Abstract
Functional neuroimaging studies have reported that the neural correlates of retrieval success (old>new effects) are larger and more widespread in older than in young adults. In the present study we investigated whether this pattern of age-related ‘over-recruitment’ continues into advanced age. Using functional magnetic resonance imaging (fMRI), retrieval-related activity from two groups (N = 18 per group) of older adults aged 84–96 yrs (‘old-old’) and 64–77 yrs (‘young-old’) was contrasted. Subjects studied a series of pictures, half of which were presented once, and half twice. At test, subjects indicated whether each presented picture was old or new. Recognition performance of the old-old subjects for twice-studied items was equivalent to that of the young-old subjects for once-studied items. Old>new effects common to the two groups were identified in several cortical regions, including medial and lateral parietal and prefrontal cortex. There were no regions where these effects were of greater magnitude in the old-old group, and thus no evidence of over-recruitment in this group relative to the young-old individuals. In one region of medial parietal cortex, effects were greater (and only significant) in the young-old group. The failure to find evidence of over-recruitment in the old-old subjects relative to the young-old group, despite their markedly poorer cognitive performance, suggests that age-related over-recruitment effects plateau in advanced age. The findings for the medial parietal cortex underscore the sensitivity of this cortical region to increasing age.
Keywords: retrieval, fMRI, compensation, over-recruitment, episodic memory, medial parietal cortex
Introduction
A wealth of cross-sectional and longitudinal studies confirm the widely held impression that cognitive performance declines with age, especially for tests of speeded cognition, word generation and long-term memory (e.g. Light, 1991; Schaie and Willis, 1993; Park et al., 1996; 2002; Lindenberger and Baltes, 1997; Nilsson et al., 2003). Among these studies, some have characterized a trajectory of cognitive decline that accelerates as older adults enter their 8th decade (e.g. Park et al, 2002; Singer et al., 2003).
The focus of the present study is on the neural correlates of long-term memory performance in individuals aged 85 years or more, as indexed by functional magnetic resonance imaging (fMRI). The overriding majority of functional neuroimaging studies that have investigated the effects of age on memory functions have employed older adults with a mean age of around 70 years. To our knowledge, no studies have described such data in samples of individuals in their ninth decade and beyond. This is despite the facts that these individuals comprise a rapidly increasing proportion of the population in developed nations (Vaupel et al., 1998), and that cognitive decline accelerates with advancing age (see above).
Functional neuroimaging studies of the effects of age on the neural correlates of episodic memory have reported that, relative to young subjects, older individuals demonstrate greater and more widespread encoding- and retrieval-related cortical activity. This phenomenon of age-related ‘over-recruitment’ has been reported in studies that differ widely in methodology and in the specific details of their results (e.g. Cabeza et al., 1997; Madden et al., 1999; Cabeza et al., 2002; Rosen et al., 2002; Maguire and Frith, 2003; Morcom et al., 2003; Grady et al., 2005; Velanova et al., 2007; Morcom et al., 2007; Duverne et al., 2008). The functional significance of these findings is unclear. Cabeza (2002) proposed that over-recruitment reflects engagement of additional neural resources that compensate for age-related decline in processing efficiency (see also Reuter-Lorenz and Cappell, 2008 and Grady, 2008). By contrast, it has also been proposed that over-recruitment is a reflection of age-related cortical ‘dedifferentiation’, and has detrimental consequences for neural efficiency and, hence, cognitive performance (Buckner and Logan, 2002; Logan et al., 2002; Morcom et al., 2003; Persson et al., 2006; Duverne et al., 2008).
The present study did not seek to arbitrate between these competing perspectives on the functional significance of over-recruitment. Rather, it addressed the question of whether adults in their mid-80s or older, when compared to adults some 20 years younger, demonstrate an analogous pattern of over-recruitment. Such a finding would suggest that the forces propelling over-recruitment continue to increase with age, perhaps tracking decline in cognitive function. Thus, using a yes/no recognition memory task, we compared the neural correlates of ‘retrieval success’ in two groups of older adults (‘old-old’ - aged 84–96, and ‘young-old’ - aged 63–77) who would be expected on the basis of prior studies (e.g. Park et al., 1996; Park and Reuter-Lorenz, 2009; Singer et al., 2003) to differ markedly in their general level of cognitive function. Event-related fMRI1 studies of recognition memory in young adults (reviewed in Rugg and Henson, 2002) have described a widespread network of cortical regions, including left lateral prefrontal cortex, and medial and lateral parietal cortex, where activity is enhanced for correctly detected ‘old’ items relative to correctly rejected ‘new’ items. Relative to young adults, the retrieval success effects of young-old subjects demonstrate over-recruitment in several of these regions. (Daselaar et al., 2003; van der Vaal et al., 2006; Morcom et al., 2007; Duverne et al., 2008)
In most prior studies of age-related differences in the neural correlates of retrieval the different age groups were not matched on retrieval performance. Without such matching the effects of performance and age are confounded, complicating the interpretation of between-group differences in neural activity (see Rugg and Morcom, 2005, for detailed discussion). Importantly, two event-related functional imaging studies that matched retrieval performance between age groups are among those to have reported age-related over-recruitment of retrieval success effects (Morcom et al., 2007; Duverne et al., 2008), although Duverne et al. (2008) reported substantially less widespread over-recruitment than did Morcom et al. (2007). Like these two prior studies, the present study utilized an event-related design and an experimental procedure that allowed memory performance to be matched between the two age groups.
Methods
Subjects
Eighteen subjects (11 female) aged between 64 and 77 years (‘young-old’, mean age: 70.2 yrs) and 18 subjects (11 female) aged between 84 and 96 years (‘old-old’, mean age: 87.4 yrs) participated in the experiment. Most subjects were recruited from the local Orange County community through newspaper advertisements and flyers. Other subjects were recruited from the control cohort of University of California, Irvine Alzheimer’s Disease Research Center, and the study cohort of an on-going longitudinal study (the ‘90+’ study; Whittle et al., 2007). Four additional subjects were excluded from the present dataset. One subject from each group was excluded because of inadequate behavioral performance, and the remaining two were excluded because of equipment malfunction.
All subjects were right-handed, English native speakers with normal or corrected-to-normal vision. The subjects had no history of significant neurological, cardiovascular, or psychiatric disease, had no contra-indications for MR imaging and were not taking CNS-active medications with the exception of the anti-hypertensive Lisinopril. No subjects were color-blind. All had MMSE scores of 26 or higher, and none scored more than two standard deviations below age-standardized means for each of the 5 sub-tests of the California Verbal Learning Test-II (CVLT). These means were available from the CVLT manual (Delis et al., 2000) in the case of the young-old subjects. Because the test manual does not provide standardized means above age 89, means for the old-old group were obtained from Whittle et al. (2007), who reported CVLT scores from a sample of 339 non-demented subjects aged 90 yrs or more. Eight young-old subjects and five of the old-old subjects were taking anti-hypertensive medication. The study was approved by the Institutional Review Board of the University of California Irvine. Informed consent was obtained prior to participation in each experimental session.
