Abstract
Toluene is a commonly abused organic solvent. Inhalant abusers are increasingly women in their prime childbearing years. Children born to mothers who abused solvents during pregnancy may exhibit characteristics of a “fetal solvent syndrome” which may include dysmorphic features. This study examined the teratological effects of an abuse pattern of binge toluene exposure during gestation on skeletal and soft tissue abnormalities, body weight, and body size in fetal rats. Pregnant Sprague–Dawley rats were exposed for 30 min, twice daily, from gestational day (GD) 8 through GD20 to either air (0 ppm), 8,000 ppm, 12,000 ppm, or 16,000 ppm toluene. Two-thirds of each litter was prepared for skeletal examination using Alizarin Red S staining while the remaining third of each litter was fixed in Bouin’s solution for Wilson’s soft tissue evaluation. Exposure to toluene at all levels significantly reduced growth, including decreases in placental weight, fetal weight, and crown-rump length. In addition, numerous gross morphological anomalies were observed such as short or missing digits and missing limbs. Skeletal examination revealed that ossification of the extremities was significantly reduced as a result of toluene exposure at all levels. Specific skeletal defects included misshapen scapula, missing and supernumerary vertebrae and ribs, and fused digits. Soft tissue anomalies were also observed at all toluene levels and there was a dose-dependent increase in the number of anomalies which included cryptorchidism, displaced abdominal organs, gastromegaly, distended/hypoplastic bladder, and delayed cardiac development, among others. These results indicate that animals exposed prenatally to levels and patterns of toluene typical of inhalant abuse are at increased risk for skeletal and soft tissue abnormalities.
1. Introduction
Toluene, an aromatic hydrocarbon (CAS #108-88-3; C7H8; methylbenzene; toluol), is one of several organic solvents that are ubiquitous in the contemporary environment and essential for industry [1]. Organic solvents such as toluene are diverse chemicals that are commonly found in many commercial products including paints, varnish, glue and gasoline [2]. With appropriate use of these products and implementation of safe practices in the workplace, only low vapor concentrations should be occurring in the household or workplace [1]. However, acute exposures to dangerously high concentrations can occur in industrial accidents [3], or increasingly, with voluntary inhalant abuse [4–6].
Administration of these compounds is not complicated and there are several means of rapidly achieving effects with voluntary inhalation/self-administration of high concentrations of these volatile products. Inhalation methods include breathing fumes from a solvent-soaked rag placed near the mouth and/or nose; from solvents placed in paper/plastic bags or balloons; from nasal inhalation directly from source containers; or by inverting spray cans and spraying aerosol compounds directly into the mouth or nose. Inhalant effects occur very quickly, usually within seconds, and produce an acute intoxication resulting in vertigo, light-headedness, and/or loss of consciousness lasting anywhere from 15 min to 60 min [7,2].
Among American adolescents, the prevalence of inhalant abuse is surpassed only by use of marijuana, alcohol and tobacco and may be attributed to the easy accessibility of products containing toluene [8,9,2]. The most recent National Survey on Drug Use and Health [10] estimates that 22.5 million Americans aged 12 years old and older deliberately abused inhalants at least once in their lifetimes (9.1% of the population aged 12 or older) and 0.8% did so in the previous year [10]. While there was no significant difference in the number of new inhalant users between 2006 and 2007, significant increases were observed in the average age at first use among recent initiates aged 12 to 49 years, up from 15.7 years (2006) to 17.1 years (2007) [10]. While males have historically had higher lifetime prevalence rates for solvent abuse, there is emerging evidence that at several ages female usage surpasses males and the most recent data show essentially equivalent prevalence rate for males and females in the 12th grade [10]. The shift to older age use among women means the rate of solvent abuse by women in their prime child-bearing years is increasing. Further, a majority of users fall into lower socioeconomic tiers [11,12,8,13] where pregnancy rates and toluene abuse in some cultures are higher [14]. There is increasing concern about the possible teratogenic effects of toluene on a developing fetus in part because organic solvents such as toluene have a high affinity for lipid-rich tissues and can readily cross the placenta, allowing for direct exposure of the fetus [15].
Studies of high-dose prenatal exposure to toluene in humans have reported embryopathic effects similar to Fetal Alcohol Syndrome (FAS; [16]). The similarity between the physical, behavioral and developmental characteristics of prenatal exposure to toluene and the deficits associated with children with the Fetal Alcohol Spectrum Disorders (FASDs) compelled Toutant and Lippmann [17] to suggest that both are expressions of a “Fetal Solvent Syndrome” (FSS) and, like others, to propose that toluene and ethanol may share teratogenic mechanism(s) [18,16]. Jones and Balster [19] described more than 100 children exposed to solvents in utero as a result of maternal solvent abuse. These children variously exhibited prenatal toluene-associated craniofacial abnormalities, growth retardation, and CNS dysfunction including microencephaly, brain malformations, mental retardation, hyperactivity and attention problems [18], all of which are also seen in children with FASDs [20].
Using high-dose inhalation in animals that models the concentrations and patterns of inhalation in solvent abuse reported in human studies (cf., [17,16,18]) has allowed direct assessment of the teratogenic effects of toluene abuse [21,22]. In our previous studies focusing on deleterious neurobehavioral outcomes in rats, we had noted also that gross malformations such as missing or malformed digits, limb deficits and craniofacial abnormalities, appeared to be more common following brief high-dose prenatal toluene exposure than in non-exposed pups [23,22,24,25]. In the current study, we specifically assessed the effects of the same repeated high-level binge prenatal toluene exposures on gross dysmorphology, skeletal defects, and soft tissue anomalies in fetal rats.
2. Materials and Methods
2.1 Animals
All animal procedures had prior approval by the Wayne State University Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health (NIH) “Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy Press 1996; NIH Publication No. 85-23, revised 1996). Thirty-two timed-pregnant female Sprague-Dawley (CRL:CD(SD)) rats (200-g minimum body weight at mating; ~ 60 days old) were purchased from Charles Rivers Laboratories (Portage, MI, USA) and arrived on gestation day 6 (GD6; sperm plug positive = GD0). Pregnant rats were housed individually in polypropylene cages (52×28×22 cm) containing wood chip bedding and fitted with steel wire tops. All animals were allowed ad lib access to Rodent Lab Diet 5001 (PMI, Nutrition International, Inc., Brentwood, MO) and water while in their home cages. The AAALAC-approved vivarium was kept on a 12-h light/12-h dark cycle (lights on at 0600 hrs) with temperature controlled to 20°C – 22°C and relative humidity levels between 40% and 70%. Two days following arrival at the vivarium, dams were assigned to one of four gestational treatment groups, counter-balanced for maternal body weight on GD6.
