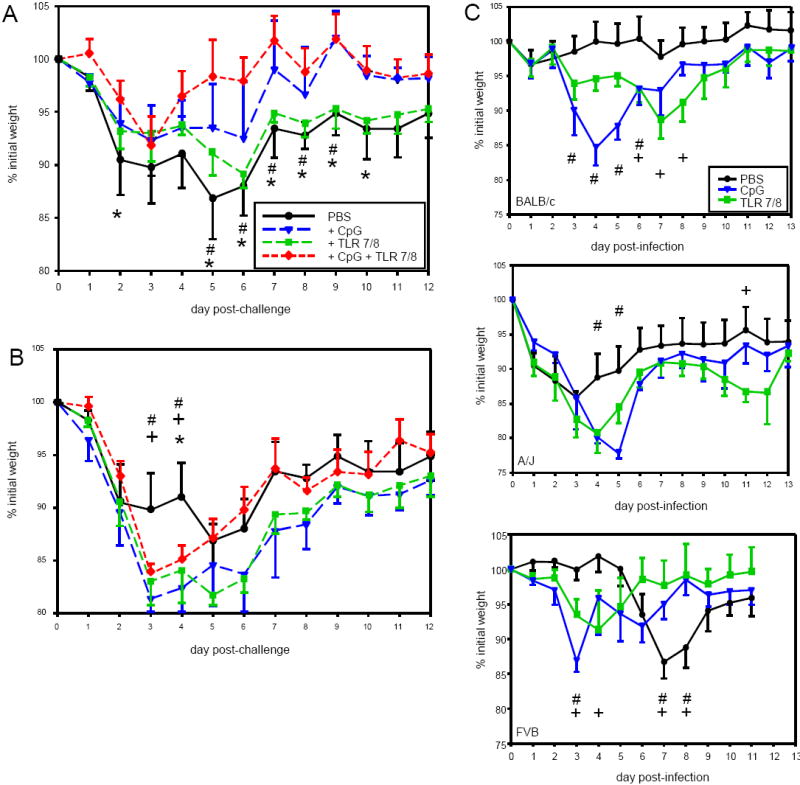
Panel A – BALB/c mice were treated with the indicated TLR agonists during FI-RSV immunization. Six weeks later the mice were challenged IN with live RSV and weighed days 0-12 after challenge. Data are represented as the percentage of weight (in grams) relative to the base weight at day 0 for n=6 mice per group in a representative experiment (of 2).
Panel B – BALB/c mice were immunized with FI-RSV IM and, six weeks later, challenged with live RSV. Two days after challenge, the mice were treated with the indicated TLR agonist. Data represent base weight for n=6 mice per group from a single experiment.
Panel C – Mice of the indicated strains were infected with live RSV IN. Two days after primary infection, the mice were treated with the indicated TLR agonist. Data represent base weight for n=5 mice per group from a single experiment
CpG and TLR7/8 agonist were administered during primary RSV infection of BALB/c mice and of FVB and A/J mice, models with increased susceptibility to lung pathology (FVB, our unpublished data) and reactive airways disease (A/J) [35], to determine whether immunity could be enhanced or disease reduced. CpG treatment resulted in a significant enhancement of weight loss early in infection, spanning days 3-6 (Figure 1C, p<0.05 relative to PBS-treated mice of the same strain). Although the magnitude of increased illness was similar with 15-20% weight loss from day 0, the duration of the effect was different between strains, with the impact in BALB/c mice being most extended. TLR7/8 agonist enhanced RSV-induced weight loss, but the timing of the effect varied (Figure 1C). In both A/J and FVB mice TLR7/8 agonist induced an early peak of weight loss that reached statistical significance in FVB mice at days 3 and 4 post-infection while weight loss in BALB/c mice did not peak until day 7 post-infection. Interestingly, in A/J mice administration of TLR7/8 agonist resulted in a second wave of weight loss very late after infection (days 10-12) with statistically significant differences observed at day 11 post-infection. Only FVB mice evidenced an advantage from TLR treatments with more rapid recovery of weight loss (p<0.05 days 7-8 relative to PBS-treated A/J mice). Thus, TLR activation has differential impact on RSV disease severity depending upon TLR specificity, host genetics, and time of administration relative to live RSV infection.
Only CpG administration during FI-RSV immunization increases protection against RSV challenge
Mice immunized with FI-RSV and treated with CpG, alone or in combination with TLR7/8 agonist, had significantly reduced peak viral titers (Figure 2A) with no detectable virus in the CpG only group. In contrast, mice treated with TLR7/8 agonist during immunization or mice treated with either TLR agonist during challenge had no significant change in viral replication.
