Abstract
Lactoferrin, an iron binding glycoprotein, possesses multiple immune modulatory activities, including the ability to promote antigen specific cell-mediated immunity. Previous studies showed that adding bovine lactoferrin to the BCG vaccine (an attenuated strain of Mycobacterium bovis Bacillus Calmette Guerin) resulted in increased host protective responses upon subsequent challenge with virulent Erdman Mycobacterium tuberculosis (MTB) in mice. The studies outlined here investigate utility of a novel recombinant human lactoferrin to enhance the BCG vaccine and protect against alveolar injury during experimental MTB infection in mice. Sialylated and non-sialylated forms of the recombinant human lactoferrin (rhLF), glycoengineered in yeast (Pichia pastoris) and expressing humanized N-glycosylation patterns, were examined for their ability to enhance efficacy of the BCG vaccine in a murine TB model system. Results indicated that the sialylated form of the recombinant human lactoferrin generated increased antigen specific recall responses to BCG antigens. Furthermore, augmented protection was demonstrated using the sialylated lactoferrin adjuvant with BCG, resulting in significant reduction in associated pathology following challenge with virulent organisms.
Keywords: Lactoferrin, BCG, Vaccine, Adjuvant, Tuberculosis
INTRODUCTION
There is a great need to improve the current Mycobacterium bovis bacillus Calmette Guerin (BCG) vaccine for tuberculosis (TB), a disease caused by the intracellular pathogen Mycobacterium tuberculosis (MTB). BCG is the most widely used vaccine in the world, however, it has remained relatively unchanged since 1921 [1, 2]. The World Health Organization (WHO) estimates that roughly one third of the world’s population is currently infected with MTB, and the incidence rate for new infections is approximately 0.6% per year [3]. Although the BCG vaccine is variably effective against childhood tuberculosis disease manifestations [4], the response wanes in adulthood [5]. The observed diminished efficacy of BCG vaccination has resulted in intensive research into developing novel strategies that could surpass the efficacy of the current BCG vaccine [6–8]. Along with a renewed interest in vaccine development is the understanding that limitation of pathology is a worthy endeavor, thus reducing spread of disease from individuals that harbor organisms [9–11]. Yet despite investigations into developing alternatives, a standard BCG protocol remains the worldwide vaccine of choice to protect against development of active tuberculosis [6–8, 12, 13].
The BCG vaccine generates host protective responses against MTB infection by promoting development of a mycobacterial antigenic specific delayed type hypersensitivity (DTH) response, characterized as a T-cell helper type-1 (TH1) immunity with increased production of IFN-γ [14]. Current dogma suggests that tuberculosis is controlled by a strong TH1 immunity which is paradoxically counter-regulated by antibody dominant TH2 responses [15, 16]. Thus, vaccine-mediated immune control should emphasize the role of adjuvants which can skew T cell differentiation as an important component of rational vaccine design [17–19]. Additionally important in vaccine design is implementation of mechanisms that limit manifestation of destructive pathology or spread of infection at a later date [9, 11]. Indeed, if vaccination can delay, reduce or eliminate caseous necrosis of pulmonary tissue, then subsequent spread of disease via aerosolized droplets would circumvent infectious transmission. There is experimental evidence that this type of vaccination would be beneficial to serve effectively to reduce pathology following challenge infection with virulent MTB, and that this event may correlate well with protection [20]. Proven experimental methods for enhancing the efficacy of the existing BCG vaccine in this manner exist, with addition of defined adjuvant components that lead to host protection against MTB infection, as observed by reduced lung inflammation and increased mycobacterial antigen specific production of IFN-γ [21–23].
It was previously demonstrated that lactoferrin, a mammalian glycoprotein, can also serve in this capacity to enhance efficacy of the BCG vaccine in murine animal models [24]. Lactoferrin, a well conserved, monomeric 80-kDa single polypeptide chain glycoprotein [25, 26] is a natural effector protein that directly participates in host defenses and in the induction of cell mediated immune responses. Thus, it differs from microbial and cytokine adjuvants. Lactoferrin bridges the innate and adaptive immune functions by regulating target cell responses. It is recognized as a significant contributor in regulation of immune homeostasis [27, 28], antigen presentation, and development of T helper response [29, 30]. Milk-derived human lactoferrin has been shown to possess significant immune modulatory activity in multiple murine experimental protocols [31]. Recently it was demonstrated that milk-derived human lactoferrin was very effective in reduction of pollen antigen-induced allergic inflammation in a murine model of asthma [32]. Overall, it has high potential for use in rationale design of vaccine development for clinical purposes. Most critical, lactoferrin augments the strong T cells responses to BCG antigens, leading to a strong induction of cell-mediated immunity in mice [31, 33–36]. The bovine lactoferrin formulated BCG vaccine was able to confer protection in the lung and spleen against aerosol MTB challenge [24, 34], accompanied by generation of increased cytokines of a TH1 profile [24, 30, 33, 34, 37]. One remarkable finding was that addition of lactoferrin to the BCG vaccine led to reduced pathological damage upon subsequent infection with virulent MTB, in essence culminating in reduction of destructive histopathology following infectious challenge.
Recently, a biologically active human recombinant lactoferrin identical in glycosylation patterns to a natural neutrophilic counterpart was successfully bioengineered using Pichia pastoris expression system [38]. More importantly the fully glycosylated recombinant lactoferrin (rhLF) demonstrated immune regulatory properties, and could effectively reduce methotrexate (MTX)-induced suppression of secondary humoral immune responses to sheep erythrocytes (SRBC). The experiments presented here investigate the utility of this novel recombinant human lactoferrin to function as an adjuvant for the BCG vaccine. Specifically, experiments were designed to identify immunomodulatory effects of rhLF to assist the BCG vaccine in augmentation of cell mediated immunity (CMI), with end results to examine protection of alveolar integrity (limited destruction pathological manifestation to lung parenchyma) upon subsequent infection with Mycobacterium tuberculosis.