2.2 Inhalation exposure procedures
For each daily exposure, pregnant rats were transported in their home cages from the vivarium to the lab and placed into an exposure chamber. The dams were weighed every other day throughout the exposure period. Dams were exposed to 0 ppm, 8,000 ppm, 12,000 ppm or 16,000 ppm toluene (#T324; Fischer Scientific, Fair Lawn, NJ; purity ≥99.5%) twice daily between 0900 and 1400 hrs, 2 hours apart, for 30 min from GD8 through GD20 to simulate the high-dose “binge” exposures in human solvent abusers. Exposures began on GD8, consistent with prior research from our laboratory, because GD8 is post implantation and limits the risk of resorption. Vapor exposures occurred in 36-liter cylindrical glass jars, with Plexiglas lids, contained within a fume hood. The lid of each exposure chamber was equipped with an injection port, fan, and a stainless steel mesh cage with filter paper located below the fan. During toluene exposure, individual dams were placed onto a grid floor 20 cm from the bottom and 30 cm from the filter paper in the lid of the chamber. After the animals were placed in the chamber, the lid was closed and a calculated volume of toluene was injected onto the filter paper. The fan was then turned on which volatilized and diffused toluene within the chamber.
Toluene vapor concentrations were calculated nominal concentrations which were confirmed periodically by single wavelength-monitoring infrared spectroscopy (Miran 1A, Foxboro Analytical, North Haven, CT). For all concentrations of toluene, mean concentrations were within 3% of nominal 2.5 min after the toluene was added and remained within 2% of the nominal concentrations throughout each 30-min session. Levels of waste gases (i.e., water vapor and CO2) were monitored during pilot studies and changes during a session were negligible. Temperature (25°C) and humidity (49%) were slightly elevated during exposure sessions as compared to ambient laboratory room levels (23.8°C and 24% humidity). Separate dams in the “air-only” control group were placed in identical exposure chambers at the same time as the toluene exposed groups, but without toluene (0 ppm). Each dam was exposed to only one solvent concentration. At the conclusion of each exposure period, dams were removed immediately and returned to their home cages until the next exposure period. Dams recovered from the sedating effects of the toluene exposure within 10–15 min after removal from the exposure chamber [23,26]. The same procedure for exposure was repeated 2 hrs later. After the second daily exposures, dams were returned to the vivarium in their home cages.
2.3 Fetal examinations
On GD 21 and immediately following euthanasia by carbon dioxide inhalation, intact gravid uteruses were removed and weighed. Beginning at the left ovary and ending at the right ovary, the amniotic sac was ruptured and the intrauterine position of each fetus was recorded as well as whether the fetus was alive, dead, or resorbed. Each fetus was removed, separated from the placenta, and live fetuses were euthanized immediately with sodium pentobarbital (120mg/kg; IP). Fetuses classified as live at the time of extraction from the uterus were blotted to remove excess amniotic fluid, sexed and weighed. Crown-rump and tail lengths were measured and each fetus was examined for gross morphological abnormalities. Two-thirds of the fetuses from each litter were selected pseudo-randomly for skeletal examination. All fetuses exhibiting external, gross morphological abnormalities were selected for the skeletal examination with the balance selected at random. The remaining one-third of each litter was designated for Wilson’s soft tissue evaluation. Maternal organs (liver, lung and kidneys) were also removed and weighed.
2.4 Specimen preparation and examination
2.4.1 Skeletal evaluation
The methods for processing and staining of the fetuses where based on previously published methods [27,28]. Fetuses selected for skeletal evaluation were eviscerated and fixed in 95% ethanol (EtOH) for 24 hours. Skin, thoracic contents and neck fat pads were removed and the fetuses returned to 95% EtOH for four additional days. Fetuses were macerated/defatted in 1% potassium hydroxide (KOH) for 48 hours or until the tissue was visibly clear and the skeleton visible. Skeletons were stained in 0.0025% Alizarin Red S in 1% KOH for 36 hours or until the skeletons were dark purple. A final soak in 0.5% KOH for 24 hours removed excess stain from the remaining cleared tissue. After a brief rinse in distilled water, the skeletons were transferred to 30% glycerin for 24 hours. Skeletons were stored in 50% glycerin until examination was completed. For permanent storage, skeletons were transferred to 100% glycerin with thymol crystals added as a preservative.
After staining was completed, each specimen was examined by an experimenter blind to gestational exposure. The ribs, sternum, limbs, various digits, and vertebral column regions were examined and the degree of ossification for each of these 10 kinds of bones was determined and scored from 0 to 1 based on degree of abnormality. Fully developed and ossified bones were given a score of 1, while a score of zero was given to a bone that was not visible and was not stained. Rudimentary, partially ossified or poorly ossified bones were given the score of 0.5. All defects other than reductions in ossification were recorded as malformations (Table 2c). Examples are presented in Figure 2.
2.4.2 Soft tissue evaluation
Fetuses for Wilson’s soft tissue examination were kept in Bouin’s solution (Sigma-Aldrich) for 3 weeks to fix and decalcify the tissue. Bouin’s solution was replaced with 70% ethanol for storage until sectioning. Fetuses were examined one at a time by an experimenter blind to the treatment condition of the fetuses. Examinations began by removing the legs and tail at the trunk. Using a gross pathology sectioning knife, the upper portion of the head was removed in a plane just above the ears. After examining for cleft palate, 1- to 2-mm sections were cut beginning at the nasal region and ending before the reproductive organs with slightly thinner sections taken through the heart and kidney regions. Sections were placed in order on glass slides and examined under a dissecting microscope. All anomalies were photographed and recorded.
2.5 Statistical Analysis
Maternal body weight was analyzed using a 4 × 8 two-way, repeated measures ANOVA with prenatal Treatment (i.e., toluene concentration – 0 ppm, 8,000 ppm, 12,000 ppm & 16,000 ppm) as the between-subjects factor and gestational Day (GD6, GD8, GD10, GD12, GD14, GD16, GD18 & GD20) as the repeated, within-subject factor. Percent maternal weight gain, litter size, fetal resorptions, sex ratio, and total number of live fetuses were analyzed by one-factor ANOVAs with prenatal Treatment as the factor. Offspring development variables, including pup weight, were analyzed using litter as the unit of measure. Scores for degree of ossification were summed across each kind of bone within each fetus. The means of all male and female pups, respectively, within a litter were calculated for each outcome and used in the analyses. Two- and three-way ANOVAs were used with prenatal Treatment and Sex as between-subjects factors. An alpha level of 0.05 was designated as the cut-point for declaration of statistical significance. Planned comparisons among the concentrations of toluene were also used, as well as Tukey’s B post hoc analyses, as appropriate (p<0.05). Frequency data for malformations were analyzed with Kruskal-Wallis one-way ANOVA (York et al., 1982).
