Abstract
The herpes simplex virus 1 (HSV-1) d106 mutant virus is a multiple immediate-early gene deletion mutant virus that has been effective as an AIDS vaccine vector in rhesus macaques (Kaur et al. 2007. Virology 357: 199–214). Further analysis of this vector is needed to advance development into clinical trials. In this study we have defined the precise nature of the multiple IE gene mutations in the d106 viral gene and have used this information to construct a new transfer plasmid for gene transfer into d106. We tested the effect of an additional mutation in the UL41 gene on d106 immunogenicity and found that it did not improve the efficacy of the d106 vector, in contrast with results from other studies with UL41 gene mutants. The safety profile of d106 was improved by generating a new vector strain, d106S, with increased sensitivity to acyclovir. Finally, we have constructed a d106S recombinant vector that expresses the HIV clade C envelope protein. The d106S-HIVenvC recombinant has retained the sensitivity to acyclovir, indicating that this phenotype is a stable property of the d106S vector.
Introduction
Viral vaccines were historically either inactivated viruses or live, attenuated viruses [1], but these types of vaccines have not been feasible for certain viruses such as the herpes viruses or HIV. Therefore, new types of vaccines including plasmid DNA vectors and replication-defective mutant viruses have been investigated. Replication-defective mutant viruses are genetically engineered or spontaneous mutant viruses that are defective for a viral function essential for replication in normal cells, but that can replicate in cells that express the missing viral gene product [2]. Replication-defective mutant viruses have been considered for vaccines for smallpox [3, 4], genital herpes [5] and AIDS [6]. Similarly, replication-defective mutant adenoviruses [7], poxviruses [8], and alphaviruses [9] have been studied as vaccine vectors. Adenoviruses have been tested extensively as AIDS vaccine vectors [10], but the failure of the recent STEP trial utilizing Ad vectors [11] raises the need for additional vector approaches.
We have constructed replication-competent and replication-defective HSV-1 recombinant viruses that express SIV gene products and have used these to immunize rhesus macaques. The initial recombinants expressed a number of HSV gene products in addition to SIV proteins and induced SIV-specific humoral and cellular immune responses that resulted in partial protection of macaques against mucosal SIVmac239 challenge infection [12]. A second-generation HSV vector, HSV-1 d106, has multiple IE gene mutations; thus, in normal cells it expresses only two viral gene products, ICP0 and ICP6 [13]. HSV-1 d106 causes minimal host protein shutoff, and minimal cyopathic effect, and shows prolonged expression of a transgene in infected cells [14]. HSV-1 d106 recombinants expressing SIV env, gag, and a rev-tat-nef fusion protein were constructed [14] and used to immunize rhesus macaques [15]. Immunized macaques showed reduced viral loads, which correlate with certain cellular and humoral immune responses [15]. Based on these results and those of further studies (Kaur et al., unpublished results), we are developing d106 recombinants as AIDS vaccine vectors for clinical trials. In this study we have defined the IE gene mutations in d106, constructed a new plasmid transfer vector, tested the effect of mutating the virion host shutoff function (vhs) on immunogenicity, and improved the safety profile of the vector strain by making it more sensitive to a herpes antiviral drug, acyclovir.
Results
Sequencing of IE gene mutations in the d106 genome
The d106 mutant virus [13] was constructed to have mutations in the ICP4 and UL54 ORF encoding ICP27 and in the intergenic regions of the promoter/enhancer between the ICP4 and ICP22 ORFs and between the ICP4–ICP47 ORFs (Figure 1C). Because d106 is being developed as a vaccine vector [14, 15] for clinical trials, it is necessary to determine the exact sequence of all deletions and mutations in the vector strain. Therefore, we sequenced the mutations and GFP cassette insertion in the d106 strain by direct sequencing of d106 genomic DNA.
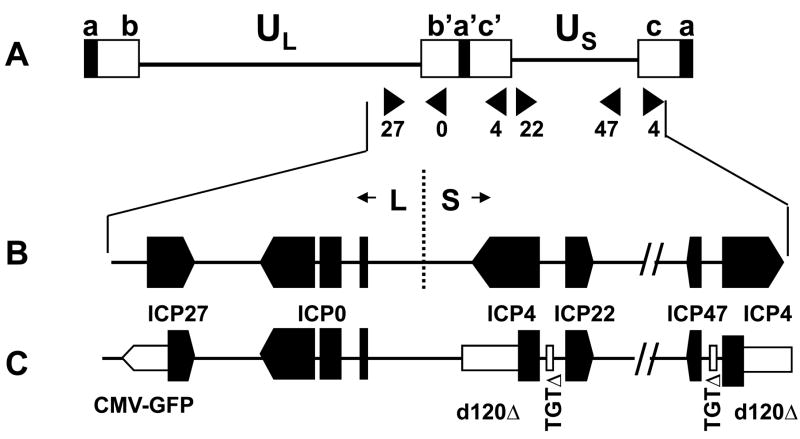
Figure 1. Organization of the HSV-1 genome.
A.Diagram of the structure of the HSV genome. The unique sequences are represented as a line, and the repeated sequences are represented as boxes. a= terminal repeats, b, b′ = L component inverted repeats, c, c′= S component inverted repeats; B. Expanded map of the right end of the genome showing the ORFs of the IE genes; C. Expanded map of the right end of the d106 genome showing the deletions (open boxes) of ICP4 and ICP22/47 gene promoters, and the CMV-GFP cassette (open arrow) insertion in the ICP27 gene.