Figure 2. RSV Titers in mice treated with TLR agonist during RSV challenge or during primary infection.
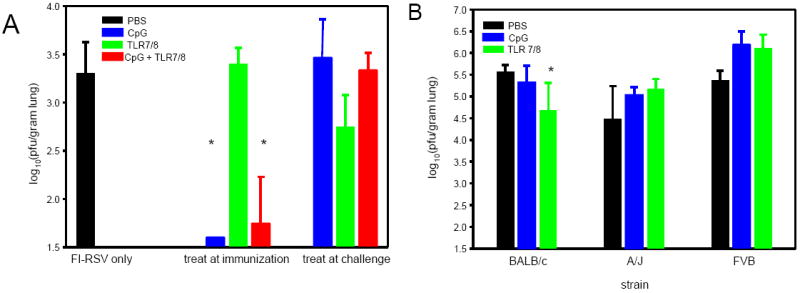
BALB/c mice were immunized with FI-RSV and treated with TLR agonists either during immunization or during RSV challenge (panel A) and mice of different strains were treated with TLR agonists during primary RSV infection (panel B) as detailed in Figure 1. RSV titers were measured in the lung at day 4 post-challenge by plaque assay. The data are represented as log10(pfu/gram lung tissue) for n=5-6 mice per group.
In mice treated during primary RSV infection (Figure 2B), only BALB/c mice treated with TLR7/8 agonist had significant reductions in day 4 viral titers, while CpG treatment had no effect. In contrast, both TLR agonists increased viral titers in A/J and FVB mice, although the differences were not statistically significant. These data demonstrate that the enhanced disease induced by TLR agonist administration during RSV infection (Figures 1B and 1C) is not due to increased viral burden and its consequent pathology.
Adding CpG to FI-RSV immunogen increases pre-challenge neutralizing antibody titers
Inclusion of CpG, either alone or in combination with TLR7/8 agonist, increased the RSV neutralizing antibody titers in pre-challenge sera (Table 1), but TLR7/8 agonist alone had no significant effect. In contrast, all FI-RSV-immunized mice (Table 1) and all mice treated with TLR agonist during primary RSV infection (Table 2) had similar levels of neutralizing antibodies in serum six weeks after RSV infection. Thus, the ability of TLR9-mediated signals triggered by CpG to augment antibody responses during immunization is lost during the strong murine antibody response to live virus infection. This loss of enhancement may not accurately predict the response in humans.
Table 1.
RSV Neutralizing Antibody in FI-RSV-Immunized Mice
| TLR Agonist | Log2(reciprocal dilution 60%) | ||
|---|---|---|---|
| At Priming | At Challenge | Day -1 Sera | Day 42 Sera |
| PBS | PBS | 2.73 ± 0.81 | 8.23 ± 1.56 |
| CpG | PBS | 5.62 ± 1.92a | 9.87 ± 0.58 |
| TLR7/8c | PBS | 3.59 ± 1.53 | 9.02 ± 0.73 |
| CpG + TLR7/8 c | PBS | 7.85 ± 0.02 a | 8.33 ± 1.56 |
| PBS | CpG | NT b | 7.24 ± 2.44 |
| PBS | TLR7/8 c | NT b | 8.34 ± 0.96 |
| PBS | CpG + TLR7/8 c | NT b | 8.93 ± 0.80 |
Statistically significant difference relative to PBS-treated group, p<0.05. N=10 in two separate experiments.
NT=not tested
Two separate experiments were performed, one using the TLR7/8 agonist R-848 and one with TLR7/8 agonist 3M-012. Results were similar between the 2 experiments.
Table 2.
RSV Neutralization Titers in Mice Treated with TLR Agonists during Primary RSV Infection
| Mouse Strain | TLR Agonist | Log2 (reciprocal dilution 60% neutralization) |
|---|---|---|
| BALB/c | PBS | 7.20 ± 1.14 |
| CpG | 7.26 ± 0.44 | |
| TLR7/8a | 8.57 ± 1.72 | |
| FVB | PBS | 7.47 ± 0.53 |
| CpG | 6.61 ± 0.52 | |
| TLR7/8a | 7.99 ± 1.37 | |
| A/J | PBS | 8.46 ± 0.73 |
| CpG | 8.93 ± 0.23 | |
| TLR7/8a | 9.13 ± 1.04 |
The TLR7/8 agonist 3M-012 was used in a single experiment. N=6 mice.