Neuropsychological Testing
A standardized neuropsychological battery was administered to all subjects in a session separate from the fMRI session. Long-term memory was assessed with the CVLT and the Immediate and Delayed NYU paragraph test (Kluger et al., 1999). Short-term memory was assessed with the Digit Span Forward and Backward Test of the WAIS-R (Wechsler, 2001). General cognitive functioning was further assessed with the Digit/Symbol Coding test of the WAIS-R, Trail Making Tests A and B and letter fluency and category fluency tests. The Wechsler Test of Adult Reading (WTAR) provided an estimate of crystallized IQ. The Beck Depression Inventory (Beck et al., 1961) was also administered. One ‘old-old’ participant declined to complete part B of the Trail Making Test and was given the maximum time possible (300s) for this measure.
Overview of Experiment
The experiment comprised a single study-test cycle, with both the study and test phases undertaken within the scanner. At study, subjects made animacy classifications on a series of pictures, half of which were presented once, and half of which were repeated after a few intervening items. During the test phase, the studied pictures were represented, intermixed with unstudied pictures, with the requirement to make a ‘yes/no’ recognition judgment on each test item. Data are reported here for the test phase only.
Materials
The experimental stimuli comprised 132 color pictures of common objects. For each subject, pictures were randomly sorted into 3 sets: 36 pictures were used in the ‘easy’ study condition, 36 pictures were used in the ‘hard’ study condition, and 60 pictures were reserved for the ‘new’ test condition. All stimulus lists contained an equal number of animate and inanimate pictures in each study and test block. Eighteen study/test list pairs were generated for each yoked ‘young-old’ and ‘old-old’ subject pair. Practice study and test lists were formed from items additional to those used to create the experimental lists described above.
Stimulus Presentation Parameters
The study phase was run as one block. A two minute rest break was interposed in the middle of the block to allow functional data to download and the scanner to be restarted. The entire study phase duration was approximately 15 min. Study items were viewed via a mirror mounted on the scanner head coil that reflected a backprojected image displayed on a screen at the head of the scanner bore. The items were presented in central vision superimposed on a grey background (subtending a visual angle of 5.72° ×5.72°) that was continuously displayed in the center of an otherwise dark monitor. A fixation cross was continuously present at the center of the background other than during item presentation. The cross changed color from black to red 500ms before the presentation of each item. The items were presented for a duration of two seconds, and were replaced by the black fixation cross. Excluding null events, stimulus onset asynchrony (SOA) was 4500ms.
Half of the study items were presented once (hard condition), while the remainder were presented twice (easy condition). The repeated items were re-presented after a minimum of four and a maximum of nine intervening trials. Additionally, 38 null events (fixation only trials) were randomly interspersed between item presentations. The study block began with two filler items. An additional two fillers followed each mid-block break.
At test, 72 previously studied and 60 new pictures (along with 46 randomly interspersed null events) were presented in a single block in an identical manner to study with the exception that the presentation duration was 1.5 s. As in the study block, a two min break was given at the halfway point. The test phase duration was approximately 17 min.
Procedure
Prior to entering the scanner, subjects practiced the study and test tasks until each task requirement was fully understood. In addition, they underwent a brief test of visual acuity and were fitted if necessary with MRI-compatible corrective lenses for use within the scanner. For the final eight old-old and two young-old subjects, the pre-scan practice session was supplemented by a prior session that took place immediately after administration of the neuropsychological test battery. No practice materials were repeated in the experiment proper. Once inside the scanner, further short practice blocks were administered just before the study and test blocks.
At study, the task requirement was to make an animacy judgment about each item. Subjects were allowed to respond any time prior to the presentation of the following item. Animacy judgments were signaled by button presses with the left and right index fingers, with the mapping of hand to response counterbalanced across subjects. Subjects were instructed to respond quickly but without sacrificing accuracy.
The test phase began approximately five minutes after the study block. Subjects were required to judge whether each test item had been presented at study (‘old’) or was new, or whether they were uncertain of its study status (‘unsure’). Old and new responses were assigned to the left and right index fingers, with the response assignment counterbalanced across subjects. The ‘unsure’ response was always assigned to the middle finger adjacent to the index finger assigned the ‘old’ response. Subjects were instructed to respond as quickly and as accurately as possible.
fMRI Data Acquisition
A Philips Intera Achieva 3T MR scanner (Philips Medical Systems, Bothell, WA), equipped with a transmit/receive radio frequency head coil was used to acquire both functional and anatomical scans. Functional data comprised T2*-weighted axial echoplanar images (EPI; flip angle 70 degrees; echo time (TE) 30ms; repetition time (TR) 2000ms) with blood oxygenation level dependent (BOLD) contrast. The ratio of TR to SOA resulted in an effective sampling rate of 2Hz. Each EPI volume consisted of 30 slices (3mm thick with 1 mm interslice gap; ascending acquisition; FOV = 240×240; matrix size = 80×79; in-plane resolution = 3 mm2) oriented parallel to the AC-PC line and positioned for full coverage of the cerebrum and most of the cerebellum. Functional data were acquired during the study phase in two scan sessions (185 volumes each). A further two sessions (220 volumes each) were employed to acquire data during the test block. The first five volumes of each session were discarded to allow tissue magnetization to achieve a steady state. Below, we describe the fMRI data from the test block only.
A T1-weighted anatomical image was collected immediately following the final functional session, using a 3-D magnetization-prepared rapid gradient echo (MP-RAGE) pulse sequence (FOV= 256 × 204; matrix size= 256 × 251; voxel size= 1×1×1.09 mm3; 150 slices; axial acquisition).
fMRI Data Analysis
Data preprocessing and statistical analyses were performed with Statistical Parametric Mapping software (SPM5; Wellcome Department of Imaging Neuroscience, London, UK: www.fil.ion.ucl.ac.uk/spm) in MATLAB R2006a (The MathWorks, Natick, MA). Functional data from the two scan sessions during test were concatenated and preprocessed as a single session. The functional volumes were realigned spatially to the first volume, and then manually reoriented to approximate the orientation of the Montreal Neurological Institute (MNI) reference brain. A sample-specific normalization template was created by first normalizing (Ashburner and Friston, 1999) the initial volume of each subject’s functional time series to the MNI EPI reference brain (Cocosco et al., 1999). These normalized volumes were separately averaged within each age group, and then averaged together to generate a template equally weighted with respect to the two groups. The template was then smoothed with an 8mm full-width half-maximum (FWHM) Gaussian kernel so as to replicate the smoothing function applied to the original MNI EPI template. Finally, each subject’s realigned volumes were normalized with respect to this sample-specific template and resampled into 3mm isotropic voxels. Normalized volumes were smoothed with an isotropic 10mm FWHM Gaussian kernel to reduce the effects of residual across-subject and across-group anatomical variation. T1 anatomical images were normalized with a procedure analogous to that applied to the EPI images. Normalized T1 images were resampled into 2mm isotropic voxels.
