Abstract
Objective
To identify potentially modifiable risk factors of placental injury reflecting maternal uteroplacental vascular compromise (UPVC) and acute and chronic placental inflammation.
Study design
A prospective epidemiologic study was conducted. A total of 1270 placentas were characterized by gross and microscopic examination. Placental pathology was coded for features of amniotic fluid infection syndrome (AFIS), chronic villitis, UPVC, and fetal vascular obstructive lesions. Odds ratios between UPVC, the acute and the chronic inflammatory lesions, and risk factors of interest were calculated.
Results
After adjusting for confounders, women with a history of preterm birth had 1.60 times the odds of chronic inflammation (95% CI: 1.10, 2.55). Women with a previous elective termination had 3.28 times the odds of acute inflammation (95% CI: 1.89, 5.70). The odds of chronic villitis increased with parity, while the odds of AFIS decreased with parity.
Conclusion
We have identified several predictors of UPVC, AFIS and chronic villitis. Further studies are needed to examine whether interventions to alter UPVC, AFIS and chronic villitis will lead to improved pregnancy outcomes.
Keywords: inflammation, pathology, placenta, preterm
Introduction
Preterm delivery is a major cause of perinatal morbidity and mortality, resulting in a significant amount of medical resources being allocated for perinatal health care.1-3 Efforts to predict, prevent or delay the occurrence of preterm birth have had marginal success.4-7 Because of this, there is great interest in ascertaining the etiology of preterm delivery so that effective preventative measures can be developed. One area of study that has garnered such attention is the contribution of maternal uteroplacental vascular compromise (UPVC) and inflammation (both as amniotic fluid infection syndrome (AFIS) and as chronic villitis) as a potential pathway to preterm birth. Certain placental histopathologic findings have been associated with preterm birth and have been helpful in predicting outcomes for preterm infants.8-11 Identifying predictors of placental pathology that are potentially modifiable could result in reducing the risk of preterm delivery.
Potential risk factors for placental pathology and preterm delivery include tobacco use12, pregnancy complications, reproductive and medical history, and socioeconomic and psychosocial attributes.13 How these influences affect the placenta and contribute to early delivery is poorly understood. Large epidemiologic studies addressing this question have been lacking. Our goal was to prospectively study a large representative population of women to evaluate what impact these factors of interest have on the placenta. We will examine the role of known and suspected risk factors for preterm birth in relation to UPVC and inflammation representing both AFIS and chronic villitis.
Materials and Methods
Placentas were obtained from women participating in the Pregnancy, Infection and Nutrition (PIN) study at the University of North Carolina-Chapel Hill. A total of 2006 women were enrolled at a prenatal clinic visit before 20 weeks gestation at the University of North Carolina medical clinics from January of 2001 to November of 2005. Of the 2006 women enrolled, 1847 (92.1%) were eligible to donate placentas, of whom 1542 (76.9%) were recruited. We obtained placentas from 1270 (63.3%) of these women. Demographic and medical information was obtained through a series of telephone and face-to-face interviews, questionnaires, and medical chart review. The basic study protocol for PIN has been described in detail elsewhere.14 This study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Placental pathology was characterized by gross and microscopic examination of the placenta and decidua, assigning histopathology to one of three categories as outlined by Salafia: inflammation (AFIS and chronic villitis) and uteroplacental vascular lesions.15-18 Details regarding categorization of uteroplacental vascular pathology have been previously described.19 For the purposes of this study, AFIS was considered present when there was any fetal inflammatory response (i.e., umbilical vasculitis, funisitis, or fetal chorionic vasculitis); these lesions have been shown to be present in <1% of uncomplicated term births.20 Chronic inflammation was diagnosed by the presence of chronic villitis, inflammation of placental villi remote from the basal plate. UPVC was diagnosed in cases via two routes: 1. All placentas with at least one non-marginal placental infarct (>2 cm from the nearest margin) >1 cm3 in volume. 2. Cases with summary scores of histology items of syncytial knotting, syncytial basophilia, villous fibrosis and excess perivillous fibrin deposition with cytotrophoblast proliferation (each scored on a 0-4 scale as previously described 15-18 ) greater than the birth cohort median value of 7. Fetal vascular obstructive lesions included chorionic and fetal stem vessel mural thrombi, the “hemorrhagic endovasculitis” group of lesions and avascular villi present in clumps of >50 villi. All placentas were examined by a single expert pathologist (CS), who was blinded to the patient characteristics when reviewing the placental pathology. For purposes of analysis, UPVC and AFIS and chronic villitis was coded as present vs. not present for each of the four categories: AFIS, chronic villitis, UPVC, and fetal vascular obstructive pathology.