In the original construction of the d106 mutant virus [13], the CMV-GFP cassette was inserted in the ICP27/UL54 gene between the BamHI (nts 113322–113327) and saII (nts 114517–114522) sites (Figure 1C). This deletion inactivates the essential ICP27/UL54 gene, making d106 replication dependent on ICP27/UL54 expressed from the gene in the complementing cell line. Our sequencing results confirmed that nts 113328–114516 were replaced by the CMV-GFP cassette, between the BamHI and SaII restriction endonuclease cleavage sites (Figure 2A). In addition, the CMV-GFP expression cassette was inserted in the opposite orientation to the UL54 ORF and flanked on the left by one copy of the PacI linker (TTAATTAA) and on the right by three copies of the Pac linker, and this entire cassette was bounded by BbaI sites (Figure 2A). Therefore, the deleted sequences, the orientation of CMV-GFP cassette insertion, and linker sequences are totally consistent with the original transfer plasmid and virus construction [13].
Figure 2. Locations of deletions and GFP insertion in d106 genome.
A. DNA sequence of the HSV sequences flanking CMV-GFP cassette insertion between nt 113327 and nt 114517 of BamH I and SaI I sites of ICP27 locus. B. ICP4 gene deletions from nts 126370–130593. The second deletion from nts 147641–151862 is not shown. C. Deletions within ICP22/47 promoters from nts 131538–131804 and 146430–146694.
The ICP4 open reading frame deletions in d106 virus (Figure 1C) were isolated originally in the d120 mutant virus and estimated to be 4.12– 4.15 kbp each [16], but the exact size of the deletions had not been defined. This deletion inactivates both copies of the essential ICP4 gene, making d106 virus replication dependent on ICP4 provided by the complementing cell line. Our sequencing results identified a 4222 bp deletion of nts 126371–130592 (Figure 2B) and 147641–151862. These deletions represent 3361 bp deletion from each of the two ICP4 ORFs, leaving 535 bp or 178 codons in the 5′ end of the ICP4 ORF followed by a frame-shift.
d106 was also constructed to have 270 bp deletions in the S component repeated sequences in the promoters between the ICP4 and ICP22 genes and between the ICP4 and ICP47 genes [13]; Figure 1C). These deletions remove an Oct-1 site, effectively making the ICP22 and ICP47 genes into early genes whose expression is dependent on ICP4 provided by the complementing cell line [13]. Our sequencing results showed that a 265 bp sequence between EcoR I and BssH II restriction enzyme sites from nts 131,539–131,803 and 146429–146695 was replaced by a 16-bp sequence, GCTCTAGATTAATTAA, used as a linker in the construction of the TGTΔ mutation (Figure 2C). The 265 bp of deleted sequence contains three SP1 binding sites and a TTAATGARAT Oct-1 site within the promoter of the ICP22 and ICP47 genes from nts 131536–131543, nts 131685–131693, and nts 131786–131794. Deletion of the SP1 and Oct-1 sites is believed to result in the disruption of promoter activity and the loss of the transcription of the ICP4, ICP22, and ICP47 genes.
The mutations in the d106 viral genome were confirmed by sequencing of another d106-derived recombinant, d106-27lacZ [14]. This virus was constructed by the replacement of the GFP cassette of ICP27/UL54 region of d106 with a lacZ cassette using homologous recombination by co-transfection of d106 genomic DNA and pd27-bgal plasmid DNA, which has HSV sequences from nts 112700–113326 (625 bp) and nts 114591–115957 (1366 bp) flanking the lacZ expression cassette. The IE gene mutations in d106-27lacZ viral DNA were sequenced and found to contain the same sequence alterations as d106 described above.
Derivation of a new transfer plasmid
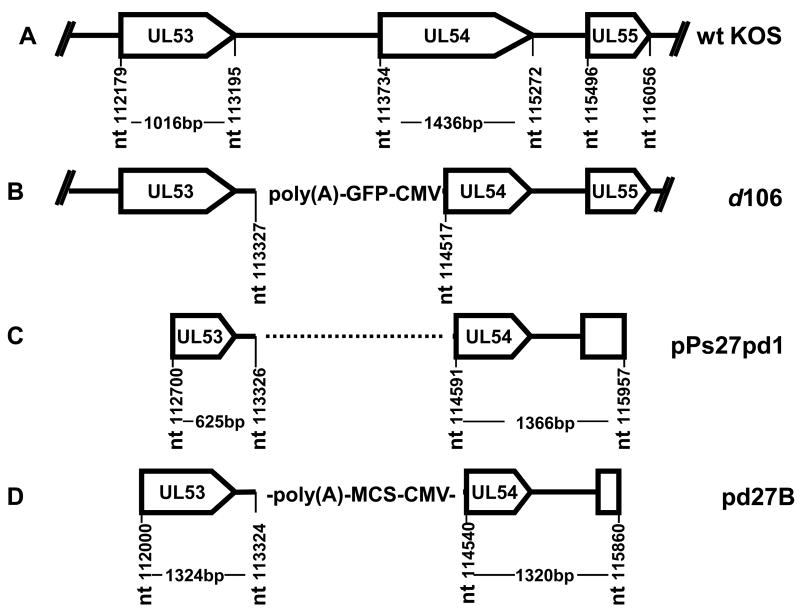
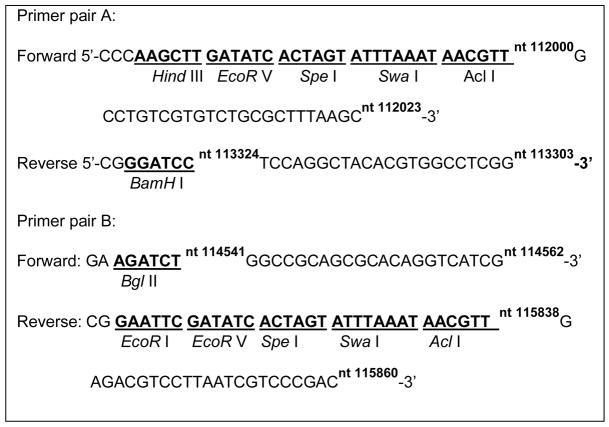