Analysis was performed using a General Linear Model (GLM) in which a delta function was used to model neural activity at stimulus onset. These functions were convolved with two hemodynamic response functions (HRFs) to yield regressors that modeled the BOLD response to each event-type. These functions consisted of a canonical HRF (Friston et al., 1998) and a delayed HRF, generated by shifting the canonical HRF one TR (2s) later in time. The delayed HRF was orthogonalized with respect to the canonical function so that variance common to both functions was allocated to the canonical HRF (Andrade et al., 1999). Thus, loadings on the orthogonalized delayed function accounted only for variance unexplained by the canonical function. The delayed HRF was included to capture variance associated with item-related responses that were either delayed in onset, or prolonged over time to an extent that meant they were not well characterized by the canonical HRF alone (see Henson et al., 2000; Morcom et al., 2007; and Rugg et al., 2003, for relevant examples)
The design matrix of the GLM consisted of four covariates and four delayed covariates that modeled events as defined by the combination of stimulus type and the subject’s response. The four events were: (i) correctly identified old words that had been presented once at study (‘hard’ hit); (ii) correctly identified old words that had been presented twice at study (‘easy’ hit); (iii) correctly rejected new items (CR) and (iv) events of no interest (unsure responses, misses, false alarms, absent or multiple responses, and fillers). The model also included as covariates six regressors modeling motion-related variance (three for rigid-body translation and three for rotation), and a further regressor to account for session effects. Functional data from volumes exhibiting a transient displacement of >1mm in any direction were eliminated by their inclusion as covariates in the estimation of item-related effects (mean number of volumes excluded= 1.25; range of volumes excluded= 0–8).
The time series in each voxel was high-pass filtered to 1/128 Hz and scaled within session to a grand mean of 100 across both voxels and scans. An AR(1) model was used to estimate and correct for non-sphericity of the error covariance (Friston et al., 2002). The GLM was used to obtain parameter estimates representing the activity elicited by the events of interest. An uncorrected statistical threshold of p < 0.001, combined with a cluster extent threshold of 10 contiguous voxels, was employed for the principal contrasts. Contrasts employed as exclusive masks were thresholded at p < 0.05 one-tailed (note that the more liberal the threshold of an exclusive mask, the more conservative is the masking procedure). Additionally, as described later, the principal between-group contrasts were repeated with gray matter volume employed as a global covariate. Time-courses of item-related responses from voxels of interest (see Results) were estimated with a Finite Impulse Response (FIR) model.
Analysis of Cortical Atrophy
Estimates of gray matter, white matter and total intracranial volume (ICV) were derived from individual MP-RAGE images using procedures described in detail in a previous publication (Kruggel, 2006). The segmentation procedure yielded three classes of probability volumes that roughly corresponded to background and cerebrospinal fluid (CSF; ‘class 0’ tissue), gray matter, muscles and connective tissue (‘class 1’ tissue), and white matter and fat (‘class 2’ tissue). A raw mask for the cerebrum was extracted from class 2 tissue by removing the outer hulls of the brain and cutting the brainstem at an axial plane 15 mm below the posterior commissure. A mask representing ICV was generated from each intensity-corrected MPRAGE image by a non-linear registration approach (Hentschel and Kruggel, 2004). An estimation of ICV was computed as the sum of all voxels in the mask, and the CSF volume was estimated as the sum of all probability values in class 0, conditional to the ICV mask. Cerebral gray matter volume (CGV) volume was determined as the sum of all probability values in class 1 tissue contained within the cerebral mask. Similarly, cerebral white matter volume (CWV) was determined as the sum of all probability values in class 2 tissue, conditional to the cerebral mask.
Results
Neuropsychological Performance
Mean raw scores on the neuropsychological test battery are summarized in Table 1. As is evident from the table, old-old subjects’ scores were significantly lower than those of young-old subjects on all indices of cognitive performance with the exception of the WTAR estimate of full-scale IQ. Three old-old subjects obtained scores on the BDI within the range for ‘mild’ or ‘moderate’ depression. The scores of these subjects on both the neuropsychological test battery and the experimental task fell comfortably within the range of the remaining subjects, and we therefore retained them in the analyses described below.
Table 1.
Subject characteristics by age group and performance on standardized neuropsychological tests.
| Young-Old Adults | Old-Old Adults | ||||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | P | |
| Age | 70.2 (3.9) | 64–77 | 87.4 (3.3) | 84–96 | |
| Years of education | 16.1 (2.2) | 12–21 | 15.4 (3.1) | 10–22 | n.s. |
| Mini Mental State Examination | 29.4 (0.7) | 28–30 | 28.1 (0.7) | 26–30 | <.001 |
| CVLT 1 immediate free recall | 11.8 (2.6) | 7–16 | 7.8 (1.1) | 3–14 | <.001 |
| CVLT 1 immediate cued recall | 13.2 (1.9) | 9–16 | 9.4 (2.6) | 6–15 | <.001 |
| CVLT 1 delayed free recall | 12.7 (2.5) | 9–16 | 7.4 (3.5) | 3–14 | <.001 |
| CVLT 1 delayed cued recall | 13.3 (2.2) | 9–16 | 9.1 (2.9) | 4–14 | <.001 |
| CVLT 1 delayed recognition | 14.8 (1.5) | 11–16 | 13.6 (1.9) | 10–16 | =0.05 |
| NYU2 paragraph immediate recall | 7.5 (2.0) | 4–11 | 5.0 (1.4) | 3–8 | <.001 |
| NYU2 paragraph delayed recall | 9.3 (2.1) | 6–14 | 6.7 (2.4) | 4–12 | <.005 |
| Forward/backward Digit Span | 19.3 (3.7) | 15–27 | 17.1 (2.5) | 14–23 | <.05 |
| Digit/Symbol substitution test | 50.8 (8.7) | 33–70 | 36.7 (9.0) | 20–53 | <.001 |
| Trail Making test A | 24.6 (7.1) | 18–44 | 39.8 (11.4) | 25–69 | <.001 |
| Trail Making test B | 65.9 (18.6) | 41–114 | 120.7 (62.1) | 56–300 | <.005 |
| Letter Fluency | 48.2 (9.8) | 28–68 | 39.8 (10.1) | 21–56 | <.05 |
| Category fluency | 21.6 (3.3) | 16–27 | 15.5 (3.6) | 9–24 | <.001 |
| WTAR3 Raw | 45.7 (4.3) | 33–50 | 42.2 (7.2) | 31–50 | n.s. |
| Beck Depression inventory | 1.9 (2.5) | 0–13 | 7.4 (6.7) | 0–25 | <.01 |
California Verbal Learning Test
New York University
Wechsler Test of Adult Reading Full Scale Intellectual Quotient
Behavioral Performance on Experimental Task
Study performance was indexed by classification accuracy and response time (RT). For twice-presented items, analysis was restricted to first presentations (a more detailed description of findings from the study phase will be presented elsewhere). Multiple responses and failures to respond were omitted from the analysis. There was no difference between young-old and old-old adults in classification accuracy (t(34)= 1.32, p >.1; mean (SD) of.93 (.05) and.95 (.04) respectively), but there was a significant difference in RT (t(27)= 3.94, p <.001), reflecting faster responding in the younger group (802 ms (162) versus 975 ms (93)).
