Abstract
Estrogen-related receptor alpha (ERRα) is an orphan member of the nuclear receptor family of transcription factors. In addition to its function as a metabolic regulator, ERRα has been implicated in the growth and progression of several malignancies. In the setting of breast cancer, not only is ERRα a putative negative prognostic factor, but we have recently found that knockdown of its expression retards tumor growth in a xenograft model of this disease. The specific aspects of ERRα function that are responsible for its actions in breast cancer, however, remain unclear. Using the coactivator PGC-1α as a protein ligand to regulate ERRα activity, we analyzed the effects of this receptor on gene expression in the ERα-positive MCF-7 cell line. This analysis led to the identification of a large number of potential ERRα target genes, many of which were subsequently validated in other breast cancer cell lines. Importantly, we demonstrate in this study that activation of ERRα in several different breast cancer cell lines leads to a significant increase in VEGF mRNA expression, an activity that translates into an increase in VEGF protein secretion. The induction of VEGF results from the interaction of ERRα with specific ERR-responsive elements within the VEGF promoter. These findings suggest that ERRα-dependent induction of VEGF may contribute to the overall negative phenotype observed in tumors in which ERRα is expressed and provide validation for its use as a therapeutic target in cancer.
Keywords: estrogen-related receptor, breast cancer, ESRRA, vascular endothelial growth factor, VEGF
Introduction
The estrogen-related receptors (ERRs) are members of the orphan nuclear receptor family of transcription factors. Although ERRα (NR3B1) and ERRβ (NR3B2) were initially identified based on homology with the classical estrogen receptors, their activity is not regulated by any known physiologically relevant estrogens [1]. Given their sequence similarities, however, multiple levels of cross-talk between the ERRs and ERs have been proposed. The convergence of the ERRα and estrogen signaling pathways may be particularly relevant in breast cancer, where it has been determined that ERRα expression is a negative prognostic factor [2, 3]. Distinct from its role as a modulator of estrogen signaling, ERRα has also been shown to function as a regulator of oxidative metabolism in tissues such as muscle, liver and adipose where it is highly expressed [4–7]. The metabolic functions of the ERRs may also play a role in breast cancer as it has been suggested that tumor response to hypoxia is significantly affected by small molecule inhibitors of ERR [8]. However, given what little we know about the additional processes in breast cancer in which ERRα is involved, we performed a genome-wide analysis of ERRα biology in breast cancer cells using a microarray approach [9].
The study of ERRα function has been hampered by the lack of a high affinity, receptor-specific small molecule agonist. Indeed, it is now apparent that the regulation of ERRα activity in vivo is likely accomplished by alterations in the expression level or activity of coregulatory molecules that function as “protein ligands” of the receptor; an observation that we have capitalized on in this study. The PPARγ coactivator (PGC-1) family members are among the most potent activators of ERRα transcriptional activity. The PGC-1α-ERRα axis has, in particular, been well-characterized in cardiac and skeletal muscle [4, 10]. We have previously reported on the use of PGC-1α to activate ERRα activity in the HepG2 hepatocellular carcinoma cell line; an approach that led to the identification of pathways in which this receptor is engaged and which may have an impact on the pathogenesis of cancer [5]. Understanding how these pathways contribute to breast cancer pathogenesis is the primary focus of our efforts in this area.
The use of PGC-1α to identify ERRα target genes in an ERα-positive breast cancer cell line has allowed us to address the outstanding issue of the extent to which ERRα activity impinges on estrogen signaling. Under the conditions in which our study was performed, we found that a large portion of genes induced or repressed by PGC-1α-coactivated ERRα were unaffected by estrogen treatment [9]. However, we did find a high degree of overlap between the biological pathways altered by PGC-1α-ERRα in breast cancer cells and in other tissues. Expectedly, genes involved in oxidative metabolism were found to be induced by PGC-1α-coactivated ERRα in MCF-7 breast cancer cells as well as in other cancer cell types. The role of these metabolic pathways in breast cancer pathogenesis is currently under investigation.
Intriguingly, our microarray analysis revealed that ERRα may regulate not only the utilization of nutrients and oxygen, but also their delivery to the growing tumor through the activation of the highly angiogenic protein, vascular endothelial growth factor (VEGF). Therefore, ERRα may enhance aerobic metabolism while also functioning to regulate the angiogenic response to the concomitant hypoxic conditions. The successful clinical application of VEGF inhibitors in oncology is testament to the importance of this growth factor in tumor angiogenesis. The pivotal role of angiogenesis in tumor biology suggests that ERRα regulation of VEGF may be in part responsible for the association of high ERRα expression with poor patient prognosis in breast cancer. We present herein a study aimed to dissect the molecular mechanism of VEGF induction by ERRα with a view toward utilizing the ERRα-VEGF axis as a new therapeutic target in breast cancer.