3. Results
3.1 Maternal observations
Consistent with our prior reports [23–25,29], in response to toluene exposure, the dams showed no overt signs of illness or abnormal behavior other than immobility and sedation. There were initial qualitative increases in activity in the chambers followed by concentration-dependent decreases in activity and, as the number of toluene exposures increased, dams appeared to develop a tolerance to and more rapid recovery from these initial activating effects of toluene. Following the first day of exposures to 12,000 ppm or 16,000 ppm toluene, the dams were essentially immobile and appeared sedated with post-exposure recovery taking ~20 min. Dams exposed to 8,000 ppm toluene never fully reached sedation although ambulatory activity was reduced. After ~10 sessions, all dams showed rapid recovery in returning to pre-exposure levels of activity within ~10 min of removal from the chamber.
A total of 32 pregnant dams were used, yielding a total of 406 live fetuses. Maternal and fetal data for all four exposure conditions are presented in Table 1. There was no difference in dam weight upon arrival on GD6 (p = 0.74; mean weight = 233.4 ± 2.45 g). As expected, dams in all exposure groups gained significant weight during gestation across days, F(7,196) = 591.75, p<0.0001. Average gains were 73.6 g by GD20, or 41.6% of initial weight on GD6 (the day of arrival in the lab) and all dams exposed to toluene gained less weight than the air-only controls, F(3,31) = 8.32, p<0.0001. A significant Day × Dose interaction was also observed, F(21,196) = 3.53, p<0.0001, with toluene-exposed dams gaining less weight than their air-only counterparts across exposure days. No significant prenatal toluene group differences were found in total number of live fetuses per dam, or litter sex ratio (p’s>0.40). Significant main effects were found for prenatal toluene on gravid uterine weight, F(3,30) = 3.79, p<0.05, and post hoc analyses revealing that the 12,000- and 16,000-ppm groups were lighter than the 0-ppm control dams (p<0.05). No significant differences or interactions were observed between exposure groups for maternal liver, lung, or kidney weights (p’s >0.10).
Table 1.
Maternal and Litter Characteristics (mean ± sem)
| Toluene Exposure: | 0 ppm | 8000 ppm | 12000 ppm | 16000 ppm | p* |
|---|---|---|---|---|---|
| Number of dams (N=32) | 8 | 8 | 8 | 8 | |
| Number of live litters (N=32) | 8 | 8 | 8 | 8 | |
| Maternal weight on GD6 (g) | 228.85 ± 2.43 | 235.21 ± 7.05 | 233.18 ± 5.43 | 236.31 ± 4.12 | ns |
| Maternal weight gain to GD20 (g) | 90.81 ± 3.64 | 69.24 ± 4.91* | 71.95 ± 4.84* | 62.58 ± 3.11* | 0.001 |
| Percent weight gain | 27.64 ± 1.02 | 21.77 ± 1.37* | 23.24 ± 1.38 | 19.99 ± 0.82* | 0.001 |
| Gravid uterine weights (g) | 72.93 ± 2.97 | 65.66 ± 3.50 | 60.74 ± 3.23* | 61.31 ± 1.93* | 0.05 |
| Resorptions (mean) | 0.13 ± 0.13 | 0.13 ± 0.13 | 0.13 ± 0.13 | 0.25 ± 0.16 | ns |
| No. of litters with resorbed fetuses | 1 | 1 | 1 | 2 | |
| % Litters w/resorbed fetuses | 12.5 | 12.5 | 12.5 | 25.0 | |
| No. of fetuses resorbed | 1 | 1 | 1 | 2 | |
| Resorbed fetuses (%) | 0.97 | 0.95 | 1.0 | 1.9 | |
| Live fetuses | 12.75 ± 0.67 | 13.00 ± 0.60 | 12.13 ± 0.74 | 12.88 ± 0.44 | ns |
| Dead fetuses | -- | -- | -- | -- | -- |
| Fetal weights (g) | |||||
| Male | 3.60 ± 0.06 | 3.35 ± 0.04* | 3.38 ± 0.04* | 3.06 ± 0.04* | 0.001 |
| Female | 3.52 ± 0.05 | 3.15 ± 0.02* | 3.12 ± 0.04* | 3.02 ± 0.04* | 0.001 |
| Placental weights (g) | |||||
| Male | 0.52 ± 0.01 | 0.46 ± 0.01* | 0.43 ± 0.01* | 0.40 ± 0.01* | 0.001 |
| Female | 0.50 ± 0.01 | 0.44 ± 0.01* | 0.41 ± 0.01* | 0.40 ± 0.01* | 0.001 |
| Crown-rump length (mm) | |||||
| Male | 36.42± 0.28 | 35.66± 0.23 | 35.52± 0.26 | 34.51± 0.20* | 0.001 |
| Female | 36.11± 0.28 | 34.37± 0.21* | 34.33± 0.29* | 34.06± 0.25* | 0.001 |
| Tail length (mm) | |||||
| Male | 13.48± 0.13 | 13.30± 0.14 | 13.04± 0.15 | 12.89 ± 0.14* | 0.01 |
| Female | 13.64± 0.17 | 13.03± 0.20 | 13.27± 0.17 | 12.96 ± 0.18 | 0.057 |
| Gross dysmorphology (see Table 2a) | |||||
| No. of litters with malformed fetuses | 3 | 6 | 4 | 6 | |
| No. of fetuses affected/examined | 3/102 | 11/104 | 5/97 | 15/103 | 0.01 |
| Malformed fetuses (%) | 2.94 | 10.57 | 5.15 | 14.56 | |
| % Litters affected | 37.5 | 75 | 50 | 75 | |
| Soft tissue anomalies (see Table 2b) | |||||
| No. of litters with malformed fetuses | 1 | 4 | 7 | 8 | |
| No. of fetuses affected/examined | 2/33 | 12/36 | 17/33 | 26/36 | 0.01 |
| Malformed fetuses (%) | 6.06 | 33.33 | 51.51 | 72.22 | |
| % Litters affected | 12.5 | 50 | 87.5 | 100 | |
| Skeletal defects (see Table 2c) | |||||
| No. of litters with malformed fetuses | 2 | 4 | 7 | 7 | |
| No. of fetuses affected/examined | 3/69 | 12/68 | 21/64 | 24/67 | 0.01 |
| Malformed fetuses (%) | 4.35 | 17.65 | 32.81 | 35.82 | |
| % Litters affected | 25 | 50 | 87.5 | 87.5 | |
Toluene exposure group is significantly different from 0-ppm controls.