Test performance is summarized in Table 2. Recognition accuracy was indexed by Pr (pHit-pFalse Alarm; Snodgrass and Corwin, 1988). An ANOVA of the accuracy data (factors of group and number of study presentations) revealed main effects of age (F(1,34) = 5.13, P <.05 and difficulty (F(1,34) = 34.44, P <.001 in the absence of a significant interaction (F(1,34) = 3.49, P > 0.05). An analogous ANOVA of the RT data revealed a main effect of number of study presentations (F(1,34)=33.67, p<.001) and age (F(1,34) = 4.75, p <.05), but again no interaction between age and number of presentations (Fs < 1).
Table 2.
Recognition memory task performance
| Young-Old Adults |
Old-Old Adults |
||||
|---|---|---|---|---|---|
| Accuracy (Proportion) | Easy items | Hard items | Easy items | Hard items | |
| Old items | hits | 93.0 (6.1) | 86.5 (12.5) | 88.9 (11.2) | 76.4 (15.4) |
| misses | 5.0 (4.6) | 10.4 (8.8) | 9.4 (10.3) | 19.2 (13.6) | |
| unsure | 1.9 (3.2) | 3.1 (4.9) | 2.9 (5.8) | 4.3 (5.8) | |
| New items | false alarms | 11.6 (6.9) | 14.6 (8.6) | ||
| correct rejections | 89.1 (7.0) | 86.1 (8.8) | |||
| unsure | 3.5 (4.1) | 4.6 (5.9) | |||
| Response Latencies (ms) | |||||
| Old items | hits | 921 (166) | 986 (186) | 1062 (215) | 1110 (168) |
| misses | 1059 (451) | 1170 (256) | 1264 (297) | 1309 (224) | |
| unsure | 1314 (330) | 1532 (200) | 1733 (328) | 1588 (384) | |
| New items | false alarms | 1162 (299) | 1150 (256) | ||
| correct rejections | 1029 (200) | 1159 (211) | |||
| unsure | 1640 (254) | 1674 (257) | |||
Notes: Mean scores are shown with standard deviations in parentheses.
The foregoing ANOVAs indicate that young-old adults responded on the test task more quickly and more accurately than the old-old adults. Since the goal of the study was to compare fMRI data from the young-old and old-old age groups under conditions of matched retrieval performance, we contrasted the young-old adults’ Pr and RT measures in the hard condition (one study presentation) with the corresponding measures from the old-old adults in the easy condition ( two study presentations). In neither case was there a significant effect (t(34) < 1 and t(34)=1.15, respectively), indicating that performance was matched across the age-groups for this combination of conditions.
fMRI Findings
In the following analyses, we define ‘retrieval success effects’ as greater neural activity for correctly identified old items (hits) than correctly identified new items (correct rejections, CRs). As mentioned previously, the primary aim of the study was to compare retrieval-related activity between the two groups under conditions where performance was matched. Therefore, the main analyses were conducted on activity elicited by easy hits in the old-old group and hard hits in the young-old group.
The results reported below were derived primarily from the parameter estimates associated with the canonical HRF. Results from the delayed HRF did not add to the findings from the canonical function, and are not reported (they are available by request from the first author).
Common Effects
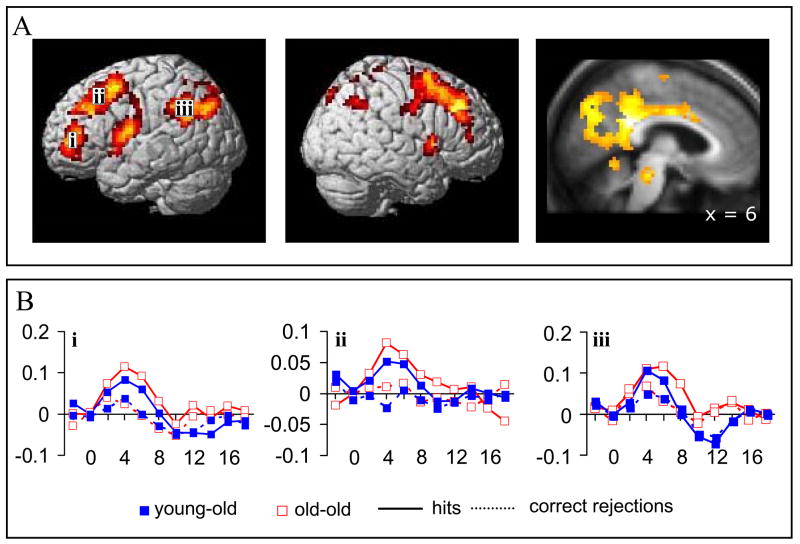
We identified regions where retrieval success effects were common to the old-old and young-old age groups as follows: first, the main effect of the old/new contrast (p<.001; cluster extent threshold of 10) was inclusively masked by the simple effect (p<.05) for each group. This ensured that the SPM for the main effect included voxels that demonstrated reliable retrieval success effects in both groups. These voxels were then exclusively masked with the two-sided contrast of the age by old/new interaction (p<.1, to give a one sided threshold of p<.05). The exclusive masking procedure eliminated voxels where the magnitude of effects differed significantly between the two groups. The outcome of this procedure revealed an extensive network of regions including prefrontal, and lateral and medial parietal cortex (see table 3, figure 1). For both the canonical and delayed HRFs, the reverse contrasts (new>old) revealed no significant effects.
Table 3.
Peak voxels of regions showing retrieval success effects common to the two groups
| Peak voxel (x,y,z) | Peak Z | No.voxels | Region | Approximate Brodmann Area(s) |
|---|---|---|---|---|
| 6 −39 27 | 4.83 | 1851 | R posterior cingulate cortex | 7/23/24/31 |
| −21 15 48 | 4.56 | 899 | L superior frontal gyrus | 6/8/9 |
| −48 −48 30 | 4.33 | 500 | L supramarginal gyrus | 40/39 |
| −33 42 12 | 4.08 | 312 | L inferior frontal gyrus | 45/46 |
| −57 −6 18 | 4.04 | 265 | L postcentral gyrus | 43/48 |
| 33 33 42 | 3.98 | 812 | R middle frontal gyrus | 9 |
| 3 −24 −15 | 3.88 | 70 | Brainstem | |
| 39 3 0 | 3.63 | 180 | R insula | 13 |
| −6 −6 0 | 3.62 | 29 | L thalamus | |
| 51 −57 39 | 3.54 | 63 | R angular gyrus | 39/40 |
| 54 −3 45 | 3.37 | 71 | R precentral gyrus | 4/6 |
| 21 18 −6 | 3.36 | 48 | R putamen | 11 |
| −27 15 9 | 3.20 | 15 | L insula | 13 |
| −21 −54 54 | 3.17 | 17 | L superior parietal cortex | 7 |
Approximate Brodmann’s areas refer to the regions subtended by the entire cluster rather than the peak of the effect.
Figure 1.