Materials and methods
Cell Culture
All cells were procured from American Type Culture Collection (Manassas, VA) through the Duke Cell Culture Facility (CCF) and cultured in media from Invitrogen (Carlsbad, CA). MDA-MB-231 and HepG2 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM). AU565 and BT474 cells were grown in Roswell Park Memorial Institute (RPMI) media. MCF-7 cells were grown in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12). All media was supplemented with 8% fetal bovine serum, 0.1 mM non-essential amino acids and 1 mM sodium pyruvate and all cells grown at 37° C in a humidified 5% CO2 atmosphere.
Plasmids
Plasmids for pcDNA3-ERRα [11], pcDNA3-PGC-1α (WT, ERRsp (2×9) and L2L3M) and VP16-ERRα [5], 3xERE-TATA-luciferase and 5xGal4-luciferase [12] were described previously. VEGF-luciferase promoter constructs were a kind gift from Dr. Salman Hyder (University of Missouri, Columbia, MO) and have been described previously [13]. Mutant constructs were generated using site-directed mutagenesis to change the 5′-TGACCT- 3′ at +411 relative to the transcription initial site to 5′-TGTTTT- 3′ and sequenced prior to use.
Generation and transduction of adenoviruses
The generation and purification of PGC-1α adenoviruses (WT, ERRsp (2×9), and L2L3M) and siRNA adenoviruses (siCON and siERRα) have been described previously [14]. Cells were infected at the indicated multiplicity of infection (MOI of 10, 20 or 50) either in suspension or adhered to the cell culture dish for 2 h at 20°C with gentle rocking. The MCF-7 microarray experiment has been described in detail previously [9]. In experiments with siRNA and PGC-1α infections, cells were infected with siRNA adenovirus 48 h prior to a second infection with PGC-1α [15].
Generation and transduction of retroviruses
ERRα siRNA oligonucleotides [15] and control siRNA oligonucleotides [14] were ligated into PSR-GFP/Neo (OligoEngine). These constructs were cotransfected (FuGene, Roche Applied Science) with the packaging vector pCL10A1 (Imigenex) into the 293TS packaging cell line [16], which was a generous gift from Dr. Christopher Counter (Duke University Medical Center, Durham, NC). 293TS viral supernatant was clarified by filtration, supplemented with 8μg/ml polybrene, and used to replace the media on MDA-MB-231 target cells for two serial 24 h infections. Positive cells were selected by two rounds of cell sorting for GFP expression, encoded with the siRNA constructs in the bicistronic pSR-GFP/Neo.
RNA preparation and analysis
Total RNA was isolated using Bio-Rad RNA purification columns, which included an on-column DNAse step (Bio-Rad, Hercules, CA). Isolated RNA (1 μg) was used to synthesize cDNA in a 20μL reaction volume using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time PCR was performed using the iCycler PCR platform with 0.25μL cDNA, 0.4μM specific primers and iQ SYBRGreen supermix (Bio-Rad, Hercules, CA). A cDNA dilution series was analyzed along with each experiment to establish a standard curve and target gene mRNA level was quantified using the 2(−ΔΔCt)method [17].
VEGF ELISA
VEGF was measured with a Quantikine ELISA kit (R&D Diagnostics, Minneapolis, MN) according to the manufacturer’s recommended protocol. Stable MDA-MB-231 siCON or siERRα cells were plated at a density of 50,000 cells in a 96-well plate (in 100 μL media) and the conditioned media was assayed 3 days later. MCF-7 cells were infected with adenovirus (MOI 100) and 10,000 cells were plated in 100 μL media; 3 days later media was subjected to VEGF ELISA. Cell number was assayed at the time of the ELISA using the using FluoReporter Blue Fluorometric dsDNA Quantitation kit (Molecular Probes, Eugene, OR) to ensure equal cell numbers. Each experiment was repeated at least 3 times and a representative experiment is shown + SEM for replicate wells. Human recombinant VEGF165 provided in the Quantikine ELISA kit was used as a standard.