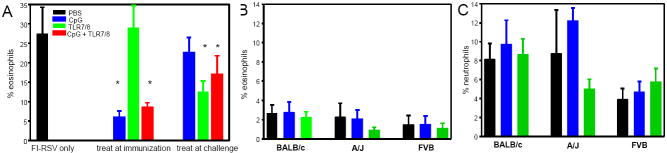
CpG treatment during FI-RSV immunization decreases eosinophil numbers in blood and BAL, while TLR7/8 agonist administered during RSV challenge reduces eosinophil recruitment
Mice immunized only with FI-RSV or with FI-RSV and TLR7/8 agonist had 23.2 ± 1.4 and 23.6 ± 4.8 percent of eosinophils in the blood, respectively, seven days after immunization while eosinophilia in mice immunized with FI-RSV + CpG or FI-RSV + CpG and TLR7/8 was 12.5 ± 4.8 and 7.8 ± 6.5, respectively. Eosinophils in BAL after RSV challenge of FI-RSV-immunized mice was significantly reduced in mice receiving CpG at immunization, either alone or in combination with TLR7/8 agonist (Figure 3A, p<0.05) while TLR7/8 agonist alone did not impact eosinophil recruitment. In marked contrast, TLR7/8 agonist, but not CpG, given during RSV challenge reduced eosinophil levels in BAL. Modulation of eosinophil recruitment observed in BAL was also observed in lung tissue (data not shown). Thus, as observed in illness, viral clearance, and pre-challenge neutralizing antibody titers, co-administration of CpG during FI-RSV immunization increased anti-viral responses and decreased immune responses associated with severe disease, but had little impact given at challenge while TLR7/8 agonist given during immunization had minimal effect, but reduced eosinophil recruitment when given at challenge.
Figure 3. BAL eosinophils and neutrophils in mice treated with TLR agonist during RSV challenge or during primary infection.
BALB/c mice were immunized with FI-RSV and treated with TLR agonists either during immunization or during RSV challenge (panel A) and mice of different strains were treated with TLR agonists during primary RSV infection (panel B and C) as detailed in Figure 1. Seven days after RSV infection, BAL was performed, and differential cell counts of the BAL cell pellets were made. The data are represented as the percentage of eosinophils (panels A and B) and neutrophils (panel C) for n=5-6 mice per group.
TLR7/8 agonists have been shown to activate and recruit eosinophils [36], and CpG has been shown to activate neutrophils [37]. Therefore, the BAL composition was examined in mice treated with TLR agonists during primary infection. Neither the TLR7/8 agonist 3M-012 nor CpG significantly altered the cellular composition in BAL (Figure 3B) or in the lung tissue (data not shown) in any strain of mice. Thus, enhanced illness induced by TLR treatment during primary RSV infection is not associated with a increased eosinophil or neutrophil recruitment in BAL or tissue, two cell populations implicated in vaccine-enhanced disease.
CpG treatment alters cytokine and chemokine production after RSV challenge
The administration of CpG during FI-RSV immunization, alone or with TLR7/8 agonist, reduced production of type 2 cytokines and eotaxin following RSV challenge with significant differences observed at day 4 post-challenge (Table 3). The impact of TLR7/8 agonist on cytokine production was less extensive with statistically significant increases observed only in day 4 IL-5 (data not shown) and day 7 IFN-γ levels. Use of CpG during challenge increased MIP-1α and MIP-1β production with statistically significant differences in MIP-1α levels. Thus, the use of CpG during FI-RSV immunization may modulate disease by reducing type 2 cytokine production while the presence of CpG during RSV challenge may enhance illness by increasing production of MIP-1α and MIP-1β While inclusion of TLR7/8 agonist at immunization or challenge increases IFN-γ levels, the inability to concomitantly decrease type 2 cytokine production may explain the for the ineffectiveness on eosinophil recruitment in TLR7/8-treated mice.
Table 3.