MATERIALS AND METHODS
Lactoferrin
Human milk lactoferrin was purchased from Sigma (St. Louis, MO). Recombinant human lactoferrins glycoengineered in yeast (Pichia pastoris), were produced as previously described [38]. In vitro sialylation of final product was achieved using methods detailed previously [38]. Briefly 200 mU sialyltransferase and 3.875 mg CMP-Sialic acid (final concentration; 52.5 μM) was added to approximately 30 mg of hLF in 50 mM morpholineethanesulfonic acid pH 6.5, 10 mM MnCl2, 0.73 μM chymostatin, and 52 ng/l pepstatin and incubated overnight at 25 °C. Sialylated rhLF was then dialyzed against 20 mM Tris (pH 8.0) followed by purification using heparin Sepharose Fast Flow 6. Endotoxin levels were assessed using a limulus amebocyte lysate endochrome assay kit from Charles River Laboratories according to the manufacturer’s instructions (Charleston, SC). The non-sialylated (rhLF-UnS) and sialylated (rhLF-Sial) forms contain 3 and 4 EU/mg of endotoxin respectively.
Immunization
Mycobacterium bovis Bacillus Calmette Guerin (BCG), Pasteur strain, (TMC 1011, ATCC, Manassas, VA) was grown in Dubos base (without addition of glycerol) with 10% supplement (5% BSA and 7.5% dextrose in saline) on an orbital shaker at 37°C for 2 weeks before use. BCG was diluted with 1× Dulbecco’s phosphate-buffered saline (PBS) (Cellgro, Herndon, VA) to 3×108 organisms/mL, the concentration was estimated using McFarland standards (Sigma), and confirmed by plating dilutions onto 7H11 agar plates (Remel, Lenesa, KS). Plates were incubated at 37°C with 5% CO2 for 3–4 weeks, and resulting colonies counted. Female C57BL/6 mice (6 weeks, Jackson Laboratories, Bar Harbor, ME) of 20–25 g initial body weight were conducted under approved guidelines of the animal ethics committee at the University of Texas, Health Science Center at Houston (HSC-AWC-03-106). Mice were immunized, according to previously published NIH-established protocols [39, 40], with 100μL of vaccine formulation, subcutaneously (s.c.) at the base of the tail, and boosted at two weeks. Each mouse received BCG only (1×106 CFU/mouse) or BCG and lactoferrin (100μg/mouse): human milk-derived human lactoferrin (hLF), non-sialylated recombinant human lactoferrin (rhLF-UnS), or sialylated recombinant human lactoferrin (rhLF-Sial). Comparisons were made to control groups receiving saline alone with no adjuvant or BCG antigen.
Splenic recall
Heat-killed BCG (HK-BCG) was achieved by autoclaving BCG suspension in 1× PBS at 121°C for 20min. Confirmation of heat-killed BCG was verified by plating on 7H11 plates and observing no growth of colonies after 3 weeks. Splenocytes were isolated from immunized and non-immunized C57BL/6 mice (6 mice/group) at 6 weeks post boost, as previously described [34]. Briefly, spleens were minced, and red blood cells lysed with ACK lysing buffer (Cambrex Bio Sciences, East Rutherford, NJ). Splenocytes were cultured at 2×106 cells/mL in Dulbecco’s Modified Eagles Medium (DMEM, Sigma, St. Louis, MO) supplemented with 2.2g/L sodium bicarbonate, 0.05g/L of HEPES (Sigma), 0.05g/L L-arginine (Sigma), 100 μg/mL penicillin G (Sigma), 50 μg/mL gentamycin sulfate (Sigma) 0.005% 2-mercaptoethanol (2-Me, Gibco, Carlsbad, CA), and 10% fetal bovine serum (FBS, Sigma). Assay controls were performed to assess potential for cellular activation, including stimulation with ConA (2 μg/mL), LPS (200 ng/mL), lactoferrin (100 μg/mL), or with HK-BCG at multiplicity of infection (MOI) 10:1 (organism:cell) ratio for 72 hrs. Supernatants were collected and stored at −20°C for analysis by ELISA.
MTB aerosol challenge
Four weeks post-immunization, mice were aerosol challenged with approximately 100 CFU of Erdman strain of Mycobacterium tuberculosis (TMC 107, ATCC). Erdman MTB was grown in Dubos base with 5.6% glycerol and 10% supplement for 3–4 weeks before use. Bacteria were taken during log phase growth, resuspended in 1× PBS, sonicated for 10 seconds to dislodge any clumping that might have occurred. Organisms were diluted in 1× PBS to 3×108 bacteria/mL; concentration was estimated using McFarland standards and confirmed by plating dilutions onto 7H11 agar plates. Plates were incubated at 37°C with 5% CO2 for 3–4 weeks, colonies were enumerated. To achieve aerosol implantation of 100 CFU, MTB was suspended in 5 mL 1× PBS (at 1×108 bacteria/mL) and aerosolized using an inhalation exposure system (IES) (GLAS-COL Model #A4212 099c Serial #377782). Inoculation dose was calculated by sacrificing 4 mice at day 1 post-infection and assessing for lung bacterial load by plating lung homogenates on 7H11 plates. Groups of mice were sacrificed at 7, 30, 65, and 150 days post-challenge, lung and spleen sectioned for colony forming units (CFU), histology, and protein analysis.
Pulmonary histological analysis
The histological analysis of the lung was performed on mice sacrificed following aerosol infection. Lungs were fixed in 10% formalin and embedded in paraffin using standard techniques. Sections, 5μm thick, were stained with hematoxilin and eosin (H&E) and subsequently reviewed histologically; the pathologist viewing and interpreting the slides was blind to the type of experiment and treatment. Multiple sections of each lung from at least 5 mice per group were digitized with a 2× objective and analyzed using digital software (NIH Image J). Relative area of occluding lesions was obtained and calculated as percent of total (100%) lung section area.
ELISA (Enzyme linked immuno-sorbant assay)
Lung sections were homogenized in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 2.2g/L sodium bicarbonate, 50mg/L HEPES, 50mg/L L-arginine, and 10% FBS. Homogenates were incubated for 2hrs at 37°C and supernatants collected for analysis using the DuoSet ELISA kits (R&D Systems, Minneapolis, MN), according to manufacturer’s instructions. Splenocytes were assayed for production of T-cell cytokines, IFN-γ, IL-2, and IL-4, proinflammatory mediators, TNF-α, IL-1βand IL-6, and the TH1 mediators IL-12p40 and IL-10. Lowest limit of detection according to manufacturer were stated as 15–32pg/mL.
Statistics
In vivo experiments were performed using sufficient animals to achieve statistical significance, using 5–10 mice per group per time point, with most experimental groups repeated at least in duplicate. All analytical assays were repeated in triplicate. ELISA determinations were performed using triplicate wells (average ± standard deviation). Statistical analysis were carried out using OneWay ANOVA followed by the Tukey post-hoc test for group-by-group comparisons, and significance was reported at p<0.05 or less. Statistical analysis was performed using the GraphPad Prizm program (La Jolla, CA), version 4.0.