Co-transfection of infectious HSV DNA with linear viral DNA sequences into cells to allow homologous recombination is one approach used to introduce new sequences into the viral genome [17], and this approach has been used to introduce expression cassettes into the HSV genome to generate vaccine vectors [12, 14]. We had used the pPs27pd1 plasmid (Figure 3) to introduce a lacZ expression cassette into the ICP27/UL54 locus of d106 to construct d106-27lacZ, as described above. This transfer plasmid has proven useful, but the recombination efficiency was low, possibly due to its short HSV sequences flanking the expression cassette (only 625 bp on one side). Therefore, we constructed a new transfer plasmid by amplifying two 1.3 kbp flanking sequences from d106 viral DNA, one from nts 112000–113303 (1324 bp) and another from nts 114541–115860 (1320 bp). Two DNA fragments from nts 112000–113303 (F1) and nts 114541–115860 (F2) were PCR-amplified from d106 DNA using primer pairs A and B, respectively (Table 1). The PCR fragments were gel purified and ligated with TA plasmid DNA. The F1 insert was removed from TA-F1 by EcoR I and Hind III digestion and inserted into pUC19 plasmid between the EcoR I and Hind III sites to construct the pUC19-F1 plasmid. The F2 insert was removed from TA-F2 by EcoR I digestion and inserted into the EcoR I site of pUC19-F1 to construct pUC19-F2-F1. The CMV immediate-early promoter/enhancer-multi-cloning site – SV40 polyadenylation signal [CMV-MCS-poly(A)] cassette was removed from pCIΔAfI III [12] by BgI II and BamHI digestion and ligated into the p54–53 plasmid cleaved with BgI II and BamH I and filled in with Klenow to generate the pd27B plasmid. The new pd27B plasmid (Figure 3) and used as the transfer plasmid for the construction of a recombinant virus expressing HIV clade C envelope, as described below, and other microbial antigens (R. Colgrove and D.M. Knipe, in preparation).
Figure 3. Structure of transfer plasmids.
A. Structure of the HSV-1 WT KOS genome from the UL53 to UL55 genes. B. Structure of the HSV-1 d106 genome from the UL53 to UL55 genes showing the CMV GFP expression cassette inserted between nts 113327–114517. C. Structure of the insert in the pPs27pd1 transfer plasmid with KOS DNA fragments from nts 112,700–113,326 and nts 114591–115,957. D. Structure of insert in transfer plasmid pd27B containing KOS DNA fragments from nts 11200–113324 and nts 114,540–115,860 flanking the CMV-multiple cloning site-poly(A) cassette.
Table 1.
Primers used in this Study
|
Effect of vhs inactivation on immunogenicity of d106 vectors
Previous studies have reported that inactivation of the vhs function can increase immunogenicity of HSV strains in some situations [18–20], although not all situations [21]. We wanted to determine if a vhs mutation could increase immunogenicity of a recombinant d106 viral vector expressing HIV gag. We first established a quantitative ELISPOT assay for CD8+ T cells specific for HIV gag. We constructed a d106-HIVgag recombinant virus by insertion of an HIV gag expression cassette into d106 as described in Materials and Methods. This recombinant expressed gag protein for at least 36 hpi in Vero cells (Figure 4A). We immunized groups of mice (n=6) with 5×102, 5×104, or 5×106 PFU of d106-HIVgag at days 0, 21, and 42. Splenocytes were collected from each recombinant-infected mice (three mice per group per time point) at 7 days after each boost and were stimulated with an MHC-I gag peptide. The gag-specific CD8+ T cell responses were proportional to the dose of vector inoculated (Figure 4B). Furthermore, the CD8+ T cell responses were higher after the priming immunization (4 weeks) than after the boosting immunization (7 weeks) (Figure 4B). Therefore, this system provided a quantitative assay for comparing the T cell responses to different d106 constructs.
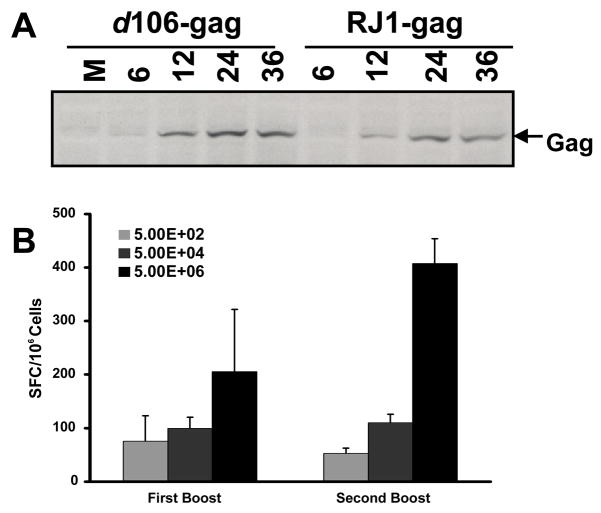
Figure 4. HIV gag expression and CD8+ T cell response induction by HSV recombinants.