Cytokine/Chemokine Production After RSV Challenge of Mice Treated with TLR Agonists during FI-RSV immunization or during RSV challenge
| Day After Challenge | Treat at Priming | Treat at Challenge | IL-13c | Eotaxin | IFN-γ | MIP-1α | MIP-1β |
|---|---|---|---|---|---|---|---|
| Day 4 | PBS | PBS | 420 ± 97 | 1515 ± 533 | 683 ± 143 | 200 ± 62 | 341 ± 67 |
| CpG | PBS | 23 ± 7a | 93 ± 35a | 1451 ± 280 | 164 ± 39 | 301 ± 64 | |
| TLR7/8 agonist | PBS | 531 ± 39 | 1699 ± 275 | 1155 ± 144 | 306 ± 29 | 488 ± 67 | |
| CpG + TLR7/8 | PBS | 110 ± 24a | 397 ± 85 a | 1813 ± 265 | 167 ± 25 | 450 ± 88 | |
| PBS | CpG | 1812 ± 42a | 816 ± 73 | 731 ± 145 | 362 ± 36a | 484 ± 30 | |
| PBS | TLR7/8 agonist | 288 ± 54 | 848 ± 96 | 1147 ± 412 | 320 ± 53 | 403 ± 41 | |
| PBS | CpG + TLR7/8 | 188 ± 48a | 910 ± 141 | 652 ± 106 | 707 ± 108a | 644 ± 105 | |
| Day 7 | PBS | PBS | 112 ± 15 | 1587.6 ± 240.4 | 58 ± 8 | 108 ± 9 | 199 ± 38 |
| CpG | PBS | ND a,b | 269 ± 18a | 83 ± 15 | 110 ± 10 | 111 ± 12 | |
| TLR7/8 agonist | PBS | 220 ± 42 | 2469 ± 432 | 122 ± 20a | 167 ± 25 | 159 ± 25 | |
| CpG + TLR7/8 | PBS | 69 ± 39 | 234 ± 42a | 47 ± 4 | 66 ± 6 a | 81 ± 31 | |
| PBS | CpG | 107 ± 7 | 2391 ± 271 | 226 ± 31a | 810 ± 27 a | 493 ± 30 a | |
| PBS | TLR7/8 agonist | 63 ± 15 | 1588 ± 377 | 137 ± 22a | 192 ± 27 | 158 ± 58 | |
| PBS | CpG + TLR7/8 | 103 ± 36 | 1708 ± 209 | 237 ± 21a | 520 ± 45 a | 262 ± 50 |
Statistically significant relative mice treated with PBS at both priming and challenge, p<0.05.
ND = not detected
Changes in IL-4, IL-5, and IL-13 levels paralleled each other. For clarity and conciseness, only IL-13 data are shown as representative of type 2 cytokine production.
No impact on IL-4, IL-5, IL-12, IL-13, or eotaxin production was observed following TLR treatment during primary RSV infection in any strain of mouse (data not shown). Similarly, the use of 3M-012 had no significant impact on the production of IL-10, IFN-γ, MIP-1α, or MIP-1β (Table 4). In contrast CpG increased IL-10, MIP-1α and MIP-1β at day 4 post-infection and IFN-γ and MIP-1α production at day 7 post-infection in all strains of mice and IFN-γ and MIP-1β (at day 4 and 7, respectively) in A/J and FVB mice (Table 4).
Table 4.
Cytokine/Chemokine Production in Mice Treated with TLR Agonists During RSV Infection
| Day 4 pi | Day 7 pi | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | Treatment | IL-10 | IFN-γ | MIP-1α | MIP-1β | IL-10 | IFN-γ | MIP-1α | MIP-1β |
| BALB/c | PBS | 4 ± 5 | 59 ± 86 | 8 ± 14 | 100 ± 78 | 6 ± 7 | 98 ± 56 | 286 ± 102 | 785 ± 365 |
| CpG | 87 ± 53 a | 133 ± 139 | 710 ± 256 a | 1623 ± 456 a | 5 ± 7 | 295 ± 34 a | 676 ± 145 a | 1015 ± 160 | |
| 3M-012 | NDb | 309 ± 237 | 86 ± 71 | 456 ± 193 | NDb | 364 ± 95 a | 401 ± 172 | 1082.2 ± 213.4 | |
| A/J | PBS | NDb | NDb | NDb | 63 ± 113 | NDb | 71 ± 75 | 19 ± 22 | 117 ± 55 |
| CpG | 39 ± 11 a | 72 ± 34 a | 256 ± 64 a | 798 ± 143 a | 2 ± 1 | 519 ± 207 a | 312 ± 118 a | 658 ± 216 a | |
| 3M-012 | 11 ± 3 | 18.7 ± 7.4 | 22 ± 22 | 154 ± 38 | NDb | 125 ± 53 | 113± 47 | 326 ± 106 | |
| FVB | PBS | NDb | 14.7 ± 10.6 | 43 ± 42 | 188 ± 92 | NDb | 22 ± 7 | 125 ± 34 | 341 ± 57 |
| CpG | 140 ± 61 a | 103.4 ± 59.0 a | 952 ± 158 a | 2344 ± 249 a | 22 ± 7 a | 182 ± 81 a | 680 ± 110 a | 1340 ± 170 a | |
| 3M-012 | 12 ± 5 | 24 ± 22 | 54 ± 22 | 183 ± 30 | NDb | 51 ± 23 | 113 ± 46 | 302 ± 82 | |
Statistically significant relative to PBS-treated mice of the same strain, p<0.05.