RESULTS
Generation of protective immunity by BCG and lactoferrin immunization
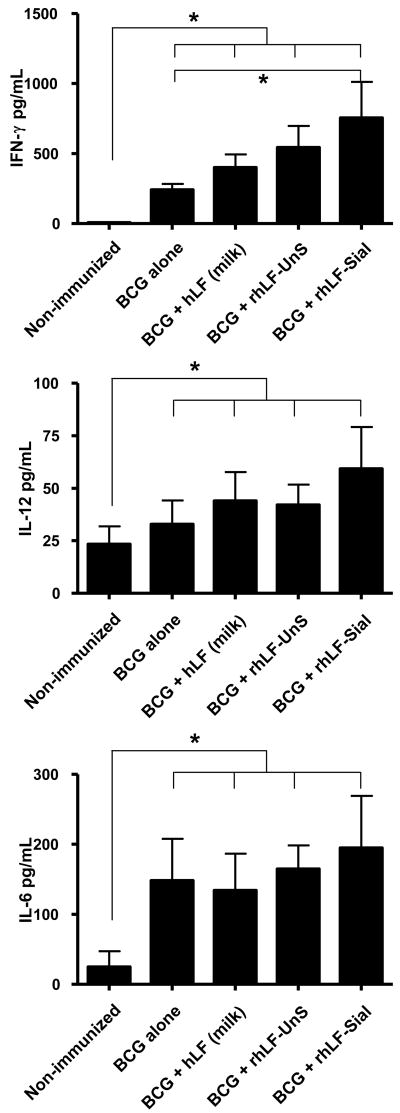
The human lactoferrin gene was cloned into Pichia pastoris engineered to produce a protein glycoform with uniform complex N-glycosylation [38], yielding a purified product that was low endotoxin for use in immunization studies. Mice immunized with BCG alone generate a modest CD4+ delayed type hypersensitive response which can be boosted when formulated with recombinant human lactoferrin (rhLF) as an adjuvant (Figure 1). Splenocyte responses obtained from BCG alone immunized mice were directly compared to mice vaccinated with BCG formulated in either a milk-derived human lactoferrin, or to those immunized with two forms of rhLF (with or without terminal N-acetylneuraminic acid). When splenocytes from immunized mice were examined for cytokine production at 6 weeks post immunization, all cultures were found to have moderately elevated IFN-γ, IL-12 and IL-6 responses to mycobacterial antigens. However, additional elevation of IFN-γ was apparent in the sialylated rhLF adjuvant vaccinated group, with higher levels produced compared to those generated in the BCG alone vaccinated mice. No significant increases over the BCG alone group were seen using either the milk-derived lactoferrin or the non-sialylated form of the rhLF.
Figure 1. Specific IFN-γ, IL-12 and IL-6 production from BCG or BCG and lactoferrin immunized mice.
C57BL/6 mice were immunized with BCG, or BCG formulated in milk-derive lactoferrin, or formulated in recombinant human lactoferrin with sialylation (rhLF-Sial) or without sialylation (rhLF-UnS). Lactoferrins were given at 100 μg/mouse. Comparisons were made to non-immunized splenocytes response. Supernatants were analyzed for IFN-γ, IL-12 and IL-6 production. Significant elevation above BCG alone was only apparent for all cytokines examined using the human recombinant sialylated lactoferrin. *p<0.05, compared to non-immunized controls.
Increased efficacy of BCG immunization using lactoferrin adjuvant in the standard NIH model
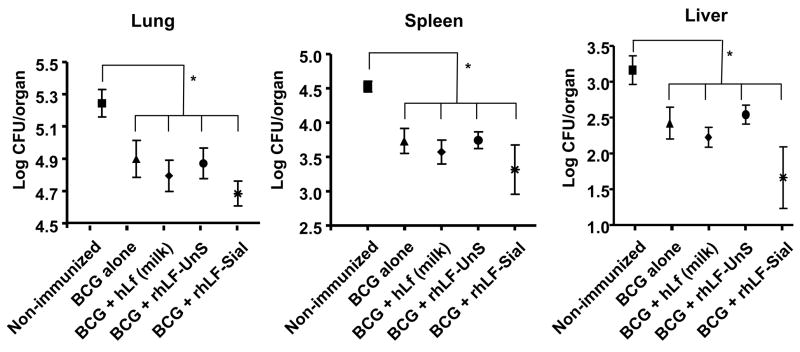
Mice immunized with BCG alone, BCG plus rhLF (sialylated or non-sialylated), or BCG plus milk-derived human lactoferrin were aerosol challenge with Mycobacterium tuberculosis (MTB, strain Erdman) at four weeks post final immunization. The effects of vaccination were assessed by examination of bacterial load and quantitative evaluation of pulmonary histopathology. Vaccination efficacy was measured at 30 days following infection by enumeration of bacterial colony count (CFU) in lung, liver and spleen. At 30 days post challenge, all vaccinated animals had significantly (p<0.05) reduced bacterial loads in the lungs (reduction of 0.34 to 0.48 log CFU) and spleen (reduction of 0.40 to 0.83 log CFU), as well as marked reduction in the liver, relative to non-vaccinated animals. Comparatively, the largest reduction in lung CFU was seen when BCG was given admixed with the rhLF sialylated formulation (Figure 2). Bacterial loads were monitored through 150 days post challenge. At 150 days, all immunized groups were similar in tissue organism loads, with CFU maintained at lower levels in lung (p<0.01) and spleen (p<0.05) compared to non-immunized groups (Table I).
Figure 2. Decreased organ bacterial load in lactoferrin vaccinated mice post-challenge with virulent MTB.
C57BL/6 mice were immunized with BCG, or with BCG and indicated lactoferrins, and aerosol challenged with <100 CFU Erdman MTB. Mice were assessed for organ bacterial load after 30 days. Mice immunized with BCG and human sialylated lactoferrin (rhLF-Sial) showed the greatest reductions in lung CFU, with reduced dissemination to other tissue. Comparisons are shown against non-immunized mice, immunization with BCG alone, human milk-derived lactoferrin (hLF), or with the non-sialylated form (rhLF-UnS). Results are shown as average ± standard deviation for 5–10 mice per group. *p<0.05, compared to non-immunized controls.
Table I.