3.2 Fetal observations
3.2.1 Gross malformations
Significant main effects were found for prenatal toluene on body weight for fetuses, F(3,405) = 40.55, p<0.0001 (Table 1). Post hoc analysis revealed that all three toluene-exposed groups of fetuses weighed significantly less than fetuses in the 0-ppm air-control group: the 16,000-ppm fetuses weighing significantly less than the 8,000- and 12,000-ppm fetuses (p’s<0.05). As expected, a significant main effect was also found for Sex, F(1,405) = 19.38, p<0.0001, with females weighing less than their male counterparts. The Sex × Treatment interaction approached but did not reach significance (p=0.08). Significant main effects for prenatal toluene exposure were also found for crown-rump length, (F(3,405) = 20.83, p<0.001, and tail length, F(3,405) = 5.29, p<0.001, with post hoc analyses revealing that toluene-exposed male fetuses and 16,000 ppm exposed female fetuses had shorter crown to rump lengths than the air-controls (p<0.05), and male fetuses that had been exposed prenatally to 16,000 ppm had shorter tails than all other exposure groups (p<0.05). There were no significant differences in the number of resorptions between control and toluene-treated litters, and there were no instances of fetal death in any group.
Gross external major malformations included short or missing digits, short/tightly curled tail, short limbs, runting, short snout and mal-positioned ears, with short or missing digits being the most commonly occurring anomaly (see Table 1 and 2; Fig. 1). Other anomalies, including proptosis, hepatomegaly and hydrocephaly, occurred in toluene-exposed groups (Tables 1, 2a & 2b). “Runting” was defined as a pup with weight > 2 SD’s below the pup’s own litter mean with the “runt” included [23]. The placentas of two fetuses in one 16,000 ppm-exposed litter were fused and shared a common amniotic sac. This was likely a spontaneous variation that would have occurred before toluene exposure began. Exposure to all levels of toluene (8,000, 12,000 & 16,000 ppm) significantly increased mean number of fetuses per litter exhibiting gross physical anomalies as compared to non-exposed fetuses, χ2=11.58, df=3, p<0.01.
Table 2.
| Table 2a. Gross Fetal Dysmorphology After Abuse Pattern Gestational Exposure to Toluene | ||||
|---|---|---|---|---|
| Toluene Exposure (ppm) | 0 | 8,000 | 12,000 | 16,000 |
| Gross anomalies (other than digital skeletal) | ||||
| No. of fetuses examined (N=406) | 102 | 104 | 97 | 103 |
| Resorbed fetuses | 1 | 1 | 1 | 2 |
| Fetuses with gross anomalies (n) | 1 | 8 | 5 | 13 |
| Litters with gross anomalies (n) | 1 | 5 | 4 | 5 |
| Percent of fetuses with anomalies (%) | 0.98 | 7.69 | 5.15 | 12.62 |
| Percent of litters with anomalies (%) | 12.5 | 62.5 | 50 | 62.5 |
| Runt | - | - | 1 | - |
| Facial anomalies (including misshapen tongue & lower jaw, rudimentary snout) | - | 3 | 2 | 5 |
| Body shape | - | 3 | - | 1 |
| Malpositioned ears | - | 1 | - | 4 |
| Hydrocephaly | 0 | 1 | 0 | 1 |
| Proptosis/displaced eyes | 0 | 0 | 2 | 2 |
| Table 2b. Fetal Soft Tissue Anomalies After Abuse Pattern Gestational Exposure to Toluene | ||||
|---|---|---|---|---|
| Toluene Exposure (ppm) | 0 | 8,000 | 12,000 | 16,000 |
| Soft tissue anomalies | ||||
| No. of fetuses examined (N=138) | 33 | 36 | 33 | 36 |
| Fetuses with soft tissue anomalies (n) | 2 | 12 | 17 | 26 |
| Total number of anomalies | 2 | 21 | 25 | 43 |
| Percent of fetuses with soft tissue anomalies (%) | 6.06% | 33.33% | 51.51% | 72.22% |
| Litters with soft tissue anomalies (n) | 1 | 4 | 7 | 8 |
| Percent of litters with soft tissue anomalies (%) | 12.5% | 50% | 87.5% | 100% |
| Hemorrhagic adrenal | - | 1 | - | - |
| Dilated cerebral ventricles | - | - | 2 | - |
| Narrowed esophagus | - | - | - | 1 |
| Large trachea | - | - | 1 | - |
| Small spinal column | - | - | - | 1 |
| Small lung | - | - | 1 | 1 |
| Dilated/displaced cardiac vessels | - | 3 | 1 | 2 |
| Cardiac defects (microcardia, cardiomegaly, hemopericardium) | - | 8 | 8 | 12 |
| Hemorrhage | - | 3 | 3 | 2 |
| Hepatomagaly | - | 1 | - | 2 |
| Microgastria/gastromegaly | - | 1 | - | 5 |
| Displaced abdominal organs | - | 1 | 1 | 5 |
| Displaced/ectopic testis | - | 1 | 5 | 5 |
| Hypoplastic/distended bladder | 1 | 2 | 1 | 4 |
| Hydroureter | - | - | 1 | 1 |
| Double ureter | - | - | - | 1 |
| Oval shaped kidney | - | - | - | 1 |
| Hydronephrosis | 1 | - | 1 | - |
| Table 2c. Fetal Skeletal Defects After Abuse Pattern Gestational Exposure to Toluene | ||||
|---|---|---|---|---|
| Toluene Exposure (ppm) | 0 | 8,000 | 12,000 | 16,000 |
| Skeletal defects | ||||
| No. of fetuses examined (N= 268) | 69 | 68 | 64 | 67 |
| Fetuses with skeletal defects (n) | 3 | 12 | 21 | 24 |
| Total number of defects (n) | 3 | 17 | 22 | 25 |
| Litters with skeletal defects (n) | 2 | 4 | 7 | 7 |
| Percent of fetuses with skeletal defects (%) | 4.35% | 17.65% | 32.81% | 35.82% |
| Percent of litters with skeletal defects (%) | 25% | 50% | 87.5% | 87.5% |
| Scapula defect | - | - | 3 | 7 |
| Hemimelia short limbs | - | 1 | - | - |
| Vertebrae agenesis | - | 2 | - | - |
| Ribs: Missing | - | - | - | 1 |
| Rudimentary | 2 | - | - | 1 |
| Supernumerary | 1 | 3 | 4 | 2 |
| Digit defects | 0 | 10 | 14 | 14 |
| Short or “tightly curled tail” | - | 1 | 1 | - |
| Ossification Ratings | ||||
| Distal phalanges (forepaws) (5) | ||||
| Right | 5.00±0.00 | 4.74±0.11* | 4.06±0.24* | 4.40±0.19* |
| Left | 5.00±0.00 | 4.65±0.15 | 4.20±0.22* | 4.54±0.17 |
| Proximal phalanges (forepaws) (4) | ||||
| Right | 0.15±0.05 | 0.11±0.05 | 0.17±0.06 | 0.13±0.05 |
| Left | 0.10±0.04 | 0.13±0.05 | 0.17±0.06 | 0.12±0.05 |
| Distal phalanges (hindpaws) (5) | ||||
| Right | 4.74±0.13 | 3.84±0.26* | 4.29±0.22 | 4.50±0.17 |
| Left | 4.52±0.17 | 3.63±0.27* | 4.25±0.22 | 4.22±0.21 |
| Proximal phalanges (hindpaws) (4) | ||||
| Right | 0 | 0 | 0 | 0 |
| Left | 0.02±0.02 | 0 | 0.00±0.00 | 0.01±0.01 |
| Metacarpals (4) | ||||
| Right | 3.58±0.06 | 3.38±0.06 | 3.45±0.08 | 3.34±0.05* |
| Left | 3.59±0.06 | 3.34±0.08* | 3.48±0.06 | 3.33±0.08* |
| Metatarsals (5) | ||||
| Right | 4.00±0.00 | 3.76±0.11* | 3.90±0.07 | 4.00±0.00 |
| Left | 4.00±0.00 | 3.85±0.08 | 3.99±0.02 | 3.88±0.08 |
| Ribs (13) | ||||
| Right | 12.99±0.01 | 13.01±0.01 | 13.01±0.01 | 12.99±0.01 |
| Left | 13.00±0.01 | 13.02±0.01 | 13.03±0.02 | 13.01±0.01 |
| Cervical, Thoracic, & Lumbar vertebrae (26) | ||||
| Right | 25.96±0.04 | 25.96±0.06 | 26.00±0.00 | 25.98±0.02 |
| Left | 25.96±0.03 | 25.96±0.06 | 26.00±0.00 | 26.00±0.00 |
| Sternebrae (6) | 4.93±0.08 | 4.66±0.10 | 4.99±0.09 | 4.67±0.08 |
| Saccral & Coccygeal vertebrae (9) | 7.71±0.08 | 7.60±0.16 | 8.04±0.10 | 7.71±0.07 |
Table represents total number of anomalies.