Transient transfections
HepG2 cells were split 24 h before transfection into 24-well plates. MCF-7 cells were split into phenol-red free media 48 h prior to infection. All cells were transfected using Lipofectin (Invitrogen, Carlsbad, CA) and luciferase analyzed 24 h thereafter as described previously [18]. Each experiment was repeated at least three times, and results are expressed as mean + SEM for biological replicates unless otherwise indicated.
Results
ERRα regulates VEGF mRNA expression in ER-positive and ER-negative breast cancer cell lines
Due to the absence of a classical small molecule to activate ERRα transcriptional activity, the coactivator PGC-1α has been used as a protein ligand to enable the identification of the genes regulated by ERRα. For the purpose of screening for ERRα targets, however, the use of PGC-1α carries with it the caveat that it also activates a host of other nuclear receptors, including ERα, the glucocorticoid receptor and PPAR family members [5] (Fig. 1). Our lab has developed a novel technology to separate the ERR-coactivating function of PGC-1α from its other activities [14]. Specifically, as reported initially by Gaillard et al, the NR-interacting domains of PGC-1α were mutated such that the resultant “customized” coactivator (named ERRspPGC1) selectively interacts with the ERR family members. The successful use of the ERR-selective ERRspPGC1 to identify novel ERRα target genes in HepG2 hepatoma cells validated the customized coactivator approach [5].
Figure 1. Customization of PGC-1α to selectively coactivate ERRα.
(A) Schematic of PGC-1α with the three LXXLL-containing motifs illustrated and the wild-type sequence shown for L2 and L3 which are responsible for interacting with nuclear receptors. To generate the ERR-selective ERRspPGC1, these sequences were replaced with the ERR-interacting peptide indicated. (B) Wild-type PGC-1α coactivates both ERRα and ERα, while ERRspPGC1 coactivates ERRα only. HeLa cells were transfected with the indicated receptor, coactivator, and a 3XERE-luciferase reporter construct. ERα transfected cells were treated for 24 hours with 17β-estradiol (100nM). Results are plotted as fold induction of luciferase expression normalized to β-galactosidase for transfection efficiency + SEM for a representative experiment. (C) Wild-type PGC-1α coactivates each of the indicated receptors on its cognate response element (with agonist as indicated) as described for ERα in (B). The mutated PGC-1α, ERRspPGC1, displays a much restricted activity, coactivating ERRα and with some cross-reactivity with HNF4α. Modified from Gaillard et al [9].
We first determined that the overexpression of both wild-type PGC-1α and ERRspPGC1 induced VEGF mRNA as suggested in our initial HepG2 and MCF-7 microarray studies [9]. In order to further validate that PGC-1α induction of VEGF is ERRα-dependent, MCF-7 cells were infected with an siRNA to ERRα (or control siRNA) and subsequently with ERRspPGC1. As illustrated in Figure 2A, both basal and PGC-1α-dependent induction of VEGF were completely abrogated by ERRα ablation. A similar result was observed when this experiment was repeated under identical conditions in the ERα-negative MDA-MB-231 breast cancer cell line (Fig. 2B).
Figure 2. PGC-1α induction of VEGF is dependent on ERRα expression.
MCF-7 (A) and MDA-MB-231 (B) cells were serially infected with the siRNA adenovirus indicated followed by ERRspPGC1 or the control β-galactosidase virus. mRNA levels were assessed by quantitative RT-PCR, normalized to the expression of 36B4 and represented + SEM.
To determine whether VEGF expression was similarly regulated in other cells types, ERα-positive (BT474 and MCF-7) and ERα-negative (AU565 and MDA-MB-231 cell lines) were infected with PGC-1α (Fig. 3A). It was observed that VEGF mRNA was induced in all of these cell lines and that basal VEGF expression could be inhibited in all cases by adenoviral infection with an siRNA to ERRα (Fig. 3B). We concluded from these experiments that VEGF is a bona fide transcriptional target of ERRα.
Figure 3. Activation of ERRα induces VEGF while ERRα siRNA suppresses basal VEGF expression.
(A) Each breast cancer cell line was infected with PGC-1α adenovirus or β-galactosidase (β-gal) control for 2 days and then RNA was collected. (B) Breast cancer cell lines were infected with siRNA-expressing adenovirus, either directed to ERRα (siERRα) or a scrambled control sequence (siCON) for 2–3 days and then RNA was collected. mRNA levels were assessed by quantitative RT-PCR, normalized to the expression of 36B4 and represented + SEM.