Bacterial load (Log CFU per organ) in immunized mice infected for 150 days with M. tuberculosis.
| Lung | Avg CFU (+/− SD) | Spleen | Avg CFU (+/− SD) |
|---|---|---|---|
| Non-Immunized | 6.19 +/− 0.50 | Non-Immunized | 4.98 +/− 0.28 |
| BCG alone | 5.61 +/− 0.29 | BCG alone | 4.56 +/− 0.39 |
| BCG + milk LF | 5.71 +/− 0.28 | BCG + milk LF | 4.76 +/− 0.27 |
| BCG + rhLF(UnS) | 5.54 +/− 0.29 | BCG + rhLF(UnS) | 4.33 +/− 0.88 |
| BCG + rhLF(Sial) | 5.60 +/− 0.29 | BCG + rhLF(Sial) | 4.46 +/− 0.38 |
Average Organ CFU ± standard deviation for 5-mice per group shown for infected mice at 150 days post aerosol infection with M. tuberculosis.
Reduced lung histopathology in BCG/lactoferrin immunized mice
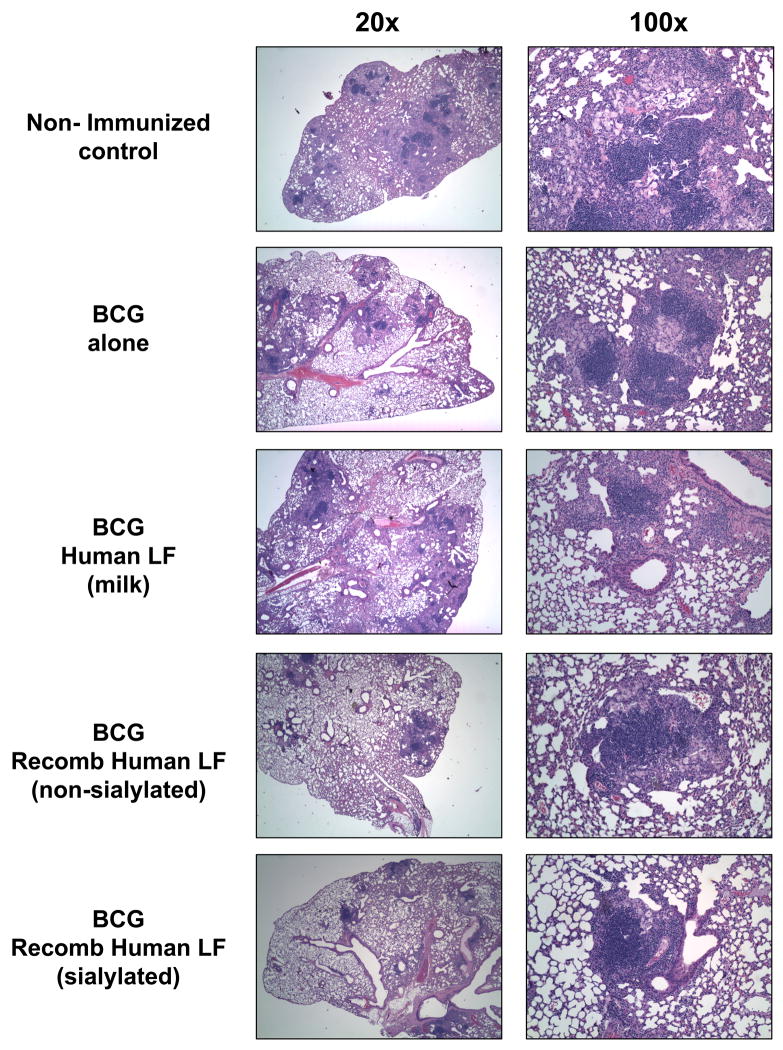
More striking were the observed differences in lung granuloma pathology between immunized and non-immunized animals. Examination of lung pathology at day 150 post-challenge showed that non-immunized mice developed loosely compacted granulomas comprised of clusters of lymphocytes and activated foamy macrophages (Figure 3). Non-immunized controls demonstrated granulomas surrounded by an overall inflamed parenchyma characterized by thickened alveoli walls. Lungs from MTB infected mice immunized only with BCG demonstrated inflammatory responses that differed from the non-immunized group, showing a granulomatous response with apparent increased lymphocytic content; lymphocytes were primarily localized to within centers of inflammatory response. Mice immunized with BCG admixed with the milk-derived human lactoferrin demonstrated similar pathologies to the BCG alone groups. In contrast, lungs from MTB infected mice immunized with BCG and recombinant human lactoferrin developed granulomas that appeared to be smaller in size compared to both the non-immunized and BCG alone immunized groups. Specifically, the mice immunized with the sialylated form of the recombinant human lactoferrin demonstrated smaller lesions and higher consolidation of inflammation. In agreement with the CFU data reported above, the sialylated form of the recombinant human lactoferrin was more effective in limiting development of pathology than the non-sialylated form.
Figure 3. Mice immunized with BCG and lactoferrin demonstrate long lasting diminished inflammation and destructive pulmonary histopathology upon challenge with virulent MTB.
C57BL/6 mice demonstrated reduced histological manifestation of disease in the BCG and recombinant lactoferrin immunized groups at day 150 post aerosol challenge. The panels depict histopathology from non-immunized controls and those immunized with BCG vaccine alone, or immunized with the human milk-derived lactoferrin, or recombinant human lactoferrins (non-sialylated or sialylated). The human recombinant lactoferrin adjuvant immunized mice revealed striking reduction in granulomas with evidence of lymphocytic clusters and contained focal pockets of inflamed monocytes. Tissue was sectioned and stained with H&E (20×, left; 100×, right). Representative pathology from one mouse per immunization group shown, examining 5–10 mice per group.
The apparent reduction in inflammatory pathology was quantitatively analyzed by measuring the total area of the lung sections occluded by granulomas and lesions developed during the infective process. All immunized groups demonstrated some reduction in lesions within lung tissue, relative to no-immunized controls. Comparing immunized groups, mice immunized and boosted with BCG in the presence of the sialylated form of recombinant human lactoferrin had significantly lower manifestation of lesions and vascular occlusion than all other BCG immunized groups (Figure 4).
Figure 4. Quantitative analysis of inflammation in immunized mice.