Defect data represents total number (or percent) of anomalies. Ossification data are presented as the mean ± SEM of the rating score (1.0=fully ossified; 0.5= partially ossified; 0=absent) summed for the total number of bones of each kind. The number in the parentheses after the bone name is the nominal norm for GD21 rat fetuses [27].
Significantly different from air-only control (p<0.05).
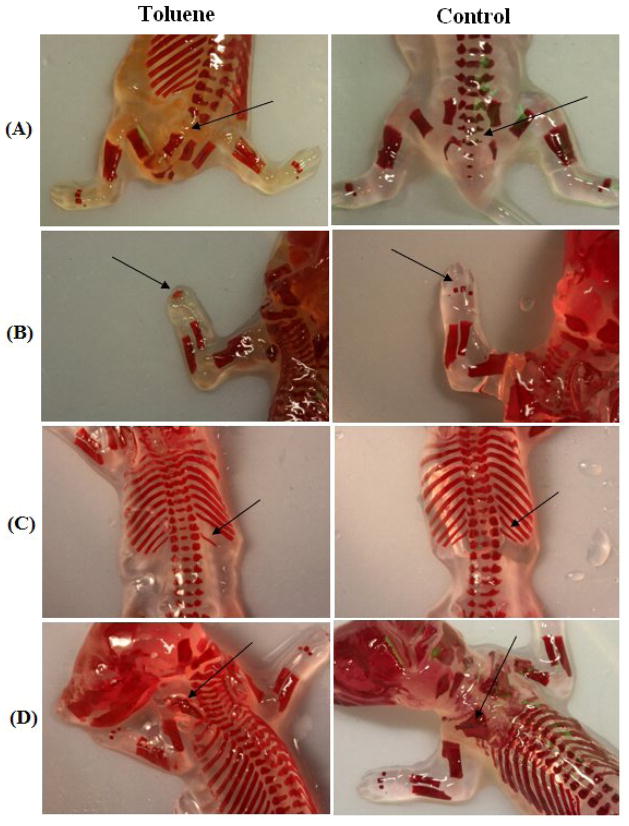
Fig. 1.
Skeletal anomalies in rat fetuses from dams treated with inhaled toluene from GD8 to GD20. (A) Absence of tail and vertebra (arrow) in pup from dam exposed to 8000 ppm. Normal (control) on right. (B) Fused metacarpals (arrow) in pup from dam exposed to 8000 ppm. Normal (control) on right. (C) Missing rib (arrow) in pup from dam exposed to 16000 ppm. Normal (control) on right. (D) Scapula defect (arrow) in pup from dam exposed to 16000 ppm. Normal (control) on right.
3.2.2 Skeletal anomalies
Numerous skeletal defects were documented during examination of the stained fetal specimens (see Tables 1 and 2; Fig. 1). These defects occurred at a significantly increased rate in toluene-exposed fetuses, χ2=20.74, df=3, p<0.01, as compared to control fetuses and litters. The highest incidence occurred after exposure to 12,000 ppm or 16,000 ppm toluene, at 32.8% and 35.8% of fetuses, respectively. The percentage of affected litters was also significantly increased in the higher toluene exposure groups, at 87.5% and 87.5%, respectively. One common defect observed was the fusion of metacarpals and metatarsals. Missing and supernumerary ribs and vertebrae were also documented. One fetus in the 8,000-ppm exposure group had no tail, or sacral or coccygeal vertebrae. Rudimentarily formed or irregularly shaped ribs, sternebrae, vertebrae, metacarpals, metatarsals, and scapula were also seen more frequently after toluene than in controls.
Degree of ossification of each bone (except in the skull) was also assessed during the skeletal examination of the fetuses. Significant reductions due to toluene were found on the degree of ossification of fetal bones in 4 of 10 skeletal areas examined, sometimes bilaterally (see Table 2c). See also Fig. 1. Right, (F(3,260) = 6.56, p<0.001), and left, (F(3,260) = 4.66, p<0.001), forepaw distal phalanges, and right, (F(3,260) = 2.51, p=0.05), metatarsal ossification were significantly decreased after toluene exposure. Ossification of the right, (F(3,260) = 2.96, p<0.05), and left, (F(3,260) = 3.58, p<0.01), metacarpals; right, (F(3,260) = 3.79, p<0.01), left hindpaw distal phalanges, (F(3,260) = 3.28, p<0.05), and sternebrae, (F(3,260) = 3.66, p<0.01), were significantly decreased after toluene exposure.