Potential risk factors for placental pathology considered in the analyses included maternal age (≥ 35 years vs. < 35 years), race (black vs. non-black), poverty to income ratio (PIR), maternal education, marital status, smoking during pregnancy, pre-pregnancy body mass index (BMI) (< 19.8, 19.8-26.0, 26.1-29.0, > 29.0), pregnancy induced hypertension (PIH), chronic hypertension, gestational diabetes (GDM), pre-pregnancy diabetes, preeclampsia, previous elective termination, previous miscarriage, previous preterm birth, and parity. GDM was defined as 2 or more abnormal values on a 3-hour glucose tolerance test (fasting ≥ 95, 1 hr ≥ 180, 2 hr ≥ 155, 3 hr ≥ 140). A previous preterm birth was defined as any birth reported by the patient occurring greater than 2 weeks before her scheduled due date. PIH was defined as an elevation in systolic blood pressure ≥ 30 mm Hg, an elevation in diastolic blood pressure ≥ 15 mm Hg, or a blood pressure ≥ 140/90 noted 2 times at least 6 hours apart. Preeclampsia was defined as PIH with either 300 mg of protein noted in a 24 hour urine collection or 1+ protein noted on a urine dipstick 2 times at least 6 hours apart. Simple associations between UPVC, AFIS and chronic villitis and covariates were analyzed and are presented in Table 1.
TABLE 1.
Prevalence of primary pathology type, according to demographic and medical factors.
| Variable | Sample size(n) |
Percent of sample (%) |
AFIS(%) | Chronic villitis(%) |
Maternal vascular pathologic condition (%) |
Fetal vascular pathologic condition (%) |
|---|---|---|---|---|---|---|
| Total | 1270 | - | 8.5 | 18.6 | 19.4 | 14.5 |
| Age (y) | ||||||
| <35 | 1070 | 84.2 | 9.2 | 18.6 | 18.6 | 14.0 |
| ≥35 | 200 | 15.8 | 4.5 | 18.5 | 23.5 | 17.0 |
| Race | ||||||
| White (including other) | 1033 | 81.3 | 7.7 | 19.1 | 18.6 | 14.9 |
| African American | 237 | 18.7 | 11.7 | 16.5 | 22.8 | 12.7 |
| Maternal education | ||||||
| ≤High school | 258 | 20.5 | 11.2 | 20.0 | 19.0 | 14.7 |
| Some college | 242 | 19.0 | 8.3 | 17.4 | 24.0 | 12.8 |
| ≥College | 770 | 60.5 | 7.7 | 15.3 | 18.0 | 15.7 |
| PIR (%) | ||||||
| <100 | 166 | 13.8 | 12.6 | 12.6 | 21.7 | 15.7 |
| 100-199 | 142 | 11.7 | 9.9 | 16.9 | 16.9 | 12.6 |
| ≥200 | 907 | 74.5 | 7.3 | 20.3 | 19.1 | 11.3 |
| Mother’s marital status | ||||||
| Married | 958 | 75.2 | 7.0 | 19.7 | 18.4 | 14.9 |
| Single, widowed, divorced, separated | 312 | 24.8 | 13.1 | 15.1 | 22.4 | 13.1 |
| Previous pregnancy | ||||||
| None | 399 | 31.5 | 10.8 | 17.5 | 21.8 | 15.0 |
| ≥1 | 864 | 68.5 | 7.5 | 19.1 | 18.1 | 14.4 |
| Previous elective termination | ||||||
| No | 1037 | 82.1 | 7.2 | 19.4 | 19.3 | 14.3 |
| Yes | 226 | 17.9 | 14.6 | 15.0 | 19.0 | 15.9 |
| Previous preterm birth | ||||||
| No | 1127 | 89.2 | 9.0 | 17.8 | 19.0 | 15.2 |
| Yes | 136 | 10.8 | 4.4 | 25.0 | 21.3 | 9.6 |
| Previous miscarriage | ||||||
| No | 924 | 73.0 | 9.2 | 18.5 | 18.9 | 14.3 |
| Yes | 339 | 27.0 | 6.8 | 18.9 | 20.1 | 15.3 |
| Body mass index (kg/m2)a | ||||||
| <19.8 | 180 | 14.3 | 8.3 | 16.1 | 21.7 | 18.4 |
| 19.8-26.0 | 640 | 51.1 | 8.9 | 18.3 | 17.8 | 12.5 |