Regions demonstrating retrieval success effects common to the old-old and young-old groups. A. Left and middle panels: Lateral surface with effects projected on a three-dimensional rendering of a single brain in MNI space (SPM5 default rendered brain). Right panel: medial section with effects projected on the average of all subjects’ normalized structural images. B. Time-courses extracted from three left hemisphere regions indicated in panel A. All time-courses were extracted using Marsbar (Brett et al., 2002) from 5mm spheres centered on the voxel showing the peak of the effect. The x-axis represents time in seconds while the y-axis is in arbitrary units.
Age-related magnitude differences in retrieval success effects
We identified age-related magnitude differences in retrieval success effects between old-old and young-old age groups by inclusively masking the contrast representing the age × old/new interaction (p<.01) with the main effect of the old/new contrast (p<.001). This procedure identified voxels demonstrating reliable retrieval success effects that, in addition, differed significantly between young-old and old-old groups (note that the main effect and interaction contrasts are orthogonal, giving a conjoint threshold of p <.0001 according to Fisher’s method for combining significance levels; Lazar et al., 2002). Young-old adults demonstrated greater retrieval success effects than old-old adults in medial parietal cortex (−9 −54 30, Z = 3.09, n = 116). Peak parameter estimates for the age × old/new effects in this region are illustrated in figure 2. As is indicated in the figure, whereas the magnitude of the correct rejection responses did not differ between the groups, activity elicited by hits was significantly different. In addition, correct rejections and easy hits did not differ in the old-old group (t(17)= 1.73, p >.1), although in both cases these responses were significantly below baseline (t(17)= 2.55, p <.05; t(17)= 3.78, p <.005 for easy hits and correct rejections respectively).
Figure 2.
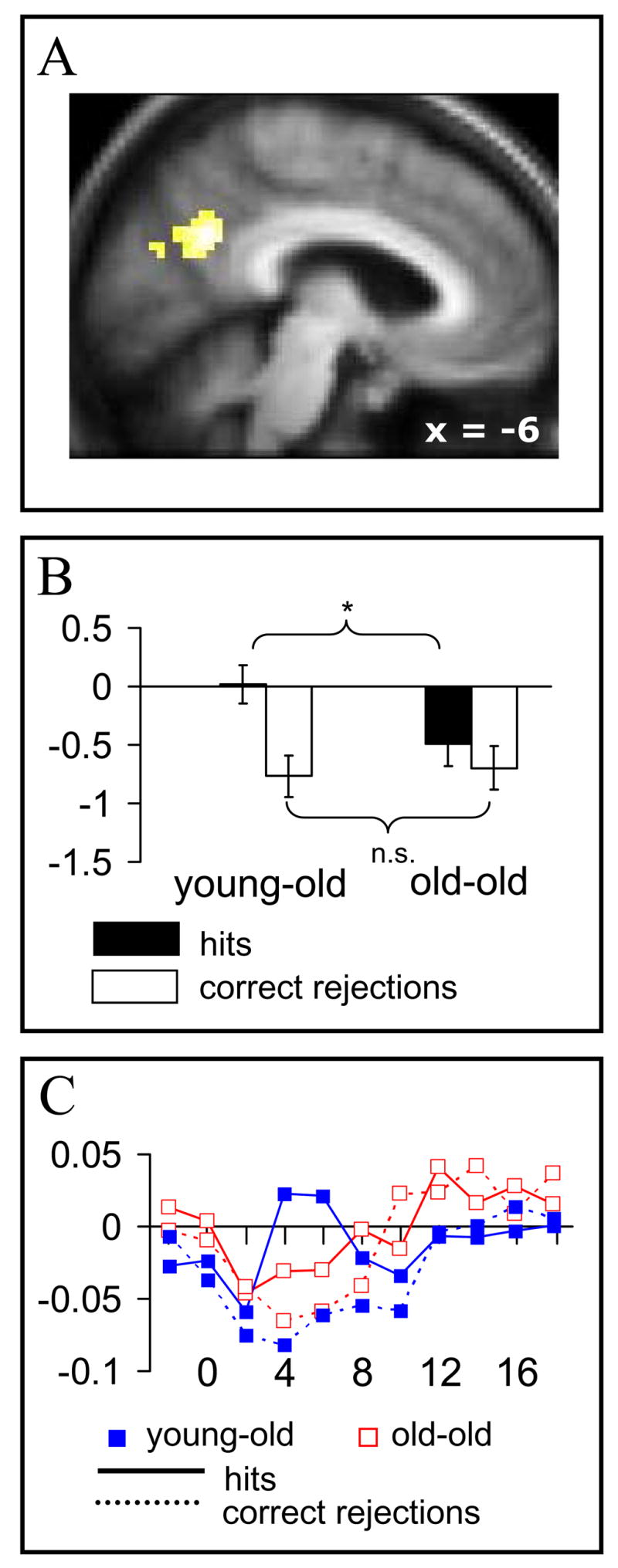
Medial parietal region where old>new effects were greater in magnitude for the young-old than old-old group. A. Outcome of the age × group interaction (p<.01 inclusively masked by main effect of old>new, p<.001) displayed on the average of all subjects’ normalized structural images. B. Parameter estimates of the peak voxel (−9 −54 30) for hits and correct rejections in each group (hits correspond to hard condition in the young-old and easy condition in the old-old.) * p<.025. C. Time-courses extracted from a 5mm sphere centered on the peak voxel (see fig. 1 legend for details).
There were no regions where retrieval success effects were greater in the old-old adults.
Effects of cortical atrophy
Mean values for ICV, CGV, and CWV volumes, along with indices of CGV and CWV corrected for ICV, are reported in table 4. As is evident from the table, CGV, as a proportion of total ICV, was significantly reduced in the old-old compared to the young-old, whereas proportional CWV did not differ between the groups. The finding for proportional CGV raises the possibility that between-group differences in cortical atrophy may have confounded the functional differences described above. Accordingly, we repeated the analyses that identified between-group differences in retrieval success effects using the group × old/new interaction estimated with a model that included CGV/ICV ratio as a covariate. Again, no clusters were identified where effects were greater for the old-old group. The reverse contrast revealed three regions where retrieval success effects were greater in the young-old group (see table 5), including a bilateral medial parietal region that overlapped the region identified in the original analysis. Thus, the finding that young-old subjects exhibit greater retrieval success effects in medial parietal cortex is unlikely to be a consequence of the confounding effects of across-group differences in gray matter volume.
Table 4.
Tissue volumetric values in ml
| Young-Old adults | Old-Old adults | P | |
|---|---|---|---|
| cerebral gray matter (cgv) | 454.3 (39.1) | 397.0 (28.8) | <.001 |
| cerebral white matter (cwv) | 499.9 (48.6) | 450.8 (47.0) | <.005 |
| intracranial volume (icv) | 1437.0 (80.0) | 1353.9 (67.2) | <.005 |
| cgv/icv | 0.31 (0.02) | 0.29 (0.02) | <.001 |
| cwv/icv | 0.34 (0.03) | 0.33 (0.03) | n.s. |
SD are in parentheses
Table 5.