ND = not detected
Cellular and cytokine responses in uninfected and RSV-infected TLR-treated mice differ
To evaluate the ability of TLR activation in the absence of RSV infection to induce pulmonary inflammation, mice were treated with CpG or TLR7/8 agonist. Cellular recruitment was examined daily for 5 days after TLR agonist treatment since the day 4 and 7 timepoints in RSV-infected mice are days 2 and 5 after treatment. Modest increases were seen in total cell number and in all cell populations but T cells (both CD4+ and CD8+) and B cells (supplemental data). Cell numbers peaked at day 1-2 after treatment. NK cell, eosinophil, neutrophil, monocyte, and macrophage levels rapidly declined to baseline (day 0) levels by day 4-5 while DC cell subsets began to decline at day 3 but did not generally baseline levels by day 5. Cytokine production also occurred rapidly, peaking by day 1 and declining by days 2-3 post-treatment (supplemental data). No type 2 cytokines were detected. While no IL-12 was detected in RSV-infected mice, CpG treatment elicited IL-12 in the absence of RSV. Conversely, IFN-γ, MIP-1α, and MIP-1β levels were higher in TLR-treated RSV-infected mice than in TLR-treated uninfected mice. Thus, the sustained increases seen with TLR-treated RSV-infected mice is not due solely to TLR-induced signaling, but reflects interactions of TLR-mediated signaling on RSV-induced responses.
Discussion
The young age of primary RSV infection, immunosenescence of the elderly, the failure of natural RSV infection to generate long-term protective immunity, and the history of vaccine-enhanced disease present considerable challenges to development of a safe and effective RSV vaccine. The ability of TLR agonists to modulate immunity provides new strategies that may improve vaccine design. In particular, TLR agonists have been shown to stimulate mature immune responses in neonates [38]. We therefore tested the ability of TLR7/8 and TLR9 agonists (1) to serve as adjuvants and modulate FI-RSV-enhanced disease immunization and (2) to serve as a potential therapeutic agent and alter pathogenesis during live RSV infection of naïve or immunized mice.
CpG has been proven an effective adjuvant in multiple models [22-26;30;39;40] inducing more type 1 CD4+ and CD8+ T cells. We demonstrate that CpG administration during FI-RSV immunization decreased disease and induced more Th1-like immune responses, reducing the Th2 bias of RSV-specific immunity induced by immunization with FI-RSV or purified RSV proteins. While TLR7/8 agonists have been successfully used as adjuvants during immunization with purfied HIV-1 proteins [30;39;40], this was not an effective strategy during FI-RSV immunization. This may be due to the complexity of alum-precipitated whole virus immunogen. It may be that the viral RNA present in FI-RSV saturated the TLR7 and TLR8 receptors in endosomal compartments. In contrast, administration of both TLR agonists tended to increase disease when given during live RSV challenge in the setting of pre-existing RSV immunity induced by FI-RSV priming. Addition of either TLR7/8 or TLR9 agonist during RSV infection enhanced disease severity as evidenced by weight loss (Figure 1) and higher levels of MIP-1α in the lung (Tables 3 and 4). Elevated levels of MIP-1α have previously been associated with severe illness in RSV-infected infants [41] and in murine models of RSV and pneumonia virus of mice pathogenesis [42;43]. Similarly, mice treated with TLR agonists during RSV infection often exhibited elevated levels of IFN-γ. This finding may suggest increased NK, NK T, and/or CD8+ T cell activity.