A. HIV Gag protein expression in Vero cells infected with d106-gag and RJ1-gag recombinant viruses at various times post infection (hours), as detected by Western blots. M=mock-infected; B. Effect of varying the dose of immunizing virus on HIV Gag specific CD8+ responses. Groups of mice (n=6) were immunized with 5×102, 5×104, or 5×106 PFU d106-gag, at days 0, 21, and 42. Splenocytes were collected from each recombinant-infected mice (three mice per group per time point) at 7 days after each boost and were stimulated with an MHC-I Gag peptide. Results are shown as the mean number of interferon-γ spot-forming cells (SFC)/106 splenocytes±standard deviation.
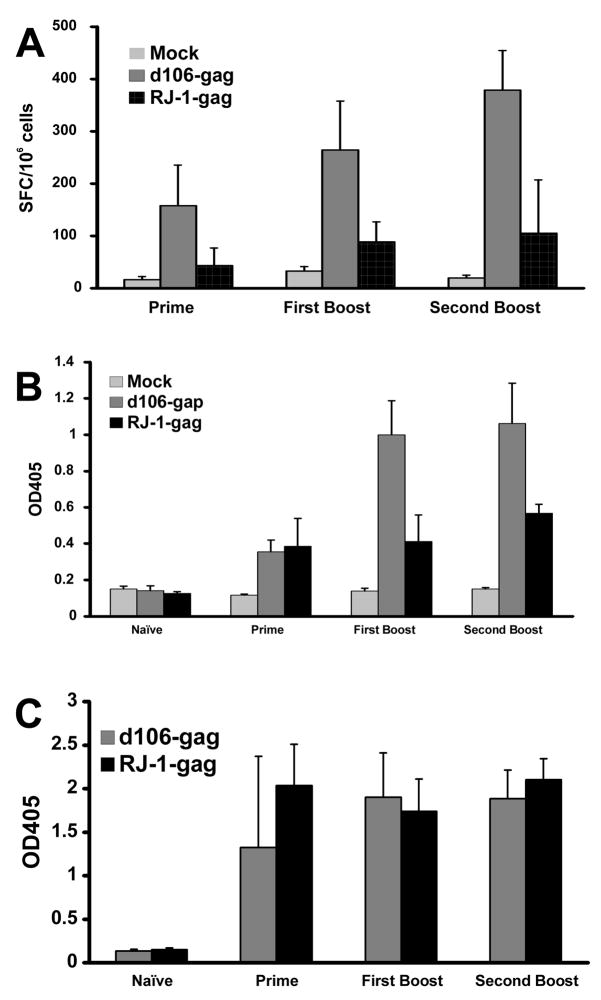
We then constructed a vector lacking vhs by inserting the HIV gag expression cassette into a vhs-deleted, d106-derived recombinant virus named RJ-1 [22], as described in Materials and Methods. The RJ-1 HIVgag recombinant expressed slightly less gag protein than the d106-gag recombinant (Figure 4A). We infected mice with the d106-HIVgag and RJ-1-HIVgag recombinants and measured CD8+ T cell responses by ELISPOT assays and antibody responses by ELISAs. In contrast with previous results with vhs mutant viruses, the RJ-1 HIV gag virus induced lower CD8+ gag-specific T cell responses (Figure 5A) and lower p24-specific antibody responses than the d106 vector (Figure 5B). HSV-specific antibody responses were similar for the two vectors (Figure 5C). These results indicated that mutation of vhs yields no improvement in immunogenicity in the context of the d106 mutant virus.
Figure 5. Effect of a vhs mutation on d106 immunogenicity.
Mice were inoculated with 2×106 PFU of d106-gag or RJ1-gag virus, followed by two booster inoculations at week 3 and week 6. Splenocytes were collected at week 4 and week 7 for ELISPOT as described in Figure 4. Serum samples were collected at week 0, week 3, and week 6 prior to booster inoculation, and week 9 after booster. A. HIV gag-specific CD8+ T cell responses as measured by ELISPOT. B. HIV Gag antibody responses, and C. anti-HSV antibody responses induced by d106-gag and d106Δvhs-gag (RJ1-gag) recombinant viruses determined by ELISA. The results are shown as mean of OD405±standard deviation.
Acyclovir resistance of and engineering of a sensitive d106S strain
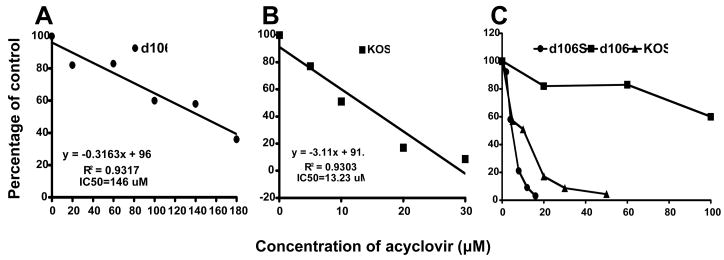
In our studies of the properties of the HSV-1 d106 virus, we observed that d106 virus was partially resistant to a herpes antiviral drug, acyclovir, in having a 50% inhibitory concentration (IC50) of 146 μM, as compared with the parental virus, HSV-1 KOS, which showed an IC50 of 13 μM (Figure 6). For an optimal safety profile, an HSV vector should be sensitive to acyclovir, so we back-crossed d106 with KOS WT virus to generate a new version of d106 that was acyclovir-sensitive. For the back-cross, we co-infected E-11 complementing cells with KOS and d106 viruses at an MOI of 3 and harvested the progeny virus. The resulting progeny virus stock was used to infect E-11 cells at high dilutions so that well-isolated viral plaques were formed. Green fluorescent plaques were picked and screened for sensitivity to 30 μM acyclovir and 60 μM acyclovir. Four of 140 plaque isolates showed sensitivity to 30 μM acyclovir and were further plaque-purified. These 4 isolates were screened for growth on E-11 cells containing the ICP4 and ICP27/UL54 genes, V827 cells containing the ICP27/UL54 and ICP8 genes, and Vero cells, the parental cell line containing no HSV genes. One plaque isolate with high sensitivity to acyclovir formed green plaques on E11 but not on Vero and V827 cells, and this virus was designated as d106S. The acyclovir sensitivity of d106S was assayed in comparison with KOS and d106 (Figure 6). The results showed that the IC50 for d106S was 6.6 μM, about 2-fold lower than KOS and 20-fold lower than d106, indicating that we had generated an acyclovir-sensitive vector strain, d106S.