| 26.1-29.0 | 142 | 11.3 | 4.2 | 20.4 | 22.5 | 16.9 |
| >29.0 | 289 | 23.3 | 9.7 | 20.4 | 20.0 | 15.5 |
| Chronic hypertension | ||||||
| No | 1166 | 92.0 | 9.0 | 18.7 | 18.9 | 13.6 |
| Yes | 99 | 8.0 | 3.0 | 18.2 | 26.3 | 24.2 |
| PIH | ||||||
| No | 950 | 75.0 | 9.0 | 18.2 | 17.8 | 12.6 |
| Yes | 315 | 25.0 | 7.0 | 20.0 | 24.4 | 20.0 |
| Preeclampsia | ||||||
| No | 1208 | 95.5 | 8.9 | 18.6 | 19.0 | 14.4 |
| Yes | 57 | 4.5 | 1.8 | 19.3 | 29.8 | 15.8 |
| GDM | ||||||
| No | 1223 | 96.3 | 8.7 | 18.4 | 19.2 | 14.5 |
| Yes | 47 | 3.7 | 4.3 | 25.5 | 25.5 | 12.8 |
| Diabetes mellitus | ||||||
| No | 1216 | 96.0 | 8.9 | 18.5 | 19.2 | 14.6 |
| Yes | 49 | 4.0 | 0.0 | 22.4 | 24.5 | 12.2 |
| Mother smoked during pregnancy | ||||||
| No | 1045 | 89.3 | 7.9 | 19.1 | 18.2 | 13.5 |
| Yes | 126 | 10.7 | 13.5 | 13.5 | 23.0 | 18.2 |
| Infant’s gender | ||||||
| Female | 596 | 46.9 | 7.7 | 17.1 | 19.6 | 16.1 |
| Male | 672 | 53.1 | 9.2 | 20.3 | 19.2 | 13.1 |
Institute of Medicine (National Research Council) categories.
Two sets of analyses were performed: one to examine the single primary pathology as determined by the pathologist and another to examine the presence or absence of any type of placental pathology, as multiple pathologies were often identified. Using generalized estimating equations to control for within subject correlation of multiple pathology types, we calculated unadjusted odds ratios (ORs) between placental pathology and covariates.21-22 Placental pathology was coded as a multivariate response variable with four types (presence or absence of acute inflammation, chronic inflammation, maternal vascular pathology, and fetal vascular pathology). Our model used interaction terms to allow the association between each covariate and placental pathology to differ by type of UPVC, AFIS and chronic villitis. Unadjusted results for the patient’s primary placental pathology are presented in Table 2. Associations found to be statistically significant at the 0.20 level were included in the final model, obtained using backward elimination. The final adjusted model for primary pathology included PIH, age, maternal smoking during pregnancy, PIR, and previous elective termination. Adjusted results are presented in Table 3. Further analysis was conducted based on the presence of any placental pathology that was noted.
TABLE 2.
Unadjusted ORs for primary placental pathologic condition, according to demographic and medical factors.
| Variable |
P value for test of association between risk factor and placental pathologic condition |
OR | |||
|---|---|---|---|---|---|
| AFIS (95% CI) | Chronic villitis (95% CI) |
Maternal vascular pathologic condition (95% CI) |
Fetal vascular pathologic condition (95% CI) |
||
| Age (y) | .0573 | ||||
| <35 | Reference | Reference | Reference | Reference | |
| ≥35 | 0.46 (0.23, 0.92) | 0.99 (0.67, 1.45) | 1.33 (0.93, 1.91) | 1.25 (0.83, 1.87) | |
| Race | .1093 | ||||
| White (including other) | Reference | Reference | Reference | Reference | |
| African American | 1.59 (1.01, 2.50) | 0.83 (0.57, 1.21) | 1.28 (0.91, 1.81) | 0.82 (0.54, 1.25) | |