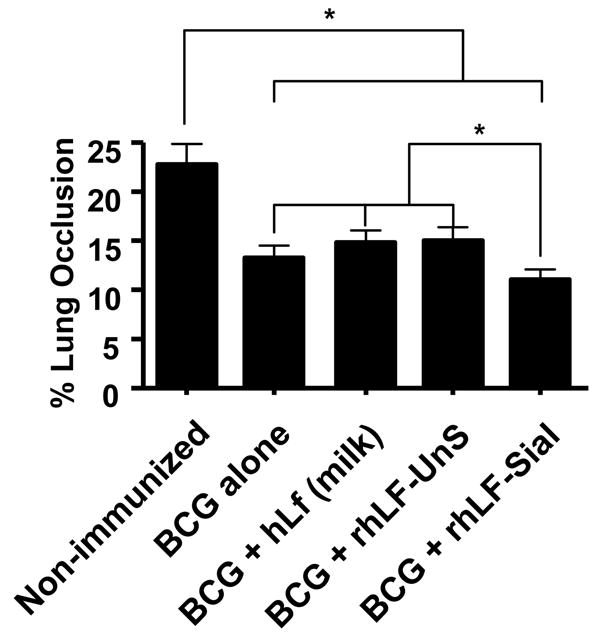
Histological sections obtained from mice infected for 150 days were assessed for lung pathology by quantitative evaluation of inflammatory lesions, represented as percent occlusion of tissue post aerosol MTB challenge. Mice immunized with BCG and human sialylated lactoferrin (rhLF-sial) showed further reduction in tissue damage than groups given BCG alone. Groups immunized with human milk lactoferrin (hLF) or given the non-sialylated form (rhLF-UnS) did not extend histological protection above that of BCG alone. Results are shown as average ± standard deviation for at least 5–10 mice per group. *p<0.001 for comparisons indicated.
Evaluation of cytokines in vivo in BCG/lactoferrin immunized mice
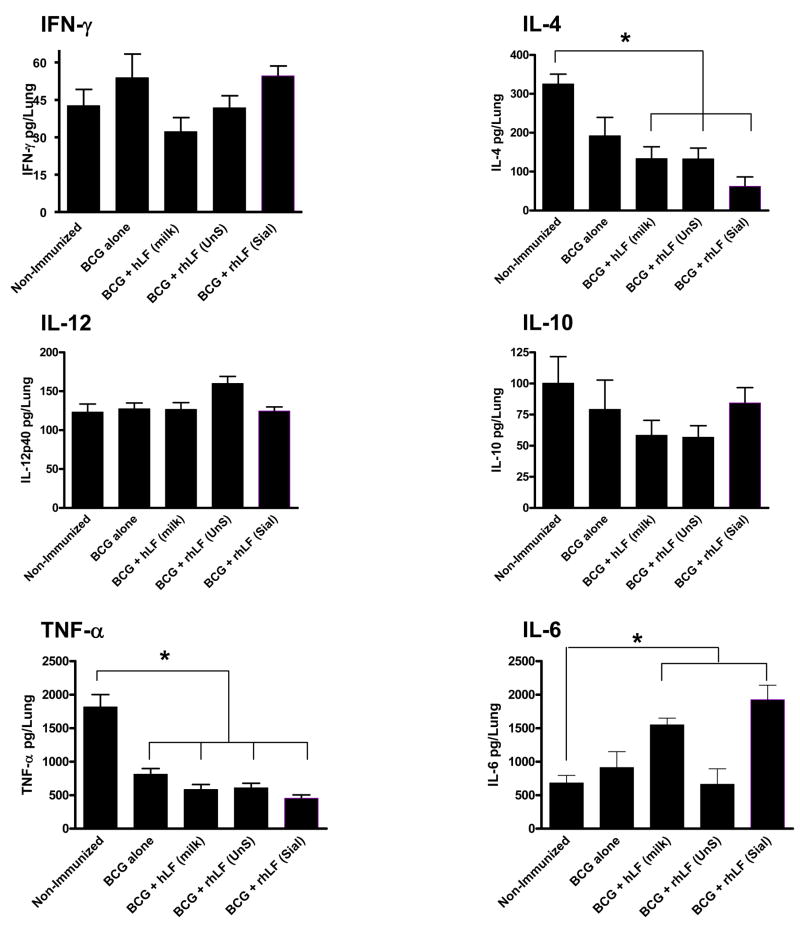
The decreased pulmonary pathology in the mice immunized with lactoferrin and BCG was similar to findings where increased CD4+ delayed type hypersensitive responses were reported [24]. To assess the effective, functional DTH, immunized mice were sacrificed one week after infection with virulent organisms, and lung tissue was assessed for cytokines by ELISA (Figure 5). All groups demonstrated low, but detectable IFN-γ at one week post challenge, with no significant increases in IFN-γ response at this early time point between groups. Likewise, there were no significant changes in IL-12 or IL-10 compared to control, non-immunized mice. As expected, TNF-α was markedly reduced in all immunized groups, relative to non-immunized mice. Unexpectedly, IL-4 production was markedly reduced (p<0.05) in mice immunized with the recombinant human sialylated lactoferrin adjuvant mice, compared to all other groups. Concurrently, IL-6 was significantly elevated in these same mice, perhaps indicating differential induction of cytokines necessary for activation of subsequent protective T helper cell responses.
Figure 5. Altered cytokine response in lungs of mice at 7 days post infection in BCG/lactoferrin immunized mice.
Cytokines were evaluated in lung tissue from C57BL/6 mice immunized and boosted with BCG alone, or with BCG and defined lactoferrins, at 7 days post aerosol infection with virulent MTB. Mice immunized with BCG and human sialylated lactoferrin (rhLF-Sial) showed patterns consistent with altered T cell cytokine response, compared to groups immunized with human milk lactoferrin (hLF) or the non-sialylated form (rhLF-UnS). Protein levels are indicated as average ± standard deviation for at least 5 mice per group. *p<0.05 or greater compared to non-immunized control mice.
DISCUSSION
Vaccines represent one of the most powerful and cost-effective mechanisms for prevention of infectious disease, with many successful efforts leading to significant reduction in morbidity and mortality due to microbial assault [41, 42]. Substantial progress has also been made during the past 15 years towards the development of improved vaccine for tuberculosis [7, 17], in large part due to the availability of genome sequences and advances in common antigenic characterization among multiple strains common to larger communities. The ultimate goal of vaccination to prevent tuberculosis disease encompasses not only long term protection to the individual, but also long term protection of the greater society [9, 11]. There are enormous obstacles to the effective control of tuberculosis [43]. However, while the causative agent, Mycobacterium tuberculosis (MTB), can infect many animals, there is no animal reservoir. Man is the only species from which the organism can escape to infect new subjects. The transmissibility of infection, and thus the organism’s continued existence, depends on its ability to escape from immune hosts to infect new ones. Therefore, the goal of a protective tuberculosis vaccine should include the ability to limit cavitary formation leading to subsequent spread of disease [10, 44, 45].