Administration of TLR agonists during RSV infection has the potential to broadly impact viral pathogenesis due to TLR expression not only on pulmonary dendritic cells [44], but also on airway epithelium [45;46] and NK cells present early during infection [47]. Interactions between RSV and several TLRs have been demonstrated as reviewed [48]. In the murine model of RSV pathogenesis NK T cells contribute to the activation and recruitment of virus-specific CD8+ T cells [49], and the magnitude of IFN-γ-producing NK cells correlates with the subsequent level of CD8+ T cell recruitment [50]. In addition, excessive numbers of RSV-specific CD8+ T cells result in severe disease during RSV infection [51]. MIP-1α has been shown to enhance CTL survival and function [52]. Thus, activation of dendritic cells, NK cells, and NK T cells and increased MIP-1α production induced by TLR agonists at the wrong time may amplify the virus-specific CD8+ T cell response, increasing immunopathology and disease. Similar factors may be involved in the enhanced pulmonary pathology observed after RSV challenge of cotton rats immunized with CpG and RSV F protein [24]. RSV has been shown to result in prolonged retention of DCs in the lung [32]. Thus, the two week interval between the second CpG-F intranasal immunization and RSV challenge may have resulted in altered T cell differentiation or recruitment, leading to severe pulmonary pathology. Additional analyses of the post-challenge immune responses would be informative in elucidating these mechanisms and contributing factors. While increased innate immunity has generally been associated with greater protection against RSV infection [20;21;53;54] and adaptive immune responses have more often been associated with disease [55], these data show innate responses can also contribute to immunopathology, depending upon the magnitude, timing, and composition. However, more extensive analyses are required to determine the precise basis for the enhanced disease severity observed here.
We also demonstrate that the genetic background of the host has consequences on the patterns of virus-specific immune responses, particularly when TLR treatment occurs during live RSV infection. While FI-RSV immunization uniformally predisposes for type 2 immune responses, the strain of mouse impacts the magnitude of the response as evidenced by at least twice as many eosinophils recruited to the lungs of FI-RSV-immunized RSV-challenged BALB/c mice compared to C57BL/6 mice [34]. Though not tested, it is hypothesized that CpG would enhance induction of Th1 immunity in all strains of mice, but that selected strains (e.g. C57BL/6) would evidence greater Th2 to Th1 shifts than would strains such as BALB/c. Studies demonstrate differences in early innate responses of particular strains induce more rapid type I responses, e.g. C57BL/6 mice have more NK cells while A/J mice have low NK cell activity [56] and 129 mice have greater numbers of pDCs [57]). Any one or a combination of these factors may then impact the induction of T cell and cytokine responses and result in different patterns of responsiveness in TLR treatment of various mouse strains.
These studies show CpG, but not TLR7/8 agonist, is an effective adjuvant capable of modulating FI-RSV-induced immune responses immunization, demonstrating a level of specificity in the ability of TLR agonists to function as vaccine adjuvants. Importantly, we demonstrate that the timing of immune interventions is of critical importance in RSV pathogenesis. While we have not identified the specific components of the immune responses resulting in increased disease, these data underscore that a precarious balance of RSV immunity exists and must be maintained to elicit immunoprotection without concomitant immunopathology.
Acknowledgments
We wish to acknowledge the technical assistance of Saran Bao. This research was supported by the Intramural Research Program of the National Institutes of Health, NIAID, Vaccine Research Center.
Footnotes
The authors have no conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cardenas S, Auais A, Piedimonte G. Palivizumab in the prophylaxis of respiratory syncytial virus infection. Expert Rev Anti Infect Ther. 2005;3:719–26. doi: 10.1586/14787210.3.5.719. [DOI] [PubMed] [Google Scholar]
- 2.Karron RA, Wright PF, Belshe RB, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Toll-like receptors and innate immunity. Nature Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann NY Acad Sci. 2008:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson TJ. Toll-like receptors, transduction-effector pathways, and disease diversity: evidence of an immunobiological paradigm explaining all human illness? Int Rev Immunol. 2008;27:255–81. doi: 10.1080/08830180801959072. [DOI] [PubMed] [Google Scholar]
- 6.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nature Rev Immunol. 2008;8:911–22. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–50. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 8.Durbin JE, Durbin RK. Respiratory syncytial virus-induced immunoprotection and immunopathology. Viral Immunol. 2004;17:370–80. doi: 10.1089/vim.2004.17.370. [DOI] [PubMed] [Google Scholar]