Peak voxels of clusters demonstrating group-wise differences (p<.01) in the magnitude of retrieval success effects with cortical atrophy as a covariate.
| Peak voxel (x,y,z) | Peak Z | No.voxels | Region | Approximate Brodmann Area |
|---|---|---|---|---|
| young-old > old-old | ||||
| 0 −63 21 | 3.12 | 188 | Medial parietal cortex | 23 |
| −9 −78 45 | 2.91 | 20 | Medial parietal cortex | 7 |
| −21 3 57 | 2.90 | 10 | L superior frontal gyrus | 6 |
Effects of study repetition on retrieval processing
To address the question whether any between–group differences might reflect, or have been obscured by, the confounding effects of number of study repetitions, we repeated the principal analyses using data from the easy condition only. Clusters demonstrating reliable across-group retrieval-success effects were smaller than in the original analysis, but were largely unchanged with respect to location. Consistent with the findings reported above, inclusive masking of the interaction contrast (p <.01) with the main old/new effect (p<.001) revealed larger retrieval success effects in the young-old than the old-old subjects in medial parietal cortex (−12 −54 33, Z = 3.11, n = 106).
Additionally, when the principal analyses were repeated using data from the hard condition only, the between-group differences in the magnitude of medial parietal retrieval success effects were again evident, albeit at a lower statistical threshold (p<.05).
Additional Analysis
A reviewer noted that it was possible that our employment of group-wise contrasts to identify age effects on retrieval-related activity might have limited our sensitivity to detect such effects because of the relatively narrow gap between the upper bound of the young-old group and the lower-bound of the old-old subjects (7 yrs). Accordingly, we performed additional analyses in which age and retrieval success effects (collapsed over hard and easy conditions) were treated as a continuous variables and entered into in a voxel-wise multiple regression model in an effort to identify regions where success effects co-varied with age. With atrophy (cgv/icv) included as a covariate, only one small cluster - in anterior cingulate gyrus (12 −33 12, Z = 3.89, n = 24) -demonstrated retrieval success effects that correlated positively with age. No clusters demonstrated the reverse relationship.
Discussion
The primary goal of the present study was to investigate whether age-related over-recruitment of the neural correlates of retrieval success continues into advanced age. In contrast to the findings of several studies that reported age-related over-recruitment in retrieval-related activity when contrasting young and young-old subjects (Madden et al., 1999; Daselaar et al., 2003; Grady et al., 2005; van der Veen et al., 2006; Morcom et al., 2007; Velanova et al., 2007; Duverne et al., 2008), we found little or no evidence of such effects in the comparison between our young-old and old-old groups. Rather, there was extensive overlap between the regions showing retrieval success effects in the two age groups. The most robust between-group difference took the form of a smaller retrieval success effect in the old-old group in medial parietal cortex. Importantly, these relatively modest differences in retrieval-related activity between the two groups were found against a backdrop of markedly poorer performance in the old-old subjects across a wide range of neuropsychological tests, including several tests of long-term memory.
Despite the fact that the old-old subjects in the present study were selected to have relatively well-preserved cognitive function, they nonetheless demonstrated markedly lower performance than the young-old group on every neuropsychological test with the exception of the WTAR. This finding highlights the extent and pervasiveness of cognitive decline in individuals approaching or entering their tenth decade. Unsurprisingly, performance on the experimental recognition memory task also demonstrated significant age effects, with young-old adults responding more quickly and accurately than the old-old group. Despite these performance differences, we were able to perform group-wise contrasts of retrieval-related neural activity under conditions where performance was matched, analogous to the procedures employed in prior studies that contrasted the neural correlates of retrieval in young and young-old adults (Morcom et al, 2007; Li et al, 2004; Duverne et al., 2008).
Turning to the fMRI data, perhaps their most striking feature is the marked similarity of the retrieval-success effects demonstrated by the two age groups. Effects of comparable magnitude were evident in several regions of parietal and frontal cortex (see Figure 1), overlapping or closely adjacent to regions where such effects have been reported in prior studies of both young subjects and young-old individuals (for reviews of young adult studies see Rugg et al., 2002; Rugg and Henson 2002; for studies comparing young and young-old adults see Daselaar et al., 2003; Cabeza et al., 2004; Grady et al., 2005; Morcom et al., 2007; Duverne et al., 2008). Differences between the groups in the magnitude of retrieval success effects were limited to a medial parietal cluster where effects were larger (and only significant) for the young-old subjects.
The present findings indicate that, despite the substantial cognitive impairment demonstrated by the old-old subjects relative to the young-old group, the retrieval success effects in the old-old subjects were no greater in magnitude or extent. Thus, age-related over-recruitment does not necessarily track cognitive decline. To the extent that over-recruitment effects support processes that compensate for age-related decline of neural efficiency (Cabeza, 2002; Reuter-Lorenz and Cappell, 2008; Grady, 2008), the association of marked cognitive impairment and the seeming absence of continuing over-recruitment in old-old individuals suggests that compensatory processes might plateau relatively early. An implication of this account is that the acceleration in cognitive impairment that accompanies advanced aging (Park et al, 1996; Singer et al., 2003) might be a consequence of the unavailability of the additional neural resources that would be required to compensate for a continuing decline in cortical efficiency.
An alternative explanation for the absence of over-recruitment effects in the old-old group is that the yes/no recognition memory task did not place sufficient demands on retrieval processing to elicit the effects. By this argument, not only did the old-old subjects fail to show over-recruitment effects relative to the young-old subjects, but the young-old subjects would have failed to show such effects in comparison to young subjects, had this contrast been conducted. Consistent with this possibility, it is noteworthy that two of the previous event-related fMRI retrieval studies (Morcom et al., 2007; Duverne et al., 2008) in which over-recruitment effects were reported in young-old relative to young subjects employed tests of source memory rather than yes/no recognition, and thus forced subjects to attempt to recollect each test item. Two lines of evidence suggest, however, that the present null findings are not attributable to the use of a yes/no recognition rather than a source memory task. First, the present retrieval success effects were remarkably similar in their locations and spatial extents to the effects reported for young-old subjects in the two aforementioned studies, implying that the present task engaged many of the same cognitive operations. Second, age-related over-recruitment effects in the neural correlates of retrieval processing have been reported previously in studies in which memory was tested with a yes/no recognition task (Madden et al., 1999; van der Veen et al., 2006), albeit not under conditions of matched performance.
As already noted, between-group contrasts identified a single medial parietal region where retrieval success effects were larger in magnitude in the young-old group. Importantly, this is a region where robust success effects are ubiquitous in studies of young subjects (see Vilberg and Rugg, 2008 for review). Thus, the present finding of statistically non-significant medial parietal effects in our old-old subjects suggests that these individuals demonstrated an absence of success-related modulation of medial parietal activity in relation not only to young-old, but also to young subjects. Crucially, the difference in medial parietal retrieval success effects between the young-old and old-old groups was still evident when an index of cortical atrophy (gray matter volume/intracranial volume) was employed as a covariate, suggesting that this difference was not merely a concomitant of greater global gray matter loss in the old-old subjects. Moreover, the difference was also evident in separate analyses of the data from each of the two difficulty conditions, indicating that it cannot be attributed to the confounding effects of study repetition in the performance-matched analyses.