The results presented are designed to provide a logical framework for development of a recombinant human lactoferrin based BCG vaccine that functions to limit immune related pathologies. It is well accepted that a cell-mediated immune (CMI) response of the TH1 type, characterized by elevated production of gamma interferon (IFN-γ) and interleukin-12 (IL-12), is critical in development of protective immunity against M. tuberculosis. Experiments reported here extend the understanding of immunomodulatory effects of lactoferrin to assist the BCG vaccine in augmentation of CMI resulting in protection of alveolar integrity upon subsequent infection. In this case, alveolar integrity is defined as limited acute destruction of lung parenchyma that culminates in a destructive pulmonary pathology. Indeed, these studies give strong support for the potential of lactoferrin, especially the fully humanized recombinant molecule, to function as an adjuvant component to augment primary interactions that lead to elevated production of TH1 cellular responses upon vaccination. The mice that were immunized with recombinant human lactoferrin of the sialylated form demonstrated antigenic recall responses with significantly increased IFN-γ, and a shift towards greater IL-12 production. Overall, this suggests that treatment with recombinant human lactoferrin adjuvant augments a local environment that would potentiate development of T cells towards a recall TH1 phenotype to BCG antigens. The mechanisms for augmentation of response are not fully understood at this time, but bovine lactoferrin-induced immune modulation of T cell responses have been presented elsewhere [30]. Specific description of modulation of activated macrophages in cytokine secretion and antigen presentation that would lead to enhancement of antigenic presentation and direction of focused T helper responses has been reported [29, 35, 37, 46]. It is hypothesized that similar mechanisms would allow human lactoferrin to function in this same manner.
Addition of lactoferrin to the BCG vaccine generated protective responses upon subsequent infection with virulent mycobacterium, with decreased immune related pathology due to infection. The effects of lactoferrin adjuvant based vaccination protocols also appeared to be long lasting; protection was maintained in the sialylated form of the recombinant human lactoferrin group through 150 days post challenge. The use of human recombinant lactoferrin in the vaccine allowed an augmented level of lymphocyte recall response to mycobacterial antigen that correlated well with observed cytokines in lung tissue soon after infection in immunized mice. Of major interest was that the group immunized with the sialylated form of the recombinant lactoferrin demonstrated a marked early increase in IL-6 with concurrent reduction in IL-4 (indicating indirectly an effective push to TH1 response) rather than a mixed format as seen in mice immunized with BCG alone. Specifically, increased level of IFN-γ was generated, which has been shown to correlate well with a reduction in disease progression [24, 34, 47]. IL-6, although not essential for development of protective immunity, is involved in stimulating early IFN-γ production [48]. The uniqueness with the novel recombinant is the drop in IL-4, indicating a functional shift away from early activated TH2 response, which also correlates well with disease progression in vaccinated humans [49], theoretically undermining TH1-mediated immunity to drive inappropriate alternative activation of macrophages and development of pathological damage in the host.
Of surprise to expected findings, the bacterial load with lung and spleen tissue was not greatly decreased in the lactoferrin vaccinated groups, compared to mice vaccinated with BCG alone. A modest reduction in liver CFU was apparent, perhaps indicative of delayed dissemination of organisms. However, the overall difference between vaccination groups was manifested primarily in reduced development of pathological damage and lung occlusion. Studies in the guinea pig suggest that survival following challenge may occur in vaccinated animals in the absence of decreased early bacillary loads [8, 44, 50]. Dascher, et al., speculate that use of defined adjuvants may alter development of T cell subsets which function more effectively over longer periods of infection. [20]. The data described here is in agreement with assessment that prolonged survival may be predicated on changes in the pathological manifestation of disease within lung tissue [51], with improvement seen in the absence of decreased early bacillary loads. In this scenario, increase in lymphocytic content is seen in vaccinated mice in the absence of help to macrophages to increase bactericidal effector function. This could limit the magnitude of pathological damage, even in the absence of markedly reduced CFU.
Multiple mechanisms of action have been described for bovine lactoferrin to function as an immune modulator [28, 52, 53], While these studies are scientifically informative, bovine lactoferrin is only 70% homologous to human lactoferrin in amino acid sequence [54], and contains markedly different glycosylation patterns [55]. Furthermore, the bovine form is not amenable for parenteral utility formulated with a human vaccine. While many options are available to produce recombinant human lactoferrin, full functionality as an immune modulator appears dependent upon both glycosylation and sialylation of the molecule [38, 56, 57]. There are two primary forms of human lactoferrin, one contained in exocrine secretions including milk, tears, saliva, bronchial and intestinal secretions. The other form is present in the secondary granules of neutrophils. While the two forms of human lactoferrin are identical in their amino acid sequence, they differ in sugar moieties. The granulocytic lactoferrin is not fucosylated thereby allowing transduction of signals that do not require fucose-specific receptors such as the mannose receptor [58–60]. In addition, while the secreted form is thought to be involved in the host defense against microbial infection at mucosal sites, the granulocytic lactoferrin has notable immunomodulatory function [31]. The comparative immunizations investigated in this study extend these observations, with the milk-derived human lactoferrin determined as less effective in adjuvant function. The different glycosylation pattern for milk-derived lactoferrin and neutrophilic form (replicated as rhLF) may be responsible for the difference in adjuvant activity. One possibility is that fucosylated lactoferrin (milk-derived) has lower affinity to the receptor that is responsible for signal transduction mechanism in those cells. While intuitive in hindsight, it would be an evolutionary advantage for neutrophils recruited to sites of inflammation to release a glycosylated-modified molecule which could both function locally to inhibit bacterial growth as well as exert effects on macrophage responses to modulate their ability to present novel antigens to subsequent interacting lymphocytes.
Overall, it is prudent to continue to search for alternative mechanisms to boost the current BCG vaccine, especially in light that no research vaccine against tuberculosis that can consistently surpass BCG in protecting the host is near FDA approval. The studies here suggest that fully humanized recombinant lactoferrin produced in Pichia pastoris may have utility to augment the BCG vaccine and confer protection against formation of cavitary disease after infection with mycobacteria. This new knowledge of lactoferrin immunomodulation paves the way to more general design of T cell-dependent vaccines that incorporate naturally occurring granulocytic components.