ERRα regulates VEGF secretion in breast cancer cell lines
After determining that PGC-1α activated ERRα induced VEGF mRNA expression in breast cancer cell lines, the impact of this effect on VEGF protein expression was assessed. As VEGF must be secreted from tumor cells to exert its pro-angiogenic effects, we assayed the levels of VEGF secreted into the media following the activation of ERRα activity in MCF-7 cells. To ensure that the effect we observed was specific to the PGC-1α/ERRα complex, we performed an analogous experiment using a vector that expressed the non NR-interacting PGC-1α L2L3M variant or a β-gal control virus (Fig. 4A). Cell growth media was harvested three days after infection with the respective viruses and subjected to VEGF ELISA analysis. In this manner, we determined that both ERRspPGC1 and wild-type PGC-1α expression led to an increase in VEGF secretion (1.5–2 fold). Notably, the magnitude of this increase is similar to that that observed when cells are grown under hypoxic conditions [19]. The inverse experiment was performed in MDA-MB-231 cells as they express a high constitutive level of ERRα activity. Specifically, we measured VEGF secretion in MDA-MB-231 cells that were engineered to stably express an siRNA to ERRα. Preliminary characterization of these cell lines demonstrated that VEGF mRNA expression was significantly reduced by ERRα RNAi. When media was collected from siCON versus siERRα MDA-MB-231 cells, ERRα knockdown resulted in an approximately 40% reduction in secreted VEGF (Fig. 4B).
Figure 4. Activation of ERRα induces VEGF secretion while knock-down of ERRα diminishes basal VEGF secretion.
(A) MCF-7 cells were infected with the indicated adenovirus (MOI 100) and conditioned media subjected to VEGF ELISA. The wild-type (WT) and ERRα-specific (ERRsp) PGC-1α induce VEGF secretion compared to the negative controls β-galactosidase (β-gal) or non NR-interacting mutant PGC-1α L2L3M. (B) Stable cell lines were derived from MDA-MB-231 breast cancer cells using retroviral expression of the indicated siRNA construct. Conditioned media from each cell line was harvested and subjected to a VEGF ELISA. The average VEGF expression + SEM for a representative experiment are shown. (* p<0.001; ** p<0.0001)
VEGF is a direct transcriptional target of ERRα
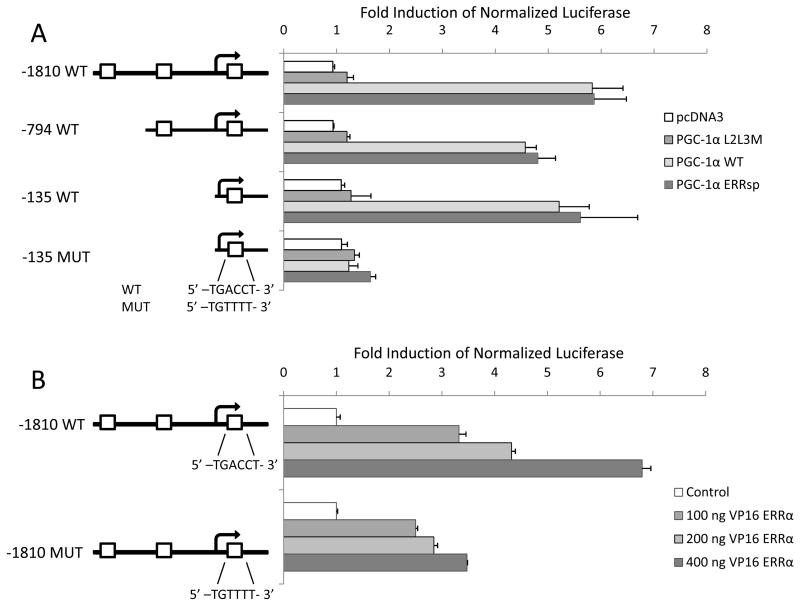
We next sought to determine if ERRα had a direct transcriptional effect on VEGF mRNA expression. ERRα binds to the promoter region of target genes through a conserved ERRα responsive element (ERRE). Pattern map searches of the VEGF gene revealed several potential ERREs within the promoter as well as one contained in an untranslated region downstream of the transcription initiation site (Fig. 5A). Although ERRα alone has low constitutive activity on the VEGF promoter region containing these elements, we observed that the coexpression of the coactivator PGC-1α, with ERRα, or of the constitutively active ERRα-VP16 fusion protein, strongly drives VEGF transcription.