| Maternal education | .3637 | ||||
| ≤High school | 1.51 (0.95, 2.41) | 0.84 (0.58, 1.21) | 1.05 (0.73, 1.51) | 0.84 (0.56, 1.28) | |
| Some college | 1.08 (0.64, 1.84) | 0.72 (0.49, 1.07) | 1.43 (1.01, 2.02) | 1.08 (0.72, 1.61) | |
| ≥College | Reference | Reference | Reference | Reference | |
| PIR (%) | .0714 | ||||
| <100 | 1.83 (1.08, 3.08) | 0.56 (0.35, 0.92) | 1.16 (0.78, 1.74) | 0.77 (0.47, 1.26) | |
| 100-199 | 1.39 (0.76, 2.54) | 0.80 (0.50, 1.27) | 0.86 (0.54, 1.37) | 0.68 (0.39, 1.18) | |
| >200 | Reference | Reference | Reference | Reference | |
| Mother’s marital status | .0055 | ||||
| Married | 0.50 (0.33, 0.76) | 1.40 (0.99, 1.99) | 0.79 (0.58, 1.08) | 1.17 (0.81, 1.71) | |
| Single, divorced widowed, separated |
Reference | Reference | Reference | Reference | |
| Body mass index (kg/m2) | .3222 | ||||
| <19.8 | 0.93 (0.51, 1.69) | 0.86 (0.55, 1.34) | 1.28 (0.85, 1.93) | 1.58 (1.01, 2.46) | |
| 19.8-26.0 | Reference | Reference | Reference | Reference | |
| 26.1-29.0 | 0.45 (0.19, 1.07) | 1.15 (0.73, 1.81) | 1.35 (0.87, 2.35) | 1.43 (0.87, 2.35) | |
| >29.0 | 1.08 (0.67, 1.74) | 1.13 (0.80, 1.60) | 1.14 (0.80, 1.62) | 1.27 (0.86, 1.89) | |
| Smoking during pregnancy | 0.0842 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 1.81 (1.04, 3.17) | 0.66 (0.39, 1.13) | 1.35 (0.87, 2.10) | 1.43 (0.88, 2.33) | |
| Previous birth | .1519 | ||||
| None | Reference | Reference | Reference | Reference | |
| ≥1 | 0.67 (0.45, 1.01) | 1.11 (0.82, 1.51) | 0.79 (0.59, 1.06) | 0.95 (0.68, 1.32) | |
| Previous preterm birth | .0128 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 0.46 (0.20, 1.07) | 1.53 (1.01, 2.32) | 1.15 (0.75, 1.78) | 0.59 (0.32, 1.07) | |
| Previous miscarriage | .6872 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 0.71 (0.44, 1.15) | 1.021 (0.74, 1.40) | 1.07 (0.78, 1.46) | 1.08 (0.76, 1.53) | |
| Previous elective termination |
.0313 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 2.19 (1.42, 3.40) | 0.74 (0.50, 1.09) | 0.98 (0.68, 1.42) | 1.14 (0.77, 1.69) | |
| Chronic hypertension | .0109 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 0.31 (0.10, 0.98) | 0.93 (0.55, 1.59) | 1.47 (0.92, 2.36) | 1.95 (1.20, .018) | |
| PIH | .0073 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 0.75 (0.46, 1.22) | 1.13 (0.81, 1.53) | 1.48 (1.10, 2.01) | 1.71 (1.22, 2.40) | |
| Preeclampsia | .0133 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 0.18 (0.03, 1.34) | 1.05 (0.54, 2.06) | 1.83 (1.02, 3.28) | 1.12 (0.54, 2.32) | |
| Prepregnancy diabetes mellitus |
.1097 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | Insufficient Data | 1.22 (0.62, 2.41) | 1.10 (0.54, 2.27) | 0.46 (0.20, 1.03) | |
| GDM | .3450 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 0.47 (0.11, 1.96) | 1.53 (0.78, 2.99) | 1.45 (0.74, 2.83) | 0.86 (0.36, 2.07) | |
| Infant’s gender | .2145 | ||||
| Female | Reference | Reference | Reference | Reference | |
| Male | 1.21 (0.81, 1.80) | 0.91 (0.61, 1.07) | 0.97 (0.73, 1.28) | 0.78 (0.57, 1.07) | |
Table 3.