Figure 6. Acyclovir sensitivity of HSV-1 KOS WT, d106, and d106S viruses.
One hundred plaque-forming units (PFUs) of HSV-1 KOS, d106 or d106S virus were plated on E-11 cells, and the cultures were incubated with the indicated concentrations of acyclovir. The mean number of plaques formed in each concentration of acyclovir was divided by the mean number of plaques in the absence of acyclovir, and the results were expressed as percent of control.
To ensure that the d106S virus contained all of the mutations engineered into d106, we sequenced the regions of mutations in d106S. Our sequencing results confirmed that d106S contained the GFP insertion in the ICP27/UL54 gene, the deletions in the ICP4 gene, and the deletions within promoter regions of the ICP22 and ICP47 genes (data not shown). Therefore, we concluded that d106S contains the five IE gene deletions in the original d106 virus but has high sensitivity to acyclovir, suggesting that d106S has WT PoI and TK genes, which define acyclovir sensitivity.
Construction of d106S recombinant viruses expressing HIV envelope protein
We wanted to use the HSV-1 d106S virus as a vector for expression of HIV gene products to serve as a candidate for a clinical trial vector. As the first application of this vector, we constructed a d106S recombinant virus that expresses the HIV clade C envelope (env) protein, d106S-HIVenvC, using homologous recombination.
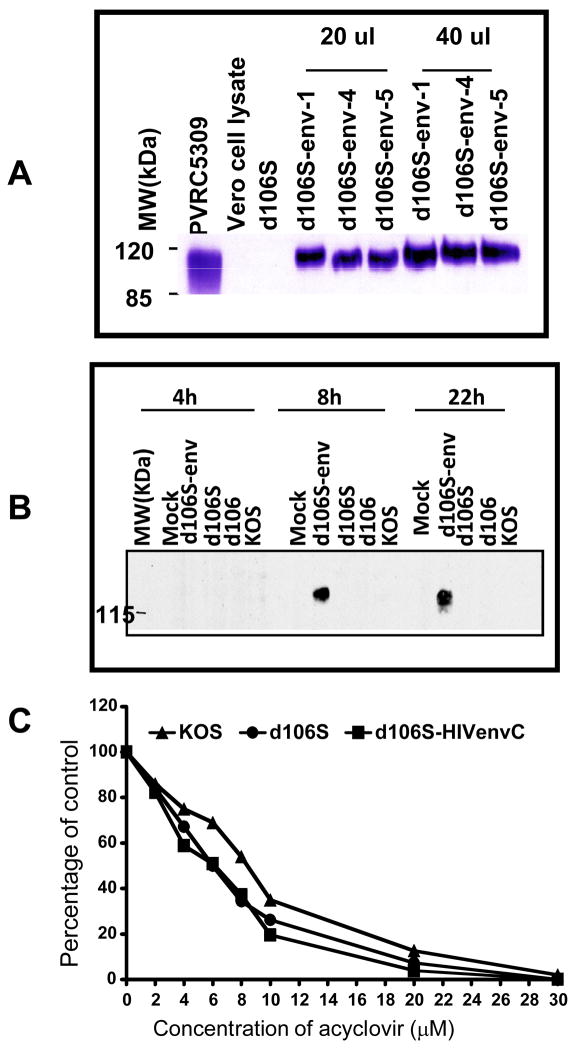
The transfer plasmid was constructed as follows. Plasmid pVRC5309, which contains an expression cassette with a codon-optimized HIV (Clade C) envelope protein (envC) ORF, was digested with BamH I and Not I, and the resulting 1880 bp fragment containing the envC ORF was inserted into the pd27B plasmid digested with XbaI and NheI and filled with Klenow. The resulting plasmid, designated pd27B-5309, was linearized by Swa I digestion and co-transfected with d106S viral DNA into E-11 cells. Viral progeny were harvested, and recombinants were identified in a screen for non-fluorescent plaques, because homologous recombination leads to the incorporation of the CMV-env cassette into the ICP27/UL54 gene locus replacing the GFP expression cassette. Three isolates were plaque-purified, and expression of env protein was assessed (Figure 7A). One of the recombinant viruses was chosen as the prototype strain and was shown to express env protein for at least 24 hours (Figure 7B). Furthermore, the recombinant virus was highly sensitive to acyclovir (Figure 7C) with an IC50 of 6.1μM. The d106S-HIVenvC recombinant viruses expressed the env protein, and retained the acyclovir sensitive property of d106S. Therefore, the acyclovir-sensitivity of the modified vector, d106S, is stable through construction of new recombinant strains.