The medial parietal region demonstrating the group-wise differences discussed above has been identified as particularly sensitive to the effects of age in several previous studies contrasting young and young-old individuals. For example, age-related differences in encoding-related activity were reported in this region in two recent studies, in each case taking the form of attenuation or reversal of the ‘subsequent memory effects’ demonstrated by young subjects (Duverne et al., in press; Miller et al., 2008). Age-related attenuation of ‘task-induced deactivations’ has also been described in this region (Lustig et al., 2003; Persson et al., 2007), as have age-related differences in correlations of resting-state activity between this and other cortical regions (Andrews-Hanna et al., 2007). Together with these prior findings, the present results highlight the sensitivity of medial parietal function to advancing age.
The reason for the attenuated medial parietal retrieval success effects in the old-old group is unclear. As noted above, differences in functional activity in this region have been reported in several prior studies contrasting young and young-old subjects. One possibility is that this region is particularly sensitive to age-related pathology. As was noted by Buckner et al. (2005), there is a convergence of cortical atrophy, hypometabolism, and amyloid deposition in this region in individuals at risk for, or in the early stages of, Alzheimer’s Disease. Thus, the present findings may reflect a higher proportion of individuals with prodromal dementia in our old-old group than in the young-old group. Weighing against this possibility, however, is the finding that there was no evidence of impaired item-related BOLD responses in the medial parietal cortex of the old-old group. As is evident from figure 2, relative to the fixation baseline, both old and new test items elicited statistically significant negative-going responses (‘deactivations’) that were comparable in magnitude to those elicited by new items in the young-old group. Thus, the attenuated retrieval success effects in the old-old subjects in this region do not seem to be due to compromised neuronal responsivity to discrete stimulus events. Resolution of this issue will require studies in which functional data are acquired in concert with direct measures of the functional and anatomical integrity of the medial parietal region.
Finally, it is important to discuss three caveats that should be borne in mind when interpreting the present findings. The first of these, which has already been alluded to, relates to the possibility of higher probability of prodromal dementia in the old-old than the young-old group. Although we took care to exclude subjects who may have been in the early stages of dementia (for example, requiring a score of 26 or more on the MMSE), this possibility cannot be ruled out. This raises the (remote, in our view) possibility that the failure to observe over-recruitment effects in our old-old subjects reflected the impact of undetected pathology, implying that such effects might be evident in subjects free from such pathology.
A second caveat arises out of a weakness common to all cross-sectional studies of aging, namely, the potentially confounding influence of cohort effects. This is a particularly acute concern in the present case because, for two reasons, it is inevitable that our sample of old-old subjects was drawn from a more restricted population than was the case for our young-old subjects. First, approximately 50% of old-young subjects (individuals < 76 yrs; ) will not survive into their ninth and tenth decades, leading to a marked survivorship effect (Arias, 2007). Second, the prevalence of cognitive impairment due to neurodegenerative disease, including Alzheimer’s, increases markedly between the sixth and ninth decades (Yesavage et al., 2002), making it highly likely that the young-old group contained subjects who, were they to survive into their late 80s/90s, would fail to meet the inclusion criteria for our old-old group. Thus, we cannot rule out the possibility that the present findings, at least to some extent, reflect the consequences of contrasting two groups containing different proportions of individuals with traits conducive to longevity and resistance to age-related cognitive impairment. This possibility can only be addressed by studies that employ longitudinal rather than cross-sectional experimental designs.
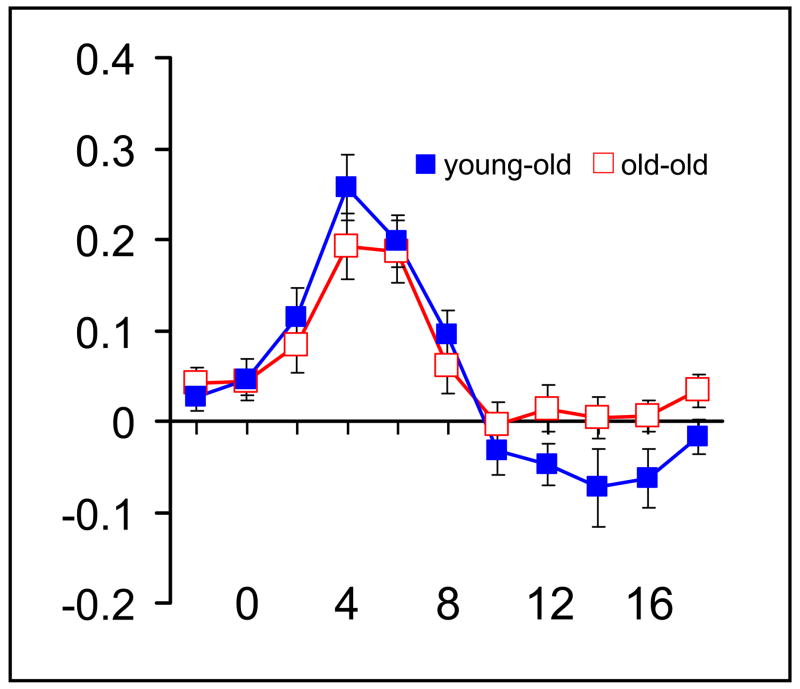
The final caveat stems from the need to assume comparability of the hemodynamic transfer functions in the two age groups. To the extent this assumption is violated, our conclusion that equivalent retrieval-related BOLD effects in the two groups imply equivalence of the associated neural activity is called into question. Whereas comparisons of the hemodynamic responses elicited in young and young-old subjects in sensory and motor cortices have generally been reassuring in this respect (D’Esposito et al., 1999; 2003 and Buckner et al., 2000; Heuttel et al., 2001 but see Ross et al., 1997 for an exception), there is a dearth of evidence concerning the stability of the BOLD response in advanced age. A single study that contrasted responses from small groups of subjects in their seventh, eighth and ninth decades found little evidence of an age effect, however (Brodtmann et al., 2003). Consistent with this finding, in the present study the time-courses associated with the experimental effects illustrated in figures 1 and 2 show little or no evidence of systematic differences in onset, shape or magnitude as a function of group. In addition, a time-course analyses of item-related BOLD responses elicited in of the region of visual cortex where these responses were maximal in the young-old subjects failed to reveal any effects of age on the latency or amplitude of the responses (see Figure 3). Thus, there is currently little reason to conclude that the results discussed above are confounded by age-related differences in the hemodynamic transfer function.
Figure 3.
Time-courses of item-related BOLD responses extracted from a 5mm sphere centered on the peak voxel (−6 −84 −3) in the visual cortex where responses were maximal in the young-old subjects (see Fig. 1 legend for details). ANOVA revealed no evidence of an age group × time point interaction (p>.1).