Adjusted ORs for primary placental pathologic, according to demographic and medical factors.a
| Variable |
P value for test of association between risk factor and placental pathologic condition |
OR | |||
|---|---|---|---|---|---|
| AFIS (95% CI) | Chronic villitis (95% CI) |
Maternal vascular pathologic condition (95% CI) |
Fetal vascular pathologic condition (95% CI) |
||
| Age (y) | .1079 | ||||
| < 35 | Reference | Reference | Reference | Reference | |
| ≥35 | 0.55 (0.26, 1.18) | 0.87 (0.58, 1.33) | 1.64 (1.11, 2.41) | 1.16 (0.74, 1.83) | |
| PIR (%) | .0864 | ||||
| >200 | Reference | Reference | Reference | Reference | |
| 100-200 | 1.03 (0.51, 2.10) | 0.75 (0.44, 1.29) | 0.79 (0.46, 1.36) | 0.60 (0.32, 1.12) | |
| <100 | 1.93 (1.05, 3.56) | 0.55 (0.32, 0.94) | 1.03 (0.63, 1.69) | 0.74 (0.40, 1.36) | |
| Smoking during pregnancy |
.1343 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 1.64 (0.85, 3.14) | 0.75 (0.41, 1.37) | 1.32 (0.78, 2.24) | 1.76 (1.00, 3.11) | |
| Previous birth | .0025 | ||||
| None | Reference | Reference | Reference | Reference | |
| ≥1 | 0.44 (0.26, 0.74) | 1.34 (0.94, 1.92) | 0.67 (0.47, 0.96) | 1.01 (0.68, 1.51) | |
| Previous preterm birth | .0218 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 0.50 (0.19, 1.30) | 1.60 (1.10, 2.55) | 1.17 (0.70, 1.94) | 0.52 (0.26, 1.04) | |
| Previous elective termination |
.0032 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 3.28 (1.89, 5.70) | 0.67 (0.43, 1.04) | 1.13 (0.74, 1.72) | 1.20 (0.76, 1.89) | |
| PIH | .0659 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 0.93 (0.55, 1.58) | 1.12 (0.77, 1.62) | 1.44 (1.01, 2.07) | 1.61 (1.10, 2.37) | |
| Preeclampsia | .1077 | ||||
| No | Reference | Reference | Reference | Reference | |
| Yes | 0.21 (0.02, 1.84) | 1.22 (0.57, 2.58) | 0.99 (0.47, 2.08) | 0.55 (0.21, 1.42) | |
Adjusted for PIH, age, maternal smoking during pregnancy, PIR percent, and previous elective termination.
Results
A total of 1270 placentas were available for examination. As Table 1 indicates, the sample was predominately white, upper income, college educated, < 35 year old women. Approximately 8.5%, 18.6%, 19.4%, and 14.5% of placentas had AFIS, chronic villitis, maternal vascular pathology, and fetal vascular pathology as the primary placental pathology, respectively (Table 1). Among examined placentas, 27.1%, 56.1%, 83.3% and 53.3% had some evidence of AFIS, chronic villitis UPVC, and fetal vascular pathology present, respectively. However, our blinded pathologist (CS) assigned a final primary pathology as stated above.
We observed unadjusted relationships between the following: AFIS and maternal age, marital status, previous elective termination, previous preterm birth, and smoking during pregnancy; chronic inflammation and PIR, marital status, and previous preterm birth; UPVC and maternal age, PIR, race, smoking during pregnancy, chronic hypertension, PIH, preeclampsia, and GDM; and fetal vascular pathology and PIR, smoking during pregnancy, chronic hypertension, and PIH (Table 1).
Table 2 describes unadjusted odds ratios between these factors of interest and the placental pathology types. Women reporting a history of a preterm birth had 1.53 (95% CI: 1.01, 2.32) times the odds of chronic villitis. Women who smoked during pregnancy had 1.81 (95% CI: 1.04, 3.17) times the odds of AFIS. Previous elective termination was associated with 2.19 (95% CI: 1.42, 3.40) times the odds of AFIS. Chronic hypertension was associated with 1.95 (95% CI: 1.20, 3.18) times the odds of fetal vascular pathology. PIH was associated with 1.48 (95% CI: 1.10, 2.01) and 1.71 (95% CI: 1.22, 2.40) times the odds of UPVC and fetal vascular compromise, respectively. Women with preeclampsia had an elevated risk (OR: 1.83; 95% CI: 1.05, 3.28) of having UPVC.
The final adjusted model for primary placental pathology is presented in Table 3. After adjustment, women with a history of preterm birth continued to have increased odds (OR: 1.60; 95% CI: 1.10, 2.55) of chronic villitis, while women with previous births seemed to be protected against AFIS (OR: 0.44; 95% CI: 0.26, 0.74). Women with a previous elective termination had increased (OR: 3.28; 95% CI: 1.89, 5.70) odds of AFIS. Maternal smoking during pregnancy was no longer a significant predictor of AFIS, with an odds ratio of 1.64 (95% CI: 0.85, 3.14), but these results are still suggestive of a potential association. PIH was significantly associated with 1.44 (95% CI: 1.01, 2.07) and 1.61 (95% CI: 1.10, 2.37) times the odds of UPVC and fetal vascular pathology.