Figure 7. Properties of the d106S-HIVenvC viruses.
A. Identification of three recombinants (1, 4, and 5) showing HIV Env p120 protein expression in Vero cells at 48 h p.i. by Western blot analysis. B. Duration of env expression in HEp-2 cells by d106S-envC. C. Acyclovir sensitivity of the d106S-HIVenvC recombinants.
Discussion
The HSV-1 d106 recombinant virus has shown good immunogenicity and protective capacity in a rhesus macaque model of SIV infection [15], and we are developing this virus as an AIDS vaccine vector for clinical trials. In this study we have determined the precise nature of the multiple IE gene mutations in the d106 viral genome and have used this information to generate a transfer plasmid containing larger flanking sequences for improved efficiency of gene transfer into recombinant virus vectors. Previous studies with HSV mutants have shown increased immunogenicity with viral strains that have the UL41 gene mutated and virion host shutoff function inactivated [18, 19], but we observed slightly lower expression of the HIV gag protein from a vector lacking UL41 and reduced cellular and humoral immunogenicity. Finally, we improved the safety profile of d106 for clinical use by generating a modified virus, d106S, that shows increased sensitivity to acyclovir.
Improved transfer plasmid
Previous studies have shown that the efficiency of gene transfer is proportional to the length of the sequences in the donor DNA [17]. We therefore used our data on the sequences surrounding the ICP27/UL54 gene deletion/insertion in d106 to make a new transfer plasmid, pd27B, with longer flanking sequences around the transgene site than the plasmid pPs27pd1, which we had previously used to insert transgenes in the ICP27/UL54 gene [14]. The plasmid pd27B contains flanking sequences of 1324bp and 1320 bp as compared with 626 bp and 1366 bp for pPs27pd1. The increased flanking sequences in pd27B have resulted in a higher frequency of recombination and greater ease of making recombinants (results not shown).
ACV sensitivity
HSV recombinants delivered for clinical purposes need to be susceptible to standard anti-HSV drugs so that the drugs, in the unlikely event that any revertant or recombinant that might arise, could control this virus. It is highly unlikely that a revertant could arise because the d106 strain has multiple deletion mutations in genes encoding functions that cannot be complemented by cellular gene products. It is also unlikely that the mutant virus could re-gain the missing viral genes from the complementing cell line due to a lack of homologous sequences for recombination. The missing functions cannot be provided by another herpesvirus, except for HSV-1 and HSV-2. However, a drug resistance mutation could be transferred from the vaccine strain to a naturally occurring HSV-1 or HSV-2 strain by homologous recombination. Therefore, it is essential that HSV strains used for clinical applications be sensitive to antiviral drugs. We observed that the d106 virus was moderately resistant to acyclovir so we backcrossed it with WT KOS virus to obtain a d106-derived strain, d106S, with acyclovir-sensitivity similar to the WT KOS parental strain. The acyclovir sensitivity phenotype was maintained in d106S after construction of an HIV gag-expressing recombinant, indicating that this is a stable property of d106S.
Lack of an effect of vhs on d106 immunogenicity
The HSV UL41 gene encodes the virion host shutoff function, a function which reduces host mRNA translation in HSV-infected cells [23]. The UL41 protein is incorporated into the tegument layer of the virion and when delivered into the cytoplasm is activated to degrade mRNAs on polyribosome. Inactivation of the UL41 gene and loss of the vhs function increases immunogenicity of certain virus strains [19, 20, 24] so it was surprising that this additional mutation did not increase immunogenicity of d106. This is likely explained by the fact that d106 already shows a defect in shutoff of host translation because of the mutation in the ICP27/UL54 gene. Shutoff of host protein synthesis by HSV requires both vhs (UL41) and ICP27 (UL54) [25]. ICP27/UL54 inhibits splicing of host mRNA [26] and cell gene transcription [27], so it is also required for host shutoff. The vhs function is required for the ability of HSV to block dendritic cell activation [28]. Because either ICP27/UL54 or UL41 gene inactivation seems to enhance immunogenicity of HSV vaccine vectors and both are required for shutoff of host protein synthesis, the increased immunogenicity is likely due to a lack of host shutoff, and not other effects of vhs on dendritic cell function or innate responses [29].
Comparisons of different HSV vectors
In addition to replication-defective mutant HSV strains, HSV amplicons have been constructed that express the HIV gag protein [30]. These amplicons contain no known HSV ORF and express no HSV proteins. Immunogenicity studies using amplicons in mice have been published [30, 31], but thus far no studies of immunogenicity in monkeys have been published. Recent studies have shown that the immediate-early ICP0 protein is required for chromatin modification and remodeling to allow efficient expression of genes on the viral genome [32, 33]. Therefore, expression of ICP0 by a HSV vaccine vector is likely to prevent host chromatin silencing of the viral genome and enhance trans-gene expression from the viral genome and the immune responses elicited. This hypothesis is consistent with decreased transgene expression by the d109 virus, which does not express any IE genes [13].
In summary, we have modified the HSV-1 d106 vector system by generating the d106S virus strain with increased acyclovir sensitivity. Furthermore, the d106S virus strain shows the properties of limited cytopathic effect and prolonged expression of transgenes. We are now constructing and characterizing a set of d106S vectors expressing HIV proteins for future clinical trials.