- 9.van Drunen Littel-van den Hurk S, Mapletoft JW, Arsic N, Kovacs-Nolan J. Immunopathology of RSV infection: prospects for developing vaccines without this complication. Rev Med Virol. 2007;17:5–34. doi: 10.1002/rmv.518. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TR, Graham BS. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23:S46–S57. doi: 10.1097/01.inf.0000108192.94692.d2. [DOI] [PubMed] [Google Scholar]
- 11.Miyairi I, Devincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev. 2008:686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y-W, Graham BS. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Invest. 1994;94:1953–8. doi: 10.1172/JCI117546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson TR, Parker RA, Johnson JE, Graham BS. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J Immunol. 2003;170:2037–45. doi: 10.4049/jimmunol.170.4.2037. [DOI] [PubMed] [Google Scholar]
- 14.Neuzil KM, Johnson JE, Tang Y-W, et al. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine. 1997;15:525–32. doi: 10.1016/s0264-410x(97)00218-1. [DOI] [PubMed] [Google Scholar]
- 15.Hussell T, Khan U, Openshaw PJM. IL-12 treatment attenuates T helper cell type 2 and B cell responses but does not improve vaccine-enhanced lung illness. J Immunol. 1997;159:328–34. [PubMed] [Google Scholar]
- 16.Hancock GE, Speelman DJ, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996;70:7783–91. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson TR, Rothenberg ME, Graham BS. Pulmonary eosinophilia requires interleukin-5, eotaxin-1, and CD4+ T cells in mice immunized with respiratory syncytial virus G glycoprotein. J Leuk Biol. 2008;84:748–59. doi: 10.1189/jlb.0907621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bont L, Versteegh J, Swelsen WTM, et al. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr Res. 2002;52:363–7. doi: 10.1203/00006450-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Hall CB, Long CE, Schnabel KC. Respiratory syncytial virus infections in previously healthy working adults. Clin Inf Dis. 2001;33:792–6. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 20.Valarcher J-F, Furze J, Wyld S, Cook R, Conzelmann K-K, Taylor G. Role of alpha/beta interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J Virol. 2003;77:8426–39. doi: 10.1128/JVI.77.15.8426-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spann KM, Tran K-C, Chi B, Rabin RL, Collins PL. Suppression of the induction of alpha, beta, and gamma interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J Virol. 2004;78:4363–9. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock GE, Heers KM, Smith JD, Scheuer CA, Igraghimov AR, Pryharski KS. CpG containing oligodeoxynucleotides are potent adjuvants for parenteral vaccination with the fusion (F) protein of respiratory syncytial virus (RSV) Vaccine. 2001;19:4874–82. doi: 10.1016/s0264-410x(01)00228-6. [DOI] [PubMed] [Google Scholar]
- 23.Hancock GE, Heers KM, Pryharski KS, Smith JD, Tiberio L. Adjuvants recognized by toll-like receptors inhibit the induction of polarized type 2 T cell responses by natural attachment (G) protein of respiratory syncytial virus. Vaccine. 2003;21:4348–58. doi: 10.1016/s0264-410x(03)00482-1. [DOI] [PubMed] [Google Scholar]
- 24.Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol. 2003;77:13156–60. doi: 10.1128/JVI.77.24.13156-13160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oumouna M, Mapletoft JW, Karvonen BC, Babiuk LA, van Drunen Littel-van den Hurk S. Formulation with CpG oligodeoxynucleotides prevents induction of pulmonary immunopathology following priming with formalin-inactivated or commercial killed bovine respiratory syncytial virus vaccine. J Virol. 2005;79:2024–32. doi: 10.1128/JVI.79.4.2024-2032.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mapletoft JW, Oumouna M, Townsend HG, Gomis S, Babiuk LA, van Drunen Littel-van den Hurk S. Formulation with CpG oligodeoxynucleotides increases cellular immunity and protection induced by vaccination of calves with formalin-inactivated bovine respiratory syncytial virus. Virology. 2006;353:316–23. doi: 10.1016/j.virol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Tayyari F, Sutton TC, Manson HE, Hegele RG. CpG-oligodeoxynucleotides inhibit RSV-enhanced allergic sensitisation in guinea pigs. Eur Respir J. 2005;25:295–302. doi: 10.1183/09031936.05.00016304. [DOI] [PubMed] [Google Scholar]
- 28.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–62. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 29.Graham BS, Henderson GS, Tang Y-W, Lu X, Neuzil KM, Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–40. [PubMed] [Google Scholar]
- 30.Wille-Reece U, Wu C, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV-1 gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 gag-specific Th1 and CD8+ T cell responses. J Immunol. 2005;174:7676–83. doi: 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