Acknowledgments
Research was supported by the National Institute of Allergy and Infectious Diseases (R42AI051050-02). This work was presented in part at the Keystone meeting (Tuberculosis: Biology, Pathology and Therapy, 2009), in Keystone Colorado.
This work was supported in part by NIH grants 1R41GM079810-01 and R42-AI051050-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oettinger T, Jorgensen M, Ladefoged A, Haslov K, Andersen P. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber Lung Dis. 1999;79(4):243–50. doi: 10.1054/tuld.1999.0206. [DOI] [PubMed] [Google Scholar]
- 2.Behr MA. BCG--different strains, different vaccines? Lancet Infect Dis. 2002 Feb;2(2):86–92. doi: 10.1016/s1473-3099(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 3.Maher D, Raviglione M. Global epidemiology of tuberculosis. Clin Chest Med. 2005 Jun;26(2):167–82. v. doi: 10.1016/j.ccm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, et al. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995 Jul;96(1 Pt 1):29–35. [PubMed] [Google Scholar]
- 5.Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. 1998 Mar;2(3):200–7. [PubMed] [Google Scholar]
- 6.Doherty TM. Real world TB vaccines: clinical trials in TB-endemic regions. Vaccine. 2005 Mar 18;23(17–18):2109–14. doi: 10.1016/j.vaccine.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Girard MP, Fruth U, Kieny MP. A review of vaccine research and development: tuberculosis. Vaccine. 2005 Dec 30;23(50):5725–31. doi: 10.1016/j.vaccine.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 8.McMurray DN. A coordinated strategy for evaluating new vaccines for human and animal tuberculosis. Tuberculosis (Edinb) 2001;81(1–2):141–6. doi: 10.1054/tube.2000.0265. [DOI] [PubMed] [Google Scholar]
- 9.Hudgens MG, Halloran ME. Causal Vaccine Effects on Binary Postinfection Outcomes. J Am Stat Assoc. 2006 Mar;101(473):51–64. doi: 10.1198/016214505000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locht C, Rouanet C, Hougardy JM, Mascart F. How a different look at latency can help to develop novel diagnostics and vaccines against tuberculosis. Expert Opin Biol Ther. 2007 Nov;7(11):1665–77. doi: 10.1517/14712598.7.11.1665. [DOI] [PubMed] [Google Scholar]
- 11.Glyn Hewinson R. TB vaccines for the World. Tuberculosis (Edinb) 2005 Jan-Mar;85(1–2):1–6. doi: 10.1016/j.tube.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A. 2000 Dec 5;97(25):13853–8. doi: 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann SH. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005 Dec;26(12):660–7. doi: 10.1016/j.it.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 15.Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb) 2004;84(1–2):93–101. doi: 10.1016/j.tube.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Rook GA, Dheda K, Zumla A. Do successful tuberculosis vaccines need to be immunoregulatory rather than merely Th1-boosting? Vaccine. 2005 Mar 18;23(17–18):2115–20. doi: 10.1016/j.vaccine.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 17.Gupta UD, Katoch VM, McMurray DN. Current status of TB vaccines. Vaccine. 2007 May 10;25(19):3742–51. doi: 10.1016/j.vaccine.2007.01.112. [DOI] [PubMed] [Google Scholar]
- 18.Rook GA, Hernandez-Pando R, Dheda K, Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004 Sep;25(9):483–8. doi: 10.1016/j.it.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Vogel FR. Improving vaccine performance with adjuvants. Clin Infect Dis. 2000 Jun;30( Suppl 3):S266–70. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 20.Dascher CC, Hiromatsu K, Xiong X, Morehouse C, Watts G, Liu G, et al. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int Immunol. 2003 Aug;15(8):915–25. doi: 10.1093/intimm/dxg091. [DOI] [PubMed] [Google Scholar]
- 21.McMurray DN. Recent progress in the development and testing of vaccines against human tuberculosis. Int J Parasitol. 2003 May;33(5–6):547–54. doi: 10.1016/s0020-7519(03)00061-4. [DOI] [PubMed] [Google Scholar]
- 22.Haile M, Schroder U, Hamasur B, Pawlowski A, Jaxmar T, Kallenius G, et al. Immunization with heat-killed Mycobacterium bovis bacille Calmette-Guerin (BCG) in Eurocine L3 adjuvant protects against tuberculosis. Vaccine. 2004 Mar 29;22(11–12):1498–508. doi: 10.1016/j.vaccine.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Chambers MA, Wright DC, Brisker J, Williams A, Hatch G, Gavier-Widen D, et al. A single dose of killed Mycobacterium bovis BCG in a novel class of adjuvant (Novasome) protects guinea pigs from lethal tuberculosis. Vaccine. 2004 Feb 25;22(8):1063–71. doi: 10.1016/j.vaccine.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Hwang SA, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, et al. Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis. Vaccine. 2007 Sep 17;25(37–38):6730–43. doi: 10.1016/j.vaccine.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legrand D, Pierce A, Elass E, Carpentier M, Mariller C, Mazurier J. Lactoferrin structure and functions. Adv Exp Med Biol. 2008;606:163–94. doi: 10.1007/978-0-387-74087-4_6. [DOI] [PubMed] [Google Scholar]
- 26.Baker EN, Baker HM. Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol Life Sci. 2005 Nov;62(22):2531–9. doi: 10.1007/s00018-005-5368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chodaczek G, Saavedra-Molina A, Bacsi A, Kruzel ML, Sur S, Boldogh I. Iron-mediated dismutation of superoxide anion augments antigen-induced allergic inflammation: effect of lactoferrin. Postepy Hig Med Dosw (Online) 2007;61:268–76. [PubMed] [Google Scholar]
- 28.Kruzel ML, Actor JK, Boldogh I, Zimecki M. Lactoferrin in health and disease. Postepy Hig Med Dosw (Online) 2007;61:261–7. [PubMed] [Google Scholar]
- 29.Hwang SA, Kruzel ML, Actor JK. Influence of bovine lactoferrin on expression of presentation molecules on BCG-infected bone marrow derived macrophages. Biochimie. 2008 Apl;27 doi: 10.1016/j.biochi.2008.04.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang SA, Wilk KM, Bangale YA, Kruzel ML, Actor JK. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med Microbiol Immunol. 2007 Sep;196(3):171–80. doi: 10.1007/s00430-007-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruzel ML, Zimecki M. Lactoferrin and immunologic dissonance: clinical implications. Arch Immunol Ther Exp (Warsz) 2002;50(6):399–410. [PubMed] [Google Scholar]