Materials and Methods
Plasmids
The pVRC5309 plasmid [34] containing a codon-optimized HIV clade C envelope protein (envC) ORF and pVRC4302 plasmid [35] containing the HIV gag-pol-nef expression cassette were provided by Dr. Gary Nabel, NIH. The pdl27CIA plasmid was described previously [12]. The TA (PCR 2.1) plasmid was obtained from Invitrogen (Carlsbad, CA).
Cells and viruses
Vero cells, V827 cells containing the ICP8 and ICP27 genes [36]) and E-11 cells containing the ICP4 and ICP27 genes [13] were cultured as described. The HSV-1 d106-LacZ virus [14], the HSV-1 d106 virus [13], and the RJ-1 virus (d106Δvhs) [22] were described previously. Low passage HSV-1 strain KOS virus was obtained from Dr. Priscilla Schaffer.
The HSV-1 d106-HIVgag and RJ-1-HIVgag viruses were constructed as follows. The gag-pol ORF from the plasmid VRC4302 was removed by HpaI and EcoRI digestion followed by Klenow treatment to fill in the ends. This fragment was inserted into plasmid pdl27.CIA [12] at a filled- in AfIII site. A partial digest was performed using SgrAI, allowing the removal of 2484bp of the pol gene (corresponding to base pairs 2334–4818 of HXB2). The vector was ligated using the open SgrAI sites, generating the gag expression cassette. The pdl27 gag vector was linearized with SwaII and cotransfected with d106 or RJ-1 viral DNA. Progeny viruses were harvested, and non-fluorescent plaque-forming viruses were purified three times.
Viral DNA Purification and Sequencing
Viral DNA was purified from infected cell lysates by sodium iodide density gradient centrifugation, as described previously [37] except that Proteinase K was used in place of Pronase. For sequencing, the viral DNA was partially digested with EcoRV restriction endonuclease, precipitated with ethanol, and analyzed by ABI sequencing at the Dana Farber/Harvard Cancer Center DNA Resource Core using primers based on the strain 17 reference sequence (NC-001806; [38].
Western blotting
Vero or HEp-2 cells were infected with the indicated viruses, and at the times indicated, whole cell lysates were prepared and resolved by SDS-PAGE. Western blot detection of HIV gag or clade C was performed using anti-HIV gag antibody (#4121) or anti-HIV env antibody (#1209) from the NIH AIDS Research and Reagent Program as done previously [14].
Animal procedures
Animal studies were conducted in accordance with National Institutes of Health (NIH) and Harvard University guidelines. Six-week-old female BALB/cJ mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and acclimated for one week prior to use. The mice were inoculated by subcutaneous (s.c.) infection in the left flank with the doses of viruses indicated or uninfected Vero cell lysate. The inoculations consisted of 20 μl of lysate stock diluted into sterile, endotoxin-free 0.9% sodium chloride solution (Sigma) per mouse.
Blood samples were collected by retro-orbital plexus puncture, and sera were prepared using Microtainer serum separators (Becton Dickinson) and stored at −20°C until analysis. Enzyme-linked immunosorbent assays (ELISAs) to determine antigen-specific IgG titers were conducted as described previously [39], except that 96-well microtiter plates were coated with HSV-1 viral lysate virions (Advanced Biotechnologies Inc) at 50 ng per well or HIV p24 recombinant protein (Protein Sciences Corp. Meriden, CT) at 200 ng per well.
Elispot assays were performed to measure the mouse CD8+ T cell responses against HIV Gag antigen using a BD ELISPOT Set (BD Biosciences Pharmingen) as described previously [14], except that MHC class I-restricted HIV Gag peptide (AMQMLKETI) was used in this study.
Acknowledgments
This research was supported by NIH HIV Research and Development grant AI46006. We thank Gary Nabel for providing the HIV gag-pol-nef and the clade C envelope expression plasmids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham BS, Crowe JE., Jr . Immunization against viral diseases. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Philadelphia, Lippincott: Williams and Wilkins; 2007. pp. 487–538. [Google Scholar]
- 2.Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006;344 :230–39. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–5. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci USA. 2004;101:4590–5. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen LH, Knipe DM, Finberg RW. Replication-defective mutants of herpes simplex virus (HSV) induce cellular immunity and protect against lethal HSV infection. J Virol. 1992;66:7067–72. doi: 10.1128/jvi.66.12.7067-7072.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans DT, Bricker JE, Sanford HB, Lang S, Carville A, Richardson BA, et al. Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239. J Virol. 2005;79:7707–20. doi: 10.1128/JVI.79.12.7707-7720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–29. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor J, Weinberg R, Languet B, Desmettre P, Paoletti E. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine. 1988;6:497–503. doi: 10.1016/0264-410x(88)90100-4. [DOI] [PubMed] [Google Scholar]
- 9.Berglund P, Quesada-Rolander M, Putkonen P, Biberfeld G, Thorstensson R, Liljestrom P. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res Hum Retroviruses. 1997;13:1487–95. doi: 10.1089/aid.1997.13.1487. [DOI] [PubMed] [Google Scholar]