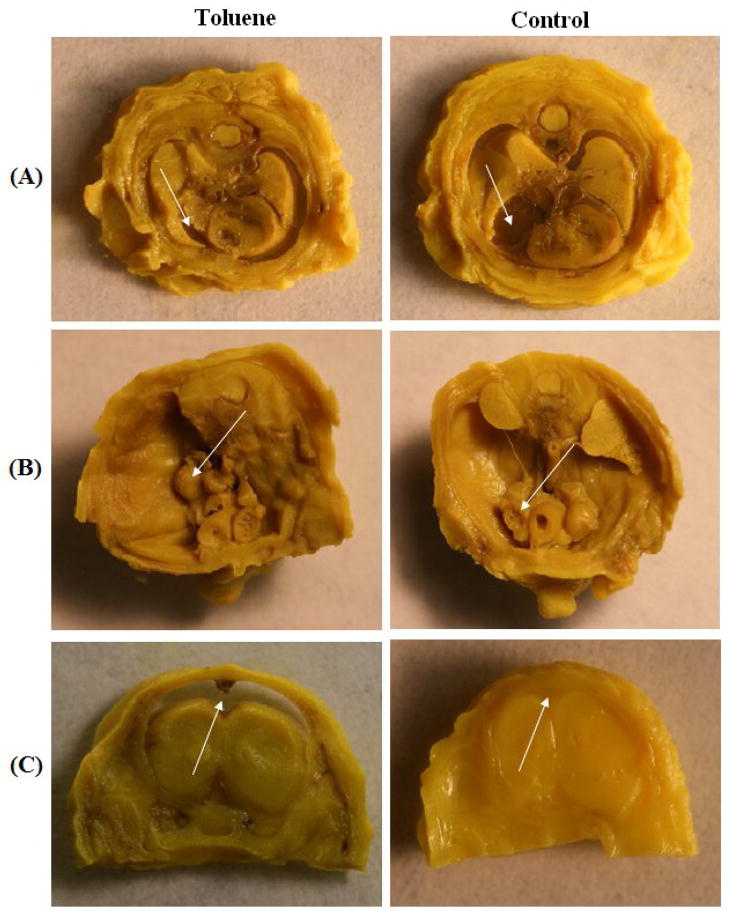
3.2.3 Soft tissue anomalies
A wide range of soft tissue anomalies were also observed throughout the fetuses (see Tables 1 and 2; Fig. 2). As with the skeletal defects, soft tissue anomalies occurred at a significantly increased rate in toluene-exposed fetuses compared to control fetuses, χ2=24.99, df=3, p<0.01. More than 75% of fetuses and 100% of the litters were affected in the 16,000-ppm group. Cardiac defects, specifically small atria, were the most commonly observed anomalies at all toluene concentrations. In addition, other common anomalies included microcardia or cardiomegaly, microgastria or gastromegaly, caudally displaced abdominal organs, displaced or ectopic testes, hypoplastic or distended bladder. Abdominal organ and renal system anomalies occurred at all doses, but as with the other observed anomalies, animals exposed to 16,000 ppm toluene were affected much more than those exposed to 8,000 ppm or 12,000 ppm.
Fig. 2.
Visceral anomalies in rat fetuses from dams treated with inhaled toluene from GD8 to GD20. (A) Small atria (arrow) in pup from dam exposed to 12000 ppm. Normal (control) atria on right. (B) Cryptorchid (arrow) in pup from dam exposed to 12000 ppm. Normal (control) on right. (C) Hydrocephalus (arrow) in pup from dam exposed to 16000 ppm. Normal (control) on right.
4. Discussion
The present study examined the preweaning developmental effects of intermittent (30 min, 2 times/day) prenatal exposure to either air (0 ppm), 8,000 ppm, 12,000 ppm, or 16,000 ppm toluene during gestational days 8–20. In previous studies in our lab using exposure parameters identical to the present study, we had noted that gross malformations such as missing or malformed digits, limb deficits and craniofacial abnormalities, appeared to be more common following brief high-dose prenatal toluene exposure than in non-exposed pups [23,22,24,25]. Our present results demonstrate that prenatal exposures to toluene in patterns and concentrations that mimic those common in inhalant abuse reduce growth and increase the incidence of dysmorphic outcomes every way they were measured, including increased rates of gross malformations and skeletal abnormalities, or soft tissues anomalies, and reduced skeletal ossification. These outcomes were found in both numbers of affected fetuses and litters, and were seen in the absence of maternal mortality, or fetal resorptions or death. Compared to the baseline control rates, the overall rate of gross malformations, skeletal abnormalities, and soft tissue anomalies were 50%–100% greater after prenatal toluene exposure. Since all fetuses exhibiting gross malformations were selected for the skeletal examination, the toluene-induced increase in the rate of soft tissue anomalies was likely to have been underestimated.
While skeletal abnormalities have been reported following long-term exposure to low levels of toluene modeling occupational settings [16], as far as we are aware, this is the first study to examine gross dysmorphology, whether skeleton or soft tissue organ systems, after repeated, high-dose binge patterns of prenatal toluene exposure. The rationale for this research is the increase in inhalant-abuse among pregnant women, which often include substances containing toluene [1,19]. Solvent abusers are known to inhale very high concentrations of toluene with some reports exceeding 10,000 ppm in brief episodes [15].
Pre- and post-natal exposures to toluene in rodents have been reported to result in neurobehavioral effects in offspring. Single daily doses of toluene by gavage (650 mg/kg) to pregnant rats from GD6 through GD19 resulted in young offspring with 15% reductions in forebrain cell numbers [30]. While these numbers returned to normal levels and developmental trajectories by weaning, reductions in neocortical myelination did not emerge until PN21. This level of exposure was thought to produce blood toluene concentrations (BTCs) equivalent to what would be seen had animals been exposed to toluene via inhalation for 3 hrs at >4100 ppm [31,32,30]. The reproductive toxicity of toluene has also been evaluated in pregnant female Sprague-Dawley rats exposed to concentrations of inhaled toluene up to 2,000 ppm for 6 hrs/day for 95 days before and during mating, throughout gestation, and until weaning at PN21 [33]. While the highest concentration tested (2,000 ppm) did not affect maternal weight gain, this concentration, but not lower concentrations (100 ppm or 500 ppm), inhibited the growth of offspring and produced a slight increase in the rate of skeletal abnormalities. While no neurobehavioral outcomes were examined, other studies have. Pregnant Wistar rats exposed to inhaled concentrations of toluene ranging from 300 ppm to 1,200 ppm for 6 hrs/day from GD9 to GD21 did not have deleterious effects on offspring behavior, even the highest concentration of toluene [34]. There were no “clear-cut” significant differences due to these levels of toluene in early maturation or locomotion, coordination, or discrimination. Wistar rats exposed to 1,800 ppm toluene for 6 hrs/day from GD7 – GD20 had no deficits in motor function or startle reflexes, but small gender-influenced differences were reported in learning [35,36]. Interestingly, perinatal exposure that included both pre- and postnatal inhalations (1,200 ppm toluene for 6 hrs/day from GD7 – PN18) produced poorer surface righting at PN3, delayed auditory startle at PN13, and altered spatial learning as adults [35]. CD-1 mice prenatally exposed during the last week of gestation (GD12-GD17) to inhaled toluene (2000 ppm for 60 min 3 times a day) gave birth to pups that weighed less than sham-exposed controls and performed more poorly on tests of behavioral development such as righting reflex, grip strength, and inverted screen [37]. Shigeta et al. [38] demonstrated that mice exposed prenatally (GD1-GD17) to inhaled toluene (1000 ppm; 6 hrs/day) gave birth to pups that were more likely to have an extra rib.
