Abstract
Purpose
Biomechanical modeling consistently indicates that superior oblique (SO) weakness alone is insufficient to explain the large hypertropia often observed in SO palsy. Magnetic resonance imaging (MRI) was used to investigate if any size or contractility changes in the inferior rectus (IR) may contribute.
Design
Prospective, case control study.
Participants
17 patients with unilateral SO palsy, and 18 orthotropic subjects.
Method
Surface coils were used to obtain sets of contiguous, 2 mm thick high-resolution, coronal MRI in different gazes. Cross sectional areas of the IR and SO muscles were determined in supraduction and infraduction for evaluation of size and contractility. Diagnosis of SO palsy was based on clinical presentations, and subnormal contractility and SO size less than the normal 95% confidence limit.
Main Outcome Measures
Cross sectional areas of the IR and SO muscles.
Results
Patients had 15.9 ± 7.2 (mean ± standard deviation) Δ central gaze hypertropia and exhibited ipsilesional SO atrophy and subnormal contractility. Mean ipsilesional, contralesional and normal IR cross-sections were 28.5 ± 3.5 mm2, 31.9 ± 3.8 mm2 and 31.8 ± 5.8 mm2, while mean contractility was 16.5 ± 3.8 mm2, 20.5 ± 4.1 mm2, and 16.6 ± 4.8 mm2, respectively. Ipsilesional IR cross-sections and contractility were significantly less than contralesional (P < 0.01).
Conclusions
In SO palsy, the contralesional IR is larger and more contractile than the ipsilesional IR, reflecting likely neurally mediated changes that augment the relatively small hypertropia due to SO weakness alone. Recession of the hyperfunctioning contralesional IR recession in SO palsy is a physiologic therapy.
Superior oblique (SO) palsy has traditionally been diagnosed indirectly on the basis of clinical observations of ocular versions and measurements of binocular misalignment.1–4 The mechanical state of the SO muscle belly itself was inaccessible to direct study. Contemporary imaging such as magnetic resonance imaging (MRI) or computed x-ray tomography now enables direct evaluation of the functional anatomy of extraocular muscles (EOMs). Horton et al. first reported qualitative evidence of SO atrophy in a patient with trochlear palsy.5 Quantitative MRI repeated in multiple gaze positions has been shown to demonstrate characteristic contractile thickening of the normal SO in infraduction, as well as reduction in SO size and contractility in SO palsy.6–9 Measurement of SO size and contractility by MRI now provides direct, objective standards confirming deficiency of SO function in SO palsy. Orbital MRI has also clarified mechanisms of cyclovertical strabismus, indicating that likely masquerading abnormalities including heterotopy of rectus EOM pulleys10 can cause incomitant strabismus by redirecting rectus forces into oblique directions.11 Unstable rectus pulleys can cause gaze-related shifts in rectus pulling directions, and thus incomitant strabismus.12 Hindrance of rectus pulleys may also cause restrictive strabismus.13
For reasons including complexity of clinical presentation and diagnosis of SO palsy, as well as potential masquerading conditions, outcomes of surgery for SO palsy have always been variable. Biomechanical modeling such as the Robinson, SQUINT, Simonsz, and See-KID models, which can help understand complex pathophysiology, consistently suggests that SO weakness alone is insufficient to explain the large hypertropia observed in SO palsy. 14–16 These theoretical studies therefore imply that currently unknown mechanisms, in addition to SO weakness, are operating to give the clinical picture of SO palsy. Additional speculative mechanisms, such as muscle overactions and length changes, have been included in mathematical models of SO palsy, but such mechanisms have not been confirmed empirically. 14–16 Contralesional inferior rectus (IR) recession is commonly performed in unilateral SO palsy, especially when the primary position vertical deviation exceeding 15.17 While contralesional IR recession is known to be effective in SO palsy, the theoretical basis for this benefit has been unclear.
The current study was designed to apply modern, quantitative, high-resolution MRI for the direct evaluation of the IR in cases of unequivocal SO palsy exhibiting SO atrophy and reduced contractility. The aim was to determine whether pathologically reduced SO contractile function is associated with IR size or contractility changes, which may help clarify the mechanism of SO palsy and provide a physiological rationale for appropriate surgical correction.