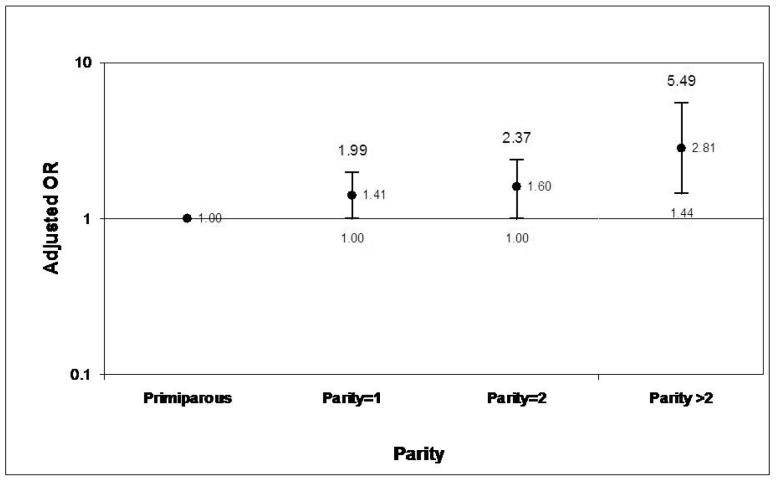
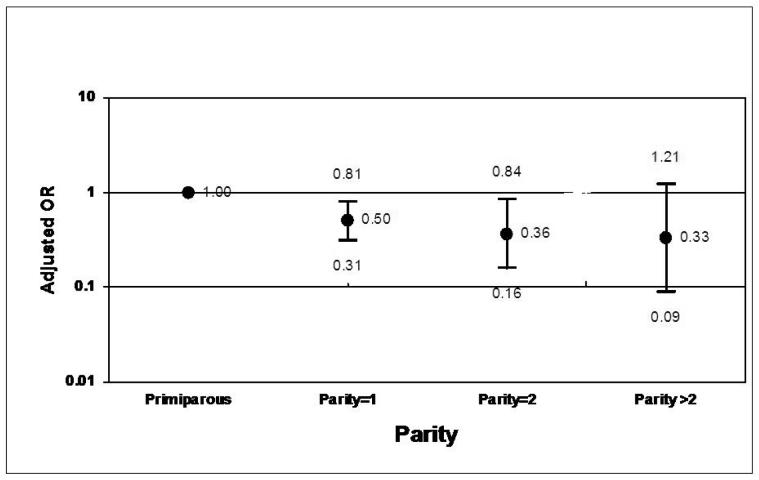
Further analyses were conducted to explore the relationship between parity and acute or chronic inflammation. When compared against primaparous mothers, the odds of chronic villitis increased with parity in a monotonic fashion (Figure 1). Conversely, the odds of AFIS decreased with parity (Figure 2).
Figure 1. Adjusted ORs for Chronic Villitis According to Parity.*.
*Adjusted for pregnancy induced hypertension, age, maternal smoking during pregnancy, poverty to income ratio percent, and previous elective termination.
Figure 2. Adjusted ORs for AFIS According to Parity.*.
*Adjusted for pregnancy induced hypertension, age, maternal smoking during pregnancy, poverty to income ratio percent, and previous elective termination.
Finally, unadjusted and adjusted odds ratios were calculated between confounders and the presence of any placental pathology that was noted histologically. Significant associations that were identified were essentially the same as those noted in the analysis of the primary placental pathology.
Comment
In this prospective epidemiologic study, we identified several factors associated with UPVC, AFIS and chronic villitis in a large representative population of pregnant women. To our knowledge, this is the largest study conducted addressing how risk factors for preterm birth affect placental pathology. The most significant findings we noted were those dealing with both AFIS and chronic villitis. A previous elective termination, smoking, and a low PIR were all risk factors for AFIS. Increased odds of chronic villitis were seen with a history of a prior preterm delivery or a prior term delivery. However, increasing parity was protective against AFIS. Finally, women with PIH were more likely to have both UPVC and fetal vascular placental lesions.
Both AFIS and chronic villitis of the placenta are common findings in preterm delivery. Recently, Ghidini and Salafia8 evaluated the placentas from 413 women delivering before 32 weeks gestation. Women with a history of a previous preterm delivery had more AFIS and chronic villitis than women without a history of preterm delivery that delivered at a similar gestational age. In a similar study by Goldenberg et al9, 457 placentas from women with both spontaneous and indicated preterm births were analyzed. They noted that AFIS was more common in spontaneous preterm births than indicated preterm births, but chronic villitis was more common in indicated preterm delivery. In our subjects, those who reported a history of having a preterm delivery were at significantly greater risk of having chronic villitis in a subsequent pregnancy.