Clinically, toluene abuse by pregnant women has resulted in toluene-related embryopathy and malformations [39–44,18,17]. Maternal solvent exposures to very high levels have been reported to lead to perinatal death [39,45] with surviving neonates showing evidence of morphological and functional teratogenicity. These affected infants were typically premature and/or growth retarded and microcephalic with facial dysmorphology (e.g., deep-set eyes, small face, low set ears, micrognathia), and spatulate fingertips and hypoplastic fingernails [39–44,18,17,46]. Other features reported after prenatal toluene included micrognathia, ear anomalies, a narrow bifrontal diameter, abnormal scalp hair patterning, down-turned corners of the mouth, enlarged fontanelle, neonatal renal tubular dysfunction and metabolic acidosis. Follow-up evaluations of the toluene-exposed children up to 3 years of age revealed developmental delays, language impairment, hyperactivity, cerebellar dysfunction, and postnatal growth retardation [39,43,44,18]. This pattern of teratogenicity, which is similar to that of FAS, is prevalent in all human studies of excessive in utero exposure to toluene [47].
In conclusion, the results of the present experiment demonstrate that repeated prenatal exposure to high concentrations of toluene is associated with developmental toxicity in the progeny of exposed rats. Teratological effects of levels and patterns of toluene typical of binge inhalant abuse were clear increases in the incidence of many and varied skeletal and soft tissue abnormalities, and of reduced body weight and body size in fetuses. The popularity of the abuse of toluene and other organic solvents, combined with what little is known about their prenatal effects, confirms the necessity for greater attention – clinically and in research - on the effects of solvent abuse on pregnancy outcomes.
Acknowledgments
This work was supported in part by NIDA R01 DA015951 to S.E. Bowen.
This research was supported by National Institute of Health (NIH) grant DA15951 to SEB and WSU Office of Undergraduate Research grant to ALS.
Footnotes
Preliminary reports of a portion of this study were presented at the 70th Annual meeting of the College on Problems of Drug Dependence, San Juan, Puerto Rico, June, 2008
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(ATSDR), AfTSaDR; U.S.D.o.H.a.H. Services. Toxicological profile for toluene. U.S. Public Health Service; Atlanta: 2000. pp. 1–357. [Google Scholar]
- 2.Anderson CE, Loomis GA. Recognition and prevention of inhalant abuse. American family physician. 2003;68:869–74. [PubMed] [Google Scholar]
- 3.Hobara T, Okuda M, Gotoh M, Oki K, Segawa H, Kunitsugu I. Estimation of the lethal toluene concentration from the accidental death of painting workers. Ind Health. 2000;38:228–31. doi: 10.2486/indhealth.38.228. [DOI] [PubMed] [Google Scholar]
- 4.Bowen SE, Daniel J, Balster RL. Deaths associated with inhalant abuse in Virginia from 1987 to 1996. Drug and alcohol dependence. 1999;53:239–45. doi: 10.1016/s0376-8716(98)00139-2. [DOI] [PubMed] [Google Scholar]
- 5.Garriott J, Petty CS. Death from inhalant abuse: toxicological and pathological evaluation of 34 cases. Clin Toxicol. 1980;16:305–15. doi: 10.3109/15563658008989954. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell JC. Deaths related to the inhalation of volatile substances in Texas: 1988-1998. The American journal of drug and alcohol abuse. 2001;27:689–97. doi: 10.1081/ada-100107662. [DOI] [PubMed] [Google Scholar]
- 7.Flanagan RJ, Ives RJ. Volatile substance abuse. Bull Narc. 1994;46:49–78. [PubMed] [Google Scholar]
- 8.Brouette T, Anton R. Clinical review of inhalants. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2001;10:79–94. doi: 10.1080/105504901750160529. [DOI] [PubMed] [Google Scholar]
- 9.SAMHSA. Results from the 2005 National Survey on Drug Use and Health: National Findings (HTML) M. Department of Health and Human Services; Rockville (Ed.): 2006. [Google Scholar]
- 10.SAMHSA. The NSDUH Report March 15, 2007 Patterns and Trends in Inhalant Use by Adolescent Males and Females:2002–2005. U.S. Department of Health and Human Services; 2007. No. NSDUH07-0315. [Google Scholar]
- 11.Beauvais F, Oetting ER. Indian youth and inhalants: an update. NIDA research monograph. 1988;85:34–48. [PubMed] [Google Scholar]
- 12.Beauvais F, Wayman JC, Jumper-Thurman P, Plested B, Helm H. Inhalant abuse among American Indian, Mexican American, and non-Latino white adolescents. The American journal of drug and alcohol abuse. 2002;28:171–87. doi: 10.1081/ada-120001287. [DOI] [PubMed] [Google Scholar]
- 13.Oetting ER, Edwards RW, Beauvais F. Social and psychological factors underlying inhalant abuse. NIDA research monograph. 1988;85:172–203. [PubMed] [Google Scholar]
- 14.Elfenbein DS, Felice ME. Adolescent pregnancy. Pediatric clinics of North America. 2003;50:781–800. viii. doi: 10.1016/s0031-3955(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 15.Bukowski JA. Review of the epidemiological evidence relating toluene to reproductive outcomes. Regul Toxicol Pharmacol. 2001;33:147–56. doi: 10.1006/rtph.2000.1448. [DOI] [PubMed] [Google Scholar]
- 16.Costa LG, Guizzetti M, Burry M, Oberdoerster J. Developmental neurotoxicity: do similar phenotypes indicate a common mode of action? A comparison of fetal alcohol syndrome, toluene embryopathy and maternal phenylketonuria. Toxicology letters. 2002;127:197–205. doi: 10.1016/s0378-4274(01)00501-x. [DOI] [PubMed] [Google Scholar]
- 17.Toutant C, Lippmann S. Fetal solvents syndrome. Lancet. 1979;1:1356. doi: 10.1016/s0140-6736(79)91997-4. [DOI] [PubMed] [Google Scholar]
- 18.Pearson MA, Hoyme HE, Seaver LH, Rimsza ME. Toluene embryopathy: delineation of the phenotype and comparison with fetal alcohol syndrome. Pediatrics. 1994;93:211–5. [PubMed] [Google Scholar]