Figure 5. PGC-1α coactivation of ERRα on the VEGF promoter.
(A) In the 1,800 bp upstream and the 955 bp downstream of the VEGF transcriptional initiation site, there are three potential ERRα-response elements (ERRE, 5′ AGGTCA 3′). Serial deletion constructs of the VEGF promoter were tested in MCF-7 cells in a transactivation assay. All conditions included transfection with an expression plasmid for ERRα, without which none of the PGC-1α constructs showed activity. Two negative controls (pcDNA3 and non-NR interacting PGC-1α (PGC-1α L2L3M)) were used in addition to the ERRα-specific PGC-1α (ERRspPGC1). While PGC-1α L2L3M did not activate ERRα on any of these fragments, ERRspPGC1 coactivation of ERRα induced even the shortest promoter construct (−135 WT) indicating that ERRα may induce VEGF through this ERRE. Furthermore, mutation of this ERRE eliminated PGC-1α-coactivation of ERRα. (B) HepG2 cells were transfected with a VEGF promoter construct in addition to increasing amounts of a constitutively active ERRα chimera formed by fusion to the VP16 activation domain (VP16-ERRα). Binding of this complex to the VEGF promoter stimulates luciferase expression. Mutation of the proximal ERRE blunts but does not eliminate the activation of the VEGF promoter by VP16-ERRα. This suggests that while ERRα can bind to the proximal ERRα, it may also drive transcription through an alternative site. Results are plotted as fold induction of luciferase expression normalized to β-galactosidase for transfection efficiency + SEM for a representative experiment.
To define in detail the mechanism(s) by which ERRα manifests its regulatory actions on the VEGF promoter, we transfected plasmids containing VEGF promoter constructs driving luciferase expression into MCF-7 cells together with vectors expressing ERRα and PGC-1α (ERRsp or L2L3M). In this manner we determined that PGC-1α expression increased transcription in an ERRα-dependent manner even on the shortest construct (−135 WT VEGF-luc, bp −135 to +955). This reporter contained a single ERRE within the transcribed region of VEGF (at +411). Mutation of this site completely abrogated PGC-1α-ERRα induction of VEGF, suggesting that ERRα likely can activate the VEGF promoter directly through this ERRE (Fig. 5A).
Having established that PGC-1α coactivates ERRα on the VEGF promoter, we sought to determine if this occurs through direct ERRα binding to the promoter. We evaluated ERRα interaction with the VEGF promoter without use of a cofactor by using a constitutively active chimera VP16-ERRα (Fig. 5B) in which transcriptional activity is a surrogate for DNA binding. In HepG2 cells, VP16-ERRα induces transcription from the full length (−1810) WT VEGF-luc (which includes three putative ERREs) 6.8 fold. However, when the proximal ERRE is mutated, the induction is reduced to a maximum of 3.5 fold. This is consistent with the analysis of a series of promoter deletion mutants (Fig. 5A), but indicates that even on the mutated promoter, VP16-ERRα retains activity. The remaining activity on the mutated promoter could be due to direct ERRα binding and activation of the promoter (at the putative ERRE despite mutation or at the upstream putative ERREs), transcriptional regulation by ERRα binding to another transcription factor which binds to the promoter (e.g., Sp1), or secondary effects of ERRα activity (e.g., ERRα induces Sp1 which induces VEGF transcription). However, taken together with the experiments using PGC-1α to coactivate ERRα on the VEGF promoter, our results indicate that the proximal ERRE within the promoter is in part responsible for the direct transcriptional activation of VEGF by ERRα.
Discussion
In this study we demonstrate that VEGF is a downstream transcriptional target of the PGC-1α/ERRα complex in breast cancer cells. Indeed we were able to implicate a specific DNA binding sequence within the VEGF promoter that is likely to be required for PGC-1α-ERRα activity. Interestingly, this ERRα-responsive site in the human VEGF promoter was also identified recently in the murine VEGF promoter and shown to bind ERRα using ChIP analysis [20].
Although it is probable that ERRα interacts directly with the VEGF promoter, it is also possible that its actions on this promoter occur in an indirect manner. The Sp1 and Sp3 binding elements (−131 to −52) that are partially responsible for mediating ERα induction of VEGF are included in the constructs tested above [21]. ERRα itself has been shown to bind to Sp1 and to activate TRα through an Sp1 binding site [22]. Moreover, ERRα has recently been shown to induce the expression of Sp1 [23]. In the context of VEGF expression in vivo, the transcriptional regulation defined herein is of biological significance whether it occurs through direct binding of ERRα to the promoter or by an alternate mechanism.