One of the strongest associations that we noted was the increased risk of AFIS in women with a history of an elective termination. Whether or not elective terminations place women at risk for future preterm deliveries is a matter of significant debate. Two recent studies did not find an increased risk of preterm delivery in patients with a history of a mid-trimester dilation and evacuation23 or in those with a history of a medical abortion.24 However, other studies have shown increased rates of preterm delivery following induced abortions.25-27 In fact, Henriet et al25 reported that the risk of preterm delivery increased with the number of previous induced abortions. In this study, we do not have information regarding the specific method used to induce the abortion. Because of this, we are not able to determine what contribution this had on the increased rate of AFIS seen in our women with a history of an elective termination. However, this finding does suggest a potential causal pathway to preterm delivery in women with a history of induced abortion if such an association truly exists.
Another interesting finding of this study was the effect of parity on AFIS and chronic villitis. While the risk of AFIS decreased with increasing parity, the risk of chronic villitis rose with each subsequent delivery. The effect of parity on placental pathology is not well described in the literature. However, a study by Lagadari et al28 did address this issue in a mouse model. They proposed that the benefits of multiparity could be explained by the presence of a protective layer of macrophages found between the decidua and trophoblast layers. In their study, these placental macrophages were found in greater number in multiparous mice compared to their primparous counterparts. The authors suggest the protective effects of these cells could be the result of the secretion of various growth factors and regulators of trophoblast function. Because macrophages represent an important part of the immune system that protects against infection, they may play an important role in placental inflammation. In our study, increasing parity was associated with decreased risk for acute placental inflammation. It may be that the increased chronic inflammation associated with parity provides some immunologic protection against infection. Obviously, more studies are needed to specifically address how parity modulates the number and function of macrophages and on the immune system itself.
Lastly, we noted an association between PIH and vascular lesions of the placenta. This is in keeping with other studies that have also shown more UPVC in pregnancies complicated by hypertensive disorders.9,29 In women with indicated preterm births, this is often a common finding and differs from the AFIS and chronic villitis associated with spontaneous preterm delivery.8-9
In summary, UPVC, AFIS and chronic villitis may play an important role in both spontaneous and indicated preterm delivery. In this study, there were several risk factors for preterm birth significantly associated with pathologic lesions of the placenta. Future research is needed to determine how much these risk factors contribute to these specific pathologic entities. Whether or not interventions aimed at altering UPVC, AFIS and chronic villitis will lead to improved pregnancy outcomes remains an unanswered question.
Acknowledgments
Supported by NIH (NICHD, NIDDK, NCI, and General Clinical Research Centers Program), the Association of Schools of Public Health, the Centers for Disease Control and Prevention, the March of Dimes Birth Defects Foundation, the Wake Area Heath Education Center, and the University of North Carolina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 28th Annual Meeting of the Society for Maternal-Fetal Medicine, Dallas, Texas, January 28-February 2, 2008.
Condensation We have identified several risk factors for placental pathology and inflammation that could aid in our understanding of preterm birth.
References
- 1.Wilcox AJ, Skjaerven R. Birth weight and perinatal mortality: The effect of gestational age. Am J Pub Health. 1992;82:378–82. doi: 10.2105/ajph.82.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15:414–43. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 3.Clements KM, Barfield WD, Avadi MF, Wilber N. Preterm birth-associated cost of early intervention services: an analysis by gestational age. Pediatrics. 2007;119(4):866–74. doi: 10.1542/peds.2006-1729. [DOI] [PubMed] [Google Scholar]
- 4.Grimes DA, Schulz KF. Randomized controlled trial of home uterine activity monitoring: A review and critique. Obstet Gynecol. 1992;79:137–42. [PubMed] [Google Scholar]
- 5.Shiono PH, Klebanoff MA. A review of risk scoring for preterm birth. Clin Perinatol. 1993;20:107–25. [PubMed] [Google Scholar]
- 6.Hauth JC, Goldenberg RL, Andrews WW, et al. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N Eng J Med. 1995;333:1732–6. doi: 10.1056/NEJM199512283332603. [DOI] [PubMed] [Google Scholar]