Methods
Subjects
We studied 18 normal, orthotropic paid volunteers recruited by advertisement, and 17 patients with unilateral incomitant hypertropia clinically diagnosed as unilateral SO palsy using standard 3-step test criteria. All gave written informed consent according to a protocol conforming to the Declaration of Helsinki and approved by the Human Subject Protection Committee at the University of California, Los Angeles. Normal volunteers underwent complete eye examinations verifying normal corrected vision, normal ocular versions, orthotropia in all gaze positions, and stereopsis of 40 arc seconds by Titmus testing. Hypertropic subjects were selected from a prospective study of EOM function in strabismus, and were selected because they had qualitative clinical evidence of atrophy of one SO muscle. All strabismic subjects had undergone complete ophthalmologic examinations by an author who is a pediatric ophthalmologist, including measurement of binocular misalignment by prism and cover testing in upright diagnostic head positions as well as with head tilting, and the Hess screen test. Cyclodeviation was measured subjectively using double Maddox rods, and objectively assessed by fundus examination. Ocular versions were quantified on a 9 point scale from −4 representing maximal underaction, to zero representing normal action, to +4 representing maximal overaction. Forced duction testing was performed in subjects who underwent strabismus surgery. Six strabismic subjects underwent ipsilesional inferior oblique (IO) weakening and contralesional IR recession, while others did not undergo surgery.
MRI
Using a 1.5 T General Electric Signa (Milwaukee, WI) scanner, high-resolution, T1-weighted orbital MRI was performed. Crucial aspects of this technique, described in detail elsewhere, include use of a dual-phased surface coil array (Medical Advances, Milwaukee, WI) to improve signal-to-noise ratio and fixation targets to avoid motion artifacts.6,10,16,18–20 To image the SO and IR, sets of 18 contiguous 2-mm thick quasi-coronal image planes were obtained perpendicular to the long axis of the orbit using a 256 ×256 matrix over an 8cm or 10 cm field of view, giving pixel resolution of 0.313 or 0.352 mm (Fig. 1). Some subjects received the intravenous paramagnetic MRI contrast agent gadodiamide (0.1 mmol/kg) to improve the contrast of EOMs against connective tissue in the anterior orbit.20, 21 During imaging, subjects fixated small, individually illuminated, afocal targets delivered by a fiberoptic system mounted on the transparent faceplate of the surface coil array. Imaging was repeatedly performed quasi-coronally in central gaze, supraduction, and infraduction with or without abduction. Noncentral targets were placed at the maximum eccentricities permitted from occlusions by the surface coil or facial anatomy, and that could be maintained for the 3.5-minute duration of each scan. For all adducted gazes, the fixation target was presented to the non scanned fellow eye.
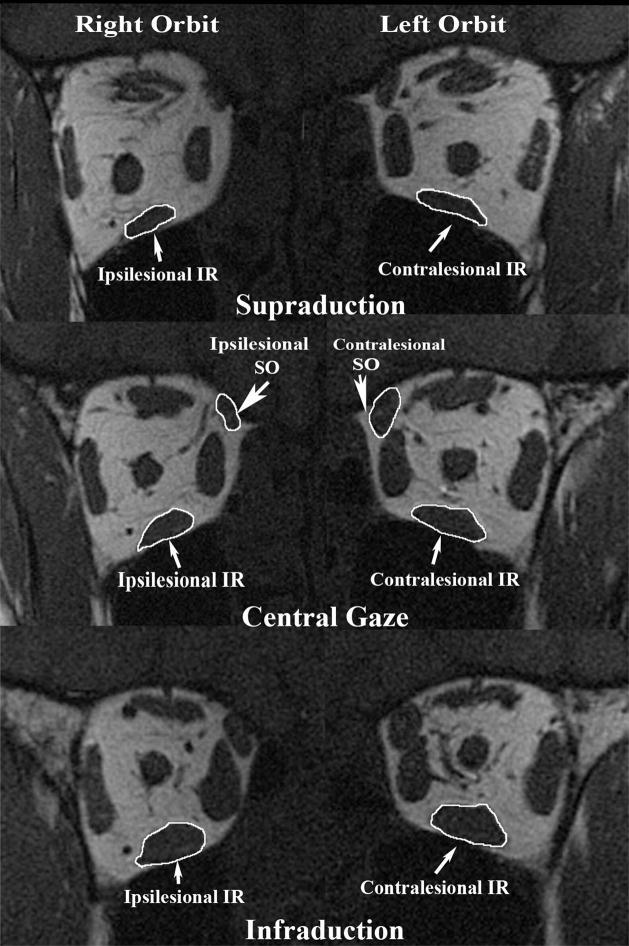
Figure 1.
Quasi-coronal MRI 8 mm posterior to the globe-optic nerve junction (ON) in supraduction, central gaze, and infraduction in subject 5 with congenital right SO palsy. Note smaller cross-section of right than left SO and less contractile thickening of right SO from supraduction to infraduction. Also note smaller cross-section of right than left IR and less contractile thickening of right IR from supraduction to infraduction. IR = inferior rectus muscle; SO = superior oblique muscle.