To conclude, the present findings failed to find evidence for over-recruitment of retrieval success effects in cognitively intact old-old adults relative to a young-old group. With the exception of the effects in a single medial parietal region, retrieval success effects in the old-old group were remarkably similar to those of the young-old subjects, despite their substantially lower level of cognitive function and gray matter volume. These findings suggest that the functional integrity of the cortical network associated with successful retrieval remains largely stable with advancing age. Whether the age-related differences found in medial parietal retrieval success effects reflect the beginnings of age-related degradation of this network, as opposed to incipient pathology, remains to be determined.
Acknowledgments
TW was supported by National Institute on Aging Training Grant 2T32AG000096-26. The research was supported by National Institute on Aging Center Grant 5P50AG016573-09. We thank Shahab Motamedinia, Brian Minton, Matt Grilli, and Brooke Rosen for assistance with subject recruitment and neuropsychological data collection, Dr Claudia Kawas for assistance with subject recruitment, and the staff of the UCI Research Imaging Center (RIC) for assistance with MRI data collection.
Footnotes
Most prior studies that investigated age-related differences in the neural correlates of retrieval employed blocked experimental designs (e.g. Cabeza et al., 1997; Madden et al., 1999; Grady et al., 2005). Unlike event-related designs, these do not allow a distinction to be drawn between activity associated with a retrieval attempt, and activity associated with successful retrieval (see Rugg and Morcom, 2005 for a detailed discussion).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade A, Paradis AL, Rouquette S, Poline JB. Ambiguous results in functional neuroimaging data analysis due to covariate correlation. Neuroimage. 1999;10:483–486. doi: 10.1006/nimg.1999.0479. [DOI] [PubMed] [Google Scholar]
- Arias E. National Vital Statistics Reports. Hyattsville, MD: National Center for Health Statistics; 2007. United States life tables, 2004; p. 56. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain. Sendai; Japan. 2002. Available on CD-ROM in NeuroImage, 16. [Google Scholar]
- Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, undemented, and demented older adults. Journal of Cognitive Neuroscience. 2000;12(Suppl):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Logan JM. Frontal contributions to episodic memory encoding in the young and elderly. In: Parker AE, Wilding EL, Bussey T, editors. The Cognitive Neuroscience of Memory Encoding and Retrieval. Philadelphia: Psychology Press; 2002. pp. 59–81. [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. Journal of Cognitive Neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in old adults: the HAROLD model. Psychology of Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RKS, Evans AC. Brainweb: online interface to a 3D MRI simulated brain database. Neuroimage. 1997;5:425. [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aquirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challeng for neuroimaging. Nature reviews Neuroscience. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiology of Aging. 2008;29:1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance and the neural correlates of successful memory encoding. Cerebral Cortex. doi: 10.1093/cercor/bhn122. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: applications. Neuroimage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Grady C, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annuals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Hentschel S, Kruggel F. Determination of the intracranial volume: a registration approach. In: Yang GZ, Jiang T, editors. Medical Imaging and Augmented Reality. Berlin/Heidelberg: Springer-Verlag; 2004. pp. 253–260. [Google Scholar]
- Heuttel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- Kluger A, Ferris SH, Golomb J, Mittleman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. Journal of Geriatric Psychiatry and Neurology. 1999;12:168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- Kruggel F. MRI-based volumetry of head compartments: normative values of healthy adults. Neuroimage. 2006;30:1–11. doi: 10.1016/j.neuroimage.2005.09.063. [DOI] [PubMed] [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: A survey of methods for statistical pooling of information. Neuroimage. 2002;16:538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- Li J, Morcom AM, Rugg MD. The effects of age on the neural correlates of successful episodic retrieval: an ERP study. Cognitive, Affective and Behavioral Neuroscience. 2004;4:279–293. doi: 10.3758/cabn.4.3.279. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging: four hypotheses in search of data. Annual Review of Psychology. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychology and Aging. 1997;12:410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale J, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in the functional neuroanatomy of verbal recognitinon memory. Human Brain Mapping. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, Depeau K, Diamond E, Dickerson BC, Rentz DM, Pihlajamäki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences USA. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cerebral Cortex. 2007;11:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Nilsson LG. Memory function in normal aging. Acta Neurologia Scandinavia Suppl. 2003;179:7–13. doi: 10.1034/j.1600-0404.107.s179.5.x. [DOI] [PubMed] [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, Earles JL, Frieske D, Zwahr M, Gaines CL. Mediators of long-term memory performance across the life span. Psychology and Aging. 1996;11:621–637. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz PR. The adaptive brain: aging and neurocognitive scaffolding. Annual Reviews of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cerebral Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: A link to cognitive control? Journal of Cognitive Neuroscience. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell K. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;3:177–182. [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O’Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JDE. Variable effects of again on frontal lobe contributions to memory. Neuroreport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Ross MH, Yurgelun-Todd DA, Renshaw PF, Maas JH, Mendelson JH, Mello NK, Cohen BM, Levin JM. Age-related Reduction in Functional MRI Response to Photic Stimulation. Neurology. 1997;48:173– 176. doi: 10.1212/wnl.48.1.173. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA. Episodic memory retrieval: an (event-related) functional neuroimaging perspective. In: Parker AE, Wilding EL, Bussey T, editors. The cognitive neuroscience of memory encoding and retrieval. Psychology Press; 2002. [Google Scholar]
- Rugg MD. Functional neuroimaging of memory. In: Baddeley A, Wilson B, Kopelman M, editors. Handbook of Memory Disorders. 2. Wiley; 2002. pp. 57–80. [Google Scholar]
- Rugg MD, Morcom AM. The relationship between brain activity, cognitive performance, and aging: The case of memory. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging. New York, NY: Oxford University Press; 2005. pp. 132–154. [Google Scholar]
- Schaie KW, Willis SL. Age difference patterns of psychometric intelligence in adulthood: generalizability within and across ability domains. Psychology and Aging. 1993;8:44–55. doi: 10.1037//0882-7974.8.1.44. [DOI] [PubMed] [Google Scholar]
- Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: six-year longitudinal findings in the Berlin Aging Study (BASE) Psychology and Aging. 2003;18:318–331. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- van der Veen FM, Nijhuis FA, Tisserand DJ, Backes WH, Jolles J. Effects of aging on recognition of intentionally and incidentally stored words: an fMRI study. Neuropsychologia. 2006;44:2477–2486. doi: 10.1016/j.neuropsychologia.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NC, Iachine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cerebral Cortex. 2007;17:1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle C, Corrada MM, Dick M, Ziegler R, Kahle-Wrobleski K, Paganini-Hill A, Kawas C. Neuropsychological data in nondemented oldest old: The 90+ study. Journal of Clinical and Experimental Neuropsychology. 2007;29:290–299. doi: 10.1080/13803390600678038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Yesavage JA, O’Hara R, Kraemer H, Noda A, Taylor JL, Ferris S, Gély-Nargeot M, Rosen A, Friedman L, Sheikh J, Derousené C. Modeling the prevelance and incidence of Alzheimer’s disease and mild cognitive impairment. Journal of Psychiatric Research. 2002;36:281–286. doi: 10.1016/s0022-3956(02)00020-1. [DOI] [PubMed] [Google Scholar]