During the course of these studies, Spiegleman and colleagues published a series of experiments demonstrating that this axis plays a crucial role in the induction of angiogenesis in skeletal muscle under ischemic conditions [20]. Furthermore, they used the murine VEGF promoter to show that the induction of angiogenesis is a result of direct transcriptional regulation of VEGF by ERRα.
Given the role of ERRα as a regulator of oxidative metabolism it is not surprising that it also regulates the expression of VEGF. Indeed if ERRα activity drives increased aerobic ATP production, which may support the high metabolic rate characteristic of highly proliferative cells, it would also increase the cellular oxygen requirement. The concomitant induction of angiogenesis by ERRα, therefore, would serve to supplement the limited oxygen and nutrient supply provided by existing vessels or by passive diffusion across cells within a tumor. ERRα has previously been shown to induce endothelial nitric oxide synthase (eNOS) which catalyzes the synthesis of nitric oxide [24]. Nitric oxide signaling has been implicated in many aspects of the tumor-stromal interaction, including increasing vascular permeability. ERRα induction of VEGF and eNOS could work together to recruit new, leaky vessels which would provide the tumor with oxygen and nutrients.
Although the best-characterized functions of VEGF relate to its ability to induce angiogenesis and enhance vascular permeability, VEGF is also thought to have an autocrine function in certain tumor cells. For instance, while VEGFR-2 (Flk-1/KDR) is primarily expressed in endothelial cells, VEGFR-1 (Flt-1) is expressed in many normal and malignant cell types, including several types of breast cancer cells [25]. Indeed, Price and colleagues demonstrated in T47D breast cancer cells that VEGF autocrine stimulation activates the MAPK (mitogen-activated protein kinase) and PI3K signaling pathways and enhances the invasive phenotype of these cells in vitro [26]. This finding is intriguing in light of our observation that activation of ERRα also increases the migration of the MDA-MB-231 breast cancer cell line and conversely that knock-down of ERRα decreases their migration [9]. However, we have not yet addressed the specific role of ERRα regulation of VEGF in this phenotypic observation. The potential link between tumor cell migration and angiogenesis in the metastatic setting has recently been explored using a dorsal skinfold window chamber to observe the dynamics of tumor cell and blood vessel interaction. Li et al. found that breast cancer cells migrate towards existing vasculature even at the earliest stages of tumor development, prior to the onset of angiogenesis [27]. The dorsal skinfold model would provide a unique view of how ERRα affects early tumor development and may reveal a role for ERRα in the control of tumor cells themselves and in altering the tumor microenvironment.
In addition to a potential role of VEGF in breast cancer cell migration other autocrine functions have been suggested, including that it may be mitogenic and anti-apoptotic in tumor cells. Although several studies report a mitogenic activity of autocrine VEGF, there is conflicting evidence in the literature. Liang and colleagues tested a panel of seven breast cancer cell lines and found that exogenous VEGF induced proliferation in the majority (five) of the cell lines [28]. This group observed that the particular cell culture conditions had a significant effect on VEGF-induced proliferation and suggested that this may account for the conflicting findings that have been published. An alternative explanation for the sensitivity of VEGF-induced proliferation to culture conditions is that the observed effects in part reflect a combination of decreased sensitivity to the withdrawal of growth factors and to the presence of apoptotic signals. This hypothesis is supported by data implicating both the anti-apoptotic Bcl-2 and the MAPK signaling pathways in the autocrine effects of VEGF [29].
While VEGF has both mitogenic and anti-apoptotic effects in many types of breast cancer, a unique role for VEGF in ERα-positive breast cancer has been suggested. In particular, VEGF has been implicated in the development of hormone-resistance in breast cancers [28]. In MCF-7 cells, exogenous VEGF blocked the anti-proliferative effects of the anti-estrogen ICI 182,780. In addition, VEGF over-expression has been shown to promote estrogen-independent growth in MCF-7 cells [30]. It has been suggested not only that VEGF regulates hormone-responsiveness, but also that hormones regulate VEGF under both physiologic and pathologic conditions. The role of hormone induced VEGF is well-established in cyclical uterine angiogenesis and proliferation [31]. Although the effects of estrogen and progesterone on VEGF expression in breast cancer are more controversial, several reports have shown that VEGF can be induced by hormone treatment in ERα-positive breast cancer cell lines [32] and tumors [33].