- 31.Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–80. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–77. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 33.Beyer M, Bartz H, Hörner K, Doths S, Koerner-Rettberg C, Schwarze J. Sustained increases in numbers of pulmonary dendritic cells after respiratory syncytial virus infection. J Allergy Clin Immunol. 2004;113:127–33. doi: 10.1016/j.jaci.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 34.Johnson TR, Graham BS. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J Virol. 1999;73:8485–95. doi: 10.1128/jvi.73.10.8485-8495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wills-Karp M, Ewart SL. The genetics of allergen-induced airway hyperresponsiveness in mice. Am J Resp Crit Care Med. 1997;156:S89–S96. doi: 10.1164/ajrccm.156.4.12-tac-3. [DOI] [PubMed] [Google Scholar]
- 36.Nagase H, Okugawa S, Ota Y, et al. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–82. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 37.Trevani AS, Chorny A, Salamone G, et al. Bacterial DNA activates human neutrophils by a CpG-independent pathway. Eur J Immunol. 2003;33:3164–74. doi: 10.1002/eji.200324334. [DOI] [PubMed] [Google Scholar]
- 38.Gold MC, Donnelly E, Cook MS, Leclair CM, Lewinsohn DA. Purified neonatal plasmacytoid dendritic cells overcome intrinsic maturation defect with TLR agonist stimulation. Pediatr Res. 2006;60:34–7. doi: 10.1203/01.pdr.0000220352.13547.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wille-Reece U, Flynn BJ, Loré K, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wille-Reece U, Flynn BJ, Loré K, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci USA. 2005;102:15190–4. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welliver RC, Garofalo RP, Ogra PL. Beta-chemokines, but neither T helper type 1 nor T helper type 2 cytokines, correlate with severity of illness during respiratory syncytial virus infection. Pediatr Infect Dis J. 2002;21:457–61. doi: 10.1097/00006454-200205000-00033. [DOI] [PubMed] [Google Scholar]
- 42.Haeberle HA, Kuziel WA, Dieterich J-J, Casola A, Gatalica Z, Garofalo RP. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1α in lung pathology. J Virol. 2001;75:878–90. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson TR, Teng MN, Collins PL, Graham BS. Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV. J Virol. 2004;78:6024–32. doi: 10.1128/JVI.78.11.6024-6032.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demedts IK, Bracke KR, Maes T, Joos GF, Brusselle GG. Different roles for human lung dendritic cell subsets in pulmonary immune defense mechanisms. Am J Respir Cell Mol Biol. 2006;35:387–93. doi: 10.1165/rcmb.2005-0382OC. [DOI] [PubMed] [Google Scholar]
- 45.Sha Q, Truoing-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by Toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–64. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 46.Guillot L, Le Goffic R, Bloch S, et al. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–80. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 47.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–42. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 48.Johnson TR. Respiratory syncytial virus and innate immunity: a complex interplay of exploitation and subversion. Expert Rev Vaccines. 2006;5:371–80. doi: 10.1586/14760584.5.3.371. [DOI] [PubMed] [Google Scholar]
- 49.Johnson TR, Hong S, Van Kaer L, Koezuka Y, Graham BS. NK T cells contribute to expansion of CD8+ T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol. 2002;76:4294–303. doi: 10.1128/JVI.76.9.4294-4303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussell T, Openshaw PJM. Intracellular IFN-γ expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J Gen Virol. 1998;79:2593–601. doi: 10.1099/0022-1317-79-11-2593. [DOI] [PubMed] [Google Scholar]
- 51.Alwan WH, Kozlowska WJ, Openshaw PJM. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–9. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones E, Price DA, Dahm-Vicker M, Cerundolo V, Klenerman P. The influence of macrophage inflammatory protein-1α on protective immunity mediated by antiviral cytotoxic T cells. Immunology. 2003;109:68–75. doi: 10.1046/j.1365-2567.2003.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, et al. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J Virol. 2006;80:1130–9. doi: 10.1128/JVI.80.3.1130-1139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bossert B, Marozin S, Conzelmann K-K. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J Virol. 2003;77:8661–8. doi: 10.1128/JVI.77.16.8661-8668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–33. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whyte AL, Miller SC. Strain differences in natural killer cell-mediated immunity among mice: a possible mechanism for the low natural killer cell activity of A/J mice. Immunobiology. 1998;199:23–38. doi: 10.1016/S0171-2985(98)80061-2. [DOI] [PubMed] [Google Scholar]
- 57.Asselin-Paturel C, Brizard G, Pin JJ, Brière F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–77. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]