- 19.Jones HE, Balster RL. Inhalant abuse in pregnancy. Obstetrics and gynecology clinics of North America. 1998;25:153–67. doi: 10.1016/s0889-8545(05)70363-6. [DOI] [PubMed] [Google Scholar]
- 20.Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neuroscience and biobehavioral reviews. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol. 2006;28:636–47. doi: 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Bowen SE, Hannigan JH. Developmental toxicity of prenatal exposure to toluene. The AAPS journal. 2006;8:E419–24. doi: 10.1007/BF02854915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowen SE, Batis JC, Mohammadi MH, Hannigan JH. Abuse pattern of gestational toluene exposure and early postnatal development in rats. Neurotoxicol Teratol. 2005;27:105–16. doi: 10.1016/j.ntt.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Bowen SE, Hannigan JH, Irtenkauf S. Maternal and fetal blood and organ toluene levels in rats following acute and repeated binge inhalation exposure. Reproductive toxicology (Elmsford, NY. 2007;24:343–52. doi: 10.1016/j.reprotox.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowen SE, Mohammadi MH, Batis JC, Hannigan JH. Gestational toluene exposure effects on spontaneous and amphetamine-induced locomotor behavior in rats. Neurotoxicol Teratol. 2007;29:236–46. doi: 10.1016/j.ntt.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowen SE, Charlesworth JD, Tokarz ME, Wright MJ, Jr, Wiley JL. Decreased sensitivity in adolescent vs. adult rats to the locomotor activating effects of toluene. Neurotoxicol Teratol. 2007;29:599–606. doi: 10.1016/j.ntt.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor P. Practical teratology. Academic; London: 1986. [Google Scholar]
- 28.Inouye M. Differential staining of cartilage and bone in fetal mouse skeleton by alcian blue and alizarin red-S. Cong Anomal. 1976;16:171–173. [Google Scholar]
- 29.Jarosz PA, Fata E, Bowen SE, Jen KL, Coscina DV. Effects of abuse pattern of gestational toluene exposure on metabolism, feeding and body composition. Physiology & behavior. 2008;93:984–93. doi: 10.1016/j.physbeh.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Gospe SM, Jr, Zhou SS. Toluene abuse embryopathy: longitudinal neurodevelopmental effects of prenatal exposure to toluene in rats. Reproductive toxicology (Elmsford, NY. 1998;12:119–26. doi: 10.1016/s0890-6238(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 31.Gospe SM, Jr, Al-Bayati MAS. A comparison of oral and inhalation exposures to toluene. Journal of the American College of Toxicology. 1994;13:21–32. [Google Scholar]
- 32.Gospe SM, Jr, Saeed DB, Zhou SS, Zeman FJ. The effects of high-dose toluene on embryonic development in the rat. Pediatric research. 1994;36:811–5. doi: 10.1203/00006450-199412000-00023. [DOI] [PubMed] [Google Scholar]
- 33.Roberts LG, Bevans AC, Schreiner CA. Developmental and reproductive toxicity evaluation of toluene vapor in the rat. I. Reproductive toxicity. Reproductive toxicology (Elmsford, NY. 2003;17:649–58. doi: 10.1016/s0890-6238(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 34.Thiel R, Chahoud I. Postnatal development and behaviour of Wistar rats after prenatal toluene exposure. Archives of toxicology. 1997;71:258–65. doi: 10.1007/s002040050385. [DOI] [PubMed] [Google Scholar]
- 35.Hass U, Lund SP, Hougaard KS, Simonsen L. Developmental neurotoxicity after toluene inhalation exposure in rats. Neurotoxicol Teratol. 1999;21:349–57. doi: 10.1016/s0892-0362(99)00013-6. [DOI] [PubMed] [Google Scholar]
- 36.Hougaard KS, Hass U, Lund SP, Simonsen L. Effects of prenatal exposure to toluene on postnatal development and behavior in rats. Neurotoxicol Teratol. 1999;21:241–50. doi: 10.1016/s0892-0362(98)00053-1. [DOI] [PubMed] [Google Scholar]
- 37.Jones HE, Balster RL. Neurobehavioral consequences of intermittent prenatal exposure to high concentrations of toluene. Neurotoxicol Teratol. 1997;19:305–13. doi: 10.1016/s0892-0362(97)00034-2. [DOI] [PubMed] [Google Scholar]
- 38.Shigeta S, Aikawa H, Misawa T. Effects of maternal exposure to toluene during pregnancy on mouse embryos and fetuses. The Tokai journal of experimental and clinical medicine. 1982;7:265–70. [PubMed] [Google Scholar]
- 39.Arnold GL, Kirby RS, Langendoerfer S, Wilkins-Haug L. Toluene embryopathy: clinical delineation and developmental follow-up. Pediatrics. 1994;93:216–20. [PubMed] [Google Scholar]
- 40.Goodwin JM, Geil C, Grodner B, Metrick S. Inhalant abuse, pregnancy, and neglected children. The American journal of psychiatry. 1981;138:1126. doi: 10.1176/ajp.138.8.1126. [DOI] [PubMed] [Google Scholar]
- 41.Goodwin TM. Toluene abuse and renal tubular acidosis in pregnancy. Obstetrics and gynecology. 1988;71:715–8. [PubMed] [Google Scholar]
- 42.Arai H, Yamada M, Miyake S, Yamashita S, Iwamoto H, Aida N, Hara M. Two cases of toluene embryopathy with severe motor and intellectual disabilities syndrome. No to hattatsu. 1997;29:361–6. [PubMed] [Google Scholar]
- 43.Hersh JH. Toluene embryopathy: two new cases. Journal of medical genetics. 1989;26:333–7. doi: 10.1136/jmg.26.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hersh JH, Podruch PE, Rogers G, Weisskopf B. Toluene embryopathy. The Journal of pediatrics. 1985;106:922–7. doi: 10.1016/s0022-3476(85)80238-9. [DOI] [PubMed] [Google Scholar]
- 45.Wilkins-Haug L, Gabow PA. Toluene abuse during pregnancy: obstetric complications and perinatal outcomes. Obstetrics and gynecology. 1991;77:504–9. [PubMed] [Google Scholar]
- 46.Lindemann R. Congenital renal tubular dysfunction associated with maternal sniffing of organic solvents. Acta Paediatr Scand. 1991;80:882–4. doi: 10.1111/j.1651-2227.1991.tb11967.x. [DOI] [PubMed] [Google Scholar]
- 47.Wilkins-Haug L. Teratogen update: toluene. Teratology. 1997;55:145–51. doi: 10.1002/(SICI)1096-9926(199702)55:2<145::AID-TERA5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]