While the intersection of the estrogenic and angiogenic pathways has been explored in breast cancer, our study is the first to describe ERRα regulation of VEGF in this context. We demonstrated that PGC-1α coactivation of ERRα induces VEGF in both ERα-positive and ERα-negative cell lines and that ERRα activity supports basal VEGF expression. Due to its dual effects on tumor metabolism and potentially on tumor angiogenesis, ERRα is uniquely situated to coordinate aerobic respiration with oxygen and nutrient delivery. Therefore, interfering with the ERRα signaling pathway may represent a novel strategy to concomitantly starve a tumor of nutrients and inhibit the metabolic pathways that provide energy to sustain tumor growth and progression.
Acknowledgments
NIH Grant DK074652 to DPM and a Department of Defense Grant W81XWH-06-1-0045 to RAS
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giguere V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, Ohuchi N, Sasano H. Estrogen-related receptor alpha in human breast carcinoma as a potent prognostic factor. Cancer Research. 2004;64(13):4670–4676. doi: 10.1158/0008-5472.CAN-04-0250. [DOI] [PubMed] [Google Scholar]
- 3.Ariazi EA, Clark GM, Mertz JE. Estrogen-related receptor alpha and estrogen-related receptor gamma associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Research. 2002;62(22):6510–6518. [PubMed] [Google Scholar]
- 4.Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor at signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Molecular And Cellular Biology. 2004;24(20):9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaillard S, Grasfeder LL, Haeffele CL, Lobenhofer EK, Chu TM, Wolfinger R, Kazmin D, Koves TR, Muoio DM, Chang CY, McDonnell DP. Receptor-selective coactivators as tools to define the biology of specific receptor-coactivator pairs. Mol Cell. 2006;24(5):797–803. doi: 10.1016/j.molcel.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Villena JA, Hock MB, Chang WY, Barcas JE, Giguere V, Kralli A. Orphan nuclear receptor estrogen-related receptor alpha is essential for adaptive thermogenesis. Proc Natl Acad Sci U S A. 2007;104(4):1418–1423. doi: 10.1073/pnas.0607696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vega RB, Kelly DP. A role for estrogen-related receptor alpha in the control of mitochondrial fatty acid beta-oxidation during brown adipocyte differentiation. Journal of Biological Chemistry. 1997;272(50):31693–31699. doi: 10.1074/jbc.272.50.31693. [DOI] [PubMed] [Google Scholar]
- 8.Ao A, Wang H, Kamarajugadda S, Lu J. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0711677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein R, Chang CY, Kazmin D, Way J, Schroeder T, Wergin M, Dewhirst M, McDonnell DP. Estrogen-related receptor alpha is critical for the growth of estrogen receptor-negative breast cancer. Cancer Research. 2008 doi: 10.1158/0008-5472.CAN-08-1594. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E, Kelly DP. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6(1):25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Zuercher WJ, Gaillard S, Orband-Miller LA, Chao EYH, Shearer BG, Jones DG, Miller AB, Collins JL, McDonnell DP, Willson TM. Identification and structure-activity relationship of phenolic acyl hydrazones as selective agonists for the estrogen-related orphan nuclear receptors ERRbeta and ERRgamma. J Med Chem. 2005;48:3107–3109. doi: 10.1021/jm050161j. [DOI] [PubMed] [Google Scholar]
- 12.Chang CY, Norris JD, Gron H, Paige LA, Hamilton PT, Kenan DJ, Fowlkes D, McDonnell DP. Dissection of the LXXLL nuclear receptor-coactivator interaction motif using combinatorial peptide libraries: Discovery of peptide antagonists of estrogen receptors alpha and beta. Molecular and Cellular Biology. 1999;19(12):8226–8239. doi: 10.1128/mcb.19.12.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Brandt S, Hyder SM. Ligand- and cell-specific effects of signal transduction pathway inhibitors on progestin-induced vascular endothelial growth factor levels in human breast cancer cells. Mol Endocrinol. 2005;19(2):312–326. doi: 10.1210/me.2004-0252. [DOI] [PubMed] [Google Scholar]