- 10.Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16:149–56. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- 11.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy CG, Lucas WT, Means R, Czajak S, Hale CL, Lifson JD, et al. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J Virol. 2000;74:7745–53. doi: 10.1128/jvi.74.17.7745-7754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samaniego LA, Neiderhiser L, DeLuca NA. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–20. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe D, Brockman MA, Ndung’u T, Mathews L, Lucas WT, Murphy CG, et al. Properties of a herpes simplex virus multiple immediate-early gene-deleted recombinant as a vaccine vector. Virology. 2007;357:186–98. doi: 10.1016/j.virol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Kaur A, Sanford HB, Garry D, Lang S, Klumpp SA, Watanabe D, et al. Ability of herpes simplex virus vectors to boost immune responses to DNA vectors and to protect against challenge by simian immunodeficiency virus. Virology. 2007;357:199–214. doi: 10.1016/j.virol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLuca NA, Schaffer PA. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Molecular & Cellular Biology. 1985;5:1997–208. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knipe DM, Ruyechan WT, Roizman B. Molecular genetics of herpes simplex virus. III. Fine mapping of a genetic locus determining resistance to phosphonoacetate by two methods of marker transfer. J Virol. 1979;29:698–704. doi: 10.1128/jvi.29.2.698-704.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiss BJ, Smith TJ, Leib DA, Morrison LA. Disruption of virion host shutoff activity improves the immunogenicity and protective capacity of a replication-incompetent herpes simplex virus type 1 vaccine strain. J Virol. 2000;74:11137–44. doi: 10.1128/jvi.74.23.11137-11144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudek T, Mathews LC, Knipe DM. Disruption of the U(L)41 gene in the herpes simplex virus 2 dl5–29 mutant increases its immunogenicity and protective capacity in a murine model of genital herpes. Virology. 2008;372(1):165–75. doi: 10.1016/j.virol.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker J, Leib DA. Protection from primary infection and establishment of latency by vaccination with a herpes simplex virus type 1 recombinant deficient in the virion host shutoff (vhs) function. Vaccine. 1998;16:1–5. doi: 10.1016/s0264-410x(97)00164-3. [DOI] [PubMed] [Google Scholar]
- 21.Hoshino Y, Pesnicak L, Dowdell KC, Lacayo J, Dudek T, Knipe DM, et al. Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5–29 and dl5-29-41L in mice and guinea pigs. Vaccine. 2008;26:4034–40. doi: 10.1016/j.vaccine.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eidson KM, Hobbs WE, Manning BJ, Carlson P, DeLuca NA. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral Infection. J Virol. 2002;76:2180–91. doi: 10.1128/jvi.76.5.2180-2191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Read GS, Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983;46:498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiss BJ, Cano GL, Tavis JE, Morrison LA. Herpes simplex virus 2 VP22 phosphorylation induced by cellular and viral kinases does not influence intracellular localization. Virology. 2004;330:74–81. doi: 10.1016/j.virol.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Song B, Yeh KC, Liu JJ, Knipe DM. Herpes simplex virus gene products required for viral inhibition of expression of G1-phase functions. Virology. 2001;290:320–28. doi: 10.1006/viro.2001.1175. [DOI] [PubMed] [Google Scholar]
- 26.Hardy WR, Sandri-Goldin RM. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–99. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer CA, Dahmus ME, Rice SA. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J Virol. 1997;71:2031–40. doi: 10.1128/jvi.71.3.2031-2040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samady L, Costigliola E, MacCormac L, McGrath Y, Cleverley S, Lilley CE, et al. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: Potential of vhs(-) HSV vectors for dendritic cell-mediated immunotherapy. J Virol. 2003;77:3768–76. doi: 10.1128/JVI.77.6.3768-3776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasieka TJ, Lu B, Crosby SD, Wylie KM, Morrison LA, Alexander DE, et al. Herpes simplex virus virion host shutoff attenuates establishment of the antiviral state. J Virol. 2008;82(11):5527–35. doi: 10.1128/JVI.02047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hocknell PK, Wiley RD, Wang X, Evans TG, Bowers WJ, Hanke T, et al. Expression of human immunodeficiency virus type 1 gp120 from herpes simplex virus type 1-derived amplicons results in potent, specific, and durable cellular and humoral immune responses. J Virol. 2002;76:5565–80. doi: 10.1128/JVI.76.11.5565-5580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorantla S, Santos K, Meyer V, Dewhurst S, Bowers WJ, Federoff HJ, et al. Human dendritic cells transduced with herpes simplex virus amplicons encoding human immunodeficiency virus type 1 (HIV-1) gp120 elicit adaptive immune responses from human cells engrafted into NOD/SCID mice and confer partial protection against HIV-1 challenge. J Virol. 2005;79:2124–32. doi: 10.1128/JVI.79.4.2124-2132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu H, Roizman B. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc Natl Acad Sci USA. 2007;104:17134–9. doi: 10.1073/pnas.0707266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cliffe AR, Knipe DM. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J Virol. 2008;82:12030–8. doi: 10.1128/JVI.01575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong WP, Huang Y, Yang ZY, Chakrabarti BK, Moodie Z, Nabel GJ. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J Virol. 2003;77:12764–72. doi: 10.1128/JVI.77.23.12764-12772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Kong W-P, Nabel GJ. Human immunodeficiency virus type 1-specific immunity after genetic immunization is enhanced by modification of Gag and Pol expression. J Virol. 2001;75:4947–51. doi: 10.1128/JVI.75.10.4947-4951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Da Costa XJ, Kramer MF, Zhu J, Brockman MA, Knipe DM. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J Virol. 2000;74:7963–71. doi: 10.1128/jvi.74.17.7963-7971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walboomers JM, ter Schegget J. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976;74(1):256–58. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- 38.McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, McNab D, et al. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. Journal of General Virology. 1988;69:1531–74. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 39.Brockman M, Knipe DM. Herpes simplex virus vectors elicit a durable antibody response in mice despite the presence of preexisting host immunity. J Virol. 2002;76:3678–87. doi: 10.1128/JVI.76.8.3678-3687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]