- 7.Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Eng J Med. 2003;348(24):2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 8.Ghidini A, Salafia CM. Histologic placental lesions in women with recurrent preterm delivery. Acta Obstet Gynecol Scand. 2005;84:547–50. doi: 10.1111/j.0001-6349.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg RL, Andrews WW, Faye-Petersen O, Cliver S, Geopfert AR, Hauth JC. The Alabama Preterm Birth Project: placental histology in recurrent spontaneous and indicated preterm birth. Am J Obstet Gynecol. 2006;195(3):792–6. doi: 10.1016/j.ajog.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 10.O’Shea TM, Klinepeter KL, Meis PJ, Dillard RG. Intrauterine infection and the risk of cerebral palsy in the very-low birthweight infants. Paediatr Perinat Epidemiol. 1998;12:72–83. [PubMed] [Google Scholar]
- 11.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Geopfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–8. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 12.Kolas T, Nakling J, Salvesen KA. Smoking during pregnancy increases the risk of preterm births among parous women. Acta Obstet Gynecol Scand. 2000;79(8):644–8. [PubMed] [Google Scholar]
- 13.Mazaki-Tovi S, Romero R, Kusanovic JP, et al. Recurrent preterm birth. Semin Perinatol. 2007;31(3):142–58. doi: 10.1053/j.semperi.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savitz DA, Dole N, Williams J, et al. Determinants of participation in an epidemiological study of preterm delivery. Paediatr Perinat Epidemiol. 1999;13(1):114–25. doi: 10.1046/j.1365-3016.1999.00156.x. [DOI] [PubMed] [Google Scholar]
- 15.Salafia CM, Vogel CA, Bantham KF, Vintzileos AM, Pezzullo J, Silberman L. Preterm delivery: Correlations of fetal growth and placental pathology. Am J Perinatol. 1992;9:190–3. doi: 10.1055/s-2007-999318. [DOI] [PubMed] [Google Scholar]
- 16.Salafia CM, Starzyk KA, Lage JM, Parkash V, Vercruysse L, Pijnenborg R. Lipoprotein(a) deposition in the uteroplacental bed and in basal plate uteroplacental arteries: Implications for atherosclerosis-associated processes in normal and complicated pregnancies. Troph Res. 1998;11:377–87. [Google Scholar]
- 17.Salafia CM, López-Zeno JA, Sherer DM, Whittington SS, Minior VK, Vintzileos AM. Histologic evidence of old intrauterine bleeding is more frequent in prematurity. Am J Obstet Gynecol. 1995;173:1065–70. doi: 10.1016/0002-9378(95)91327-0. [DOI] [PubMed] [Google Scholar]
- 18.Salafia CM, Ernst LM, Pezzullo JC, Wolf EJ, Rosenkrantz TS, Vintzileos AM. The very low birthweight infant: Maternal complications leading to preterm birth, placental lesions, and intrauterine growth. Am J Perinatol. 1995;12:106–10. doi: 10.1055/s-2007-994417. [DOI] [PubMed] [Google Scholar]
- 19.Starzyk KA, Salafia CM, Pezzullo JC, Lage JM, Parkash V, Vercruysse L, Hanssens M, Pijnenborg R. Quantitative differences in arterial morphometry define the placental bed in preeclampsia. Hum Pathol. 1997;28:353–8. doi: 10.1016/s0046-8177(97)90135-0. [DOI] [PubMed] [Google Scholar]
- 20.Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73(3 Pt 1):383–9. [PubMed] [Google Scholar]
- 21.Zeger SL, Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 22.Liang K-Y, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 23.Jackson JE, Grobman WA, Haney E, Casele H. Mid-trimester dilation and evacuation with laminaria does not increase the risk for severe subsequent pregnancy complications. Int J Gynaecol Obstet. 2007;96:12–5. doi: 10.1016/j.ijgo.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648–53. doi: 10.1056/NEJMoa070445. [DOI] [PubMed] [Google Scholar]
- 25.Henriet L, Kaminski M. Impact of induced abortions on subsequent pregnancy outcome: the 1995 French national perinatal survey. BJOG. 2001;108:1036–42. doi: 10.1111/j.1471-0528.2001.00243.x. [DOI] [PubMed] [Google Scholar]
- 26.Moreau C, Kaminski M, Ancel PY, et al. Previous induced abortions and the risk of very preterm delivery: results of the EPIPAGE study. BJOG. 2005;112:430–7. doi: 10.1111/j.1471-0528.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 27.Thorp JM, Hartmann KE, Shadigian E. Long-term physical and psychological consequences of induced abortion: review of the evidence. Obstet Gynecol Surv. 2003;58(1):67–79. doi: 10.1097/00006254-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Lagadari M, Blois S, Margni R, Miranda S. Analysis of macrophage presence in murine placenta: influence of age and parity status. AJRI. 2004;51:49–55. doi: 10.1046/j.8755-8920.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- 29.Goldenberg RL, Faye-Peterson O, Andrews WW, Goepfert AR, Cliver SP, Hauth JC. The Alabama preterm birth study: diffuse decidual leukocytoclastic necrosis of the deciduas basalis, a placental lesion associated with preeclampsia, indicated preterm birth and decreased fetal growth. J Matern Fetal Neonatal Med. 2007;20(5):391–5. doi: 10.1080/14767050701236365. [DOI] [PubMed] [Google Scholar]