- 14.Gaillard S, Dwyer MA, McDonnell DP. Definition of the molecular basis for estrogen receptor-related receptor-alpha-cofactor interactions. Mol Endocrinol. 2007;21(1):62–76. doi: 10.1210/me.2006-0179. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERR alpha) Journal Of Biological Chemistry. 2003;278(11):9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- 16.Baines AT, Lim KH, Shields JM, Lambert JM, Counter CM, Der CJ, Cox AD. Use of retrovirus expression of interfering RNA to determine the contribution of activated k-ras and ras effector expression to human tumor cell growth. Methods Enzymol. 2005;407:556–574. doi: 10.1016/S0076-6879(05)07045-X. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Norris J, Fan D, Aleman C, Marks JR, Futreal PA, Wiseman RW, Iglehart JD, Deininger PL, McDonnell DP. Identification of a new subclass of alu DNA repeats which can function as estrogen receptor-dependent transcriptional enhancers. J Biol Chem. 1995;270:22777–22782. doi: 10.1074/jbc.270.39.22777. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6(4):1433–1439. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 20.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451(7181):1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 21.Stoner M, Wormke M, Saville B, Samudio I, Qin C, Abdelrahim M, Safe S. Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor alpha and SP proteins. Oncogene. 2004;23(5):1052–1063. doi: 10.1038/sj.onc.1207201. [DOI] [PubMed] [Google Scholar]
- 22.Castet A, Herledan A, Bonnet S, Jalaguier S, Vanacker J-M, Cavailles V. RIP140 differentially regulates estrogen receptor-related receptor transactivation depending on target genes. Mol Endocrinol. 2006 doi: 10.1210/me.2005-0227. In Press. [DOI] [PubMed] [Google Scholar]
- 23.Sumi D, Ignarro LJ. Sp1 transcription factor expression is regulated by estrogen-related receptor alpha1. Biochem Biophys Res Commun. 2005;328(1):165–172. doi: 10.1016/j.bbrc.2004.12.165. [DOI] [PubMed] [Google Scholar]
- 24.Sumi D, Ignarro LJ. Estrogen-related receptor alpha 1 up-regulates endothelial nitric oxide synthase expression. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2003;100(24):14451–14456. doi: 10.1073/pnas.2235590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miralem T, Steinberg R, Price D, Avraham H. VEGF(165) requires extracellular matrix components to induce mitogenic effects and migratory response in breast cancer cells. Oncogene. 2001;20(39):5511–5524. doi: 10.1038/sj.onc.1204753. [DOI] [PubMed] [Google Scholar]
- 26.Price DJ, Miralem T, Jiang S, Steinberg R, Avraham H. Role of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cells. Cell Growth Differ. 2001;12(3):129–135. [PubMed] [Google Scholar]
- 27.Li CY, Shan S, Huang Q, Braun RD, Lanzen J, Hu K, Lin P, Dewhirst MW. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92(2):143–147. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]
- 28.Liang Y, Brekken RA, Hyder SM. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr Relat Cancer. 2006;13(3):905–919. doi: 10.1677/erc.1.01221. [DOI] [PubMed] [Google Scholar]
- 29.Baek JH, Jang JE, Kang CM, Chung HY, Kim ND, Kim KW. Hypoxia-induced VEGF enhances tumor survivability via suppression of serum deprivation-induced apoptosis. Oncogene. 2000;19(40):4621–4631. doi: 10.1038/sj.onc.1203814. [DOI] [PubMed] [Google Scholar]
- 30.Guo P, Fang Q, Tao HQ, Schafer CA, Fenton BM, Ding I, Hu B, Cheng SY. Overexpression of vascular endothelial growth factor by MCF-7 breast cancer cells promotes estrogen-independent tumor growth in vivo. Cancer Res. 2003;63(15):4684–4691. [PubMed] [Google Scholar]
- 31.Hyder SM, Stancel GM, Chiappetta C, Murthy L, Boettger-Tong HL, Makela S. Uterine expression of vascular endothelial growth factor is increased by estradiol and tamoxifen. Cancer Res. 1996;56(17):3954–3960. [PubMed] [Google Scholar]
- 32.Garvin S, Nilsson UW, Dabrosin C. Effects of oestradiol and tamoxifen on VEGF, soluble VEGFR-1, and VEGFR-2 in breast cancer and endothelial cells. Br J Cancer. 2005;93(9):1005–1010. doi: 10.1038/sj.bjc.6602824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabrosin C, Margetts PJ, Gauldie J. Estradiol increases extracellular levels of vascular endothelial growth factor in vivo in murine mammary cancer. Int J Cancer. 2003;107(4):535–540. doi: 10.1002/ijc.11398. [DOI] [PubMed] [Google Scholar]