- 32.Kruzel ML, Bacsi A, Choudhury B, Sur S, Boldogh I. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology. 2006 Oct;119(2):159–66. doi: 10.1111/j.1365-2567.2006.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL, Jr, Kruzel ML. Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol. 2002 Mar;2(4):475–86. doi: 10.1016/s1567-5769(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 34.Hwang SA, Kruzel ML, Actor JK. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int Immunopharmacol. 2005 Mar;5(3):591–9. doi: 10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Zimecki M, Kapp J, Machnicki M, Zagulski T, Wlaszczyk A, Kubler A, et al. Lactoferrin. Its role in maturation and function of cells of the immune system and protection against shock in mice. Adv Exp Med Biol. 1998;443:331–6. [PubMed] [Google Scholar]
- 36.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol Lett. 2000 Nov 1;74(3):183–8. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 37.Wilk KM, Hwang SA, Actor JK. Lactoferrin modulation of antigen-presenting-cell response to BCG infection. Postepy Hig Med Dosw (Online) 2007;61:277–82. [PMC free article] [PubMed] [Google Scholar]
- 38.Choi BK, Actor JK, Rios S, d’Anjou M, Stadheim TA, Warburton S, et al. Recombinant human lactoferrin expressed in glycoengineered Pichia pastoris: effect of terminal N-acetylneuraminic acid on in vitro secondary humoral immune response. Glycoconj J. 2008 Mar 26; doi: 10.1007/s10719-008-9123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009 Mar 1; doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 40.Copenhaver RH, Sepulveda E, Armitige LY, Actor JK, Wanger A, Norris SJ, et al. A mutant of Mycobacterium tuberculosis H37Rv that lacks expression of antigen 85A is attenuated in mice but retains vaccinogenic potential. Infect Immun. 2004 Dec;72(12):7084–95. doi: 10.1128/IAI.72.12.7084-7095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saalmuller A. New understanding of immunological mechanisms. Vet Microbiol. 2006 Oct 5;117(1):32–8. doi: 10.1016/j.vetmic.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005 Apr;11(4 Suppl):S25–32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 43.Young DB, Perkins MD, Duncan K, Barry CE., 3rd Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest. 2008 Apr;118(4):1255–65. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padilla-Carlin DJ, McMurray DN, Hickey AJ. The guinea pig as a model of infectious diseases. Comp Med. 2008 Aug;58(4):324–40. [PMC free article] [PubMed] [Google Scholar]
- 45.Hope JC, Villarreal-Ramos B. Bovine TB and the development of new vaccines. Comp Immunol Microbiol Infect Dis. 2008 Mar;31(2–3):77–100. doi: 10.1016/j.cimid.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Hwang SA, Kruzel ML, Actor JK. Influence of bovine lactoferrin on expression of presentation molecules on BCG-infected bone marrow derived macrophages. Biochimie. 2009 Jan;91(1):76–85. doi: 10.1016/j.biochi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beamer GL, Flaherty DK, Vesosky B, Turner J. Peripheral blood gamma interferon release assays predict lung responses and Mycobacterium tuberculosis disease outcome in mice. Clin Vaccine Immunol. 2008 Mar;15(3):474–83. doi: 10.1128/CVI.00408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect Immun. 2000 Jun;68(6):3322–6. doi: 10.1128/iai.68.6.3322-3326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rook GA. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med. 2007 May;7(3):327–37. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 50.Baldwin SL, D’Souza C, Roberts AD, Kelly BP, Frank AA, Lui MA, et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998 Jun;66(6):2951–9. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orme IM, McMurray DN, Belisle JT. Tuberculosis vaccine development: recent progress. Trends Microbiol. 2001 Mar;9(3):115–8. doi: 10.1016/s0966-842x(00)01949-1. [DOI] [PubMed] [Google Scholar]
- 52.Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci. 2005 Nov;62(22):2549–59. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimecki M, Artym J, Chodaczek G, Kocieba M, Kuryszko J, Houszka M, et al. Immunoregulatory function of lactoferrin in immunosuppressed and autoimmune animals. Postepy Hig Med Dosw (Online) 2007;61:283–7. [PubMed] [Google Scholar]
- 54.Kim SJ, Yu DY, Pak KW, Jeong S, Kim SW, Lee KK. Structure of the human lactoferrin gene and its chromosomal localization. Mol Cells. 1998 Dec 31;8(6):663–8. [PubMed] [Google Scholar]
- 55.van Veen HA, Geerts ME, van Berkel PH, Nuijens JH. The role of N-linked glycosylation in the protection of human and bovine lactoferrin against tryptic proteolysis. Eur J Biochem. 2004 Feb;271(4):678–84. doi: 10.1111/j.1432-1033.2003.03965.x. [DOI] [PubMed] [Google Scholar]
- 56.Thomassen EA, van Veen HA, van Berkel PH, Nuijens JH, Abrahams JP. The protein structure of recombinant human lactoferrin produced in the milk of transgenic cows closely matches the structure of human milk-derived lactoferrin. Transgenic Res. 2005 Aug;14(4):397–405. doi: 10.1007/s11248-005-3233-0. [DOI] [PubMed] [Google Scholar]
- 57.Nandi S, Yalda D, Lu S, Nikolov Z, Misaki R, Fujiyama K, et al. Process development and economic evaluation of recombinant human lactoferrin expressed in rice grain. Transgenic Res. 2005 Jun;14(3):237–49. doi: 10.1007/s11248-004-8120-6. [DOI] [PubMed] [Google Scholar]
- 58.Heegaard NH, Brimnes J. Comparison of heparin-binding to lactoferrin from human milk and from human granulocytes by means of affinity capillary electrophoresis. Electrophoresis. 1996 Dec;17(12):1916–20. doi: 10.1002/elps.1150171218. [DOI] [PubMed] [Google Scholar]
- 59.Moguilevsky N, Retegui LA, Masson PL. Comparison of human lactoferrins from milk and neutrophilic leucocytes. Relative molecular mass, isoelectric point, iron-binding properties and uptake by the liver. Biochem J. 1985 Jul 15;229(2):353–9. doi: 10.1042/bj2290353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998 Jun;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]