Analysis
Digital MRI images were transferred to Macintosh computers (Apple Computer, Cupertino, CA), converted into spatially calibrated, 8-bit tagged image file format using locally developed software, and quantified using the program ImageJ (National Institutes of Health; available at: http://rsb.info.nih.gov/ij/. Verified November 11, 2006.) Cross-sectional areas in each MRI image of the SO and IR were computed using the “area-measure” function of the program (Fig. 1). Anteroposterior SO and IR positions were normalized by defining the globe–optic nerve junction plane in central gaze as image plane 0. Values for SO and IR cross-sections were averaged across subjects in 2-mm bins referenced to image plane 0. For both SO and IR contractility, we analyzed only orbits for which there were also complete image sets in upward and downward gaze positions. Contractile change was taken to be the cross-section in infraduction minus that in supraduction for each image plane. Since quasi-coronal image planes are perpendicular to the orbit but not to the SO or IR muscles, both cross-sectional area and contractility were trigonometrically corrected to obtain a true cross-section perpendicular to the long axis of the muscles.6
Statistical analysis was performed using SPSS 10.0 (SPSS Inc., Chicago, IL).
Results
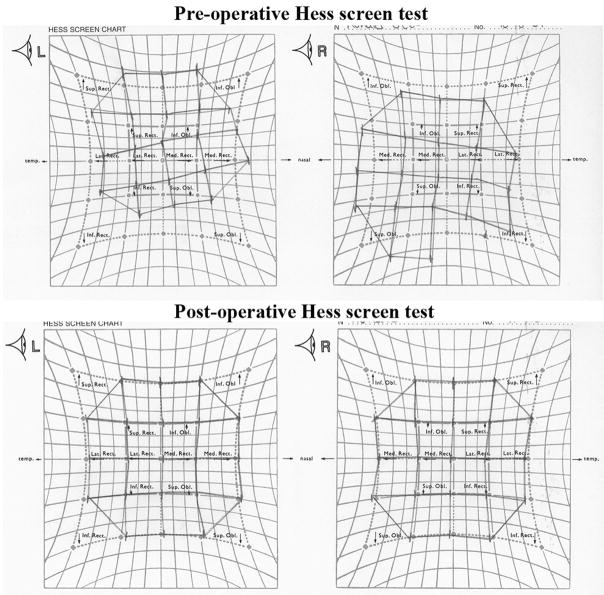
A total of 27 orbits were imaged in 18 normal subjects of mean (± standard deviation) age 34 ± 11 years old (range, 23–57 years). Before MRI, subjects with SO atrophy had been clinically diagnosed with unilateral SO palsy by strabismus specialists. Both ipsilesional and contralesional orbits were imaged in all 17 patients, who had mean age 35 ± 20 (range, 15–83) years with unilateral SO palsy. Hypertropic subjects were diagnosed as having probable congenital or acquired SO palsy on the basis of medical history of onset time of strabismus, old photographs, and existence of large fusional vergence amplitudes. Causes of SO palsy were ascertained clinically in acquired cases wherever there was a clear temporal relationship of onset to a plausibly-causative head trauma, tumor, or surgical manipulation causing trochlear nerve palsy; all other cases were diagnosed as idiopathic. Clinical characteristics of the subjects with SO palsy are summarized in Table 1. Among all subjects with SO palsy, only Subject 11 had very mild facial asymmetry. Subject 2 showed severe SO tendon laxity by intra-operative forced duction testing. In our population of strabismic patients, neither SO tendon laxity nor facial asymmetry, has correlated reliably with SO muscle size and function by MRI. 22 All hypertropic subjects showed ipsilesional deficiency of infraduction in adduction. After undergoing MRI, Subjects 1–6 achieved excellent postoperative alignment after ipsilesional IO weakening and contralesional IR recession, as shown by example pre-operative and post-operative Hess screen tests (Fig. 2).
Table 1.
Characteristics of Subjects with Superior Oblique Palsy
| Hyperdeviation (Δ) |
Subjective Excyclodeviation (°) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Sex | Age (yrs) | Lesion Side | Cause | Duration (yrs) | Spontaneous Head Tilt | Upright | Right Tilt | Left Tilt | |
| 1 | M | 50 | R | I | 0.5 | L | 8 | 15 | 0 | 2 |
| 2 | M | 70 | L | C | 70 | R | 10 | 6 | 15 | 0 |
| 3 | F | 18 | R | A | 0.9 | L | 16 | 18 | 2 | 5 |
| 4 | M | 19 | L | A | 1.8 | R | 15 | 0 | 19 | 6 |
| 5 | F | 22 | R | C | 22 | L | 25 | 35 | 10 | 9 |
| 6 | M | 18 | L | C | 17 | R | 7 | 0 | 13 | 5 |
| 7 | M | 32 | L | C | 32 | R | 25 | 3 | 30 | 9 |
| 8 | M | 32 | R | A | 1 | L | 15 | 10 | 0 | 5 |
| 9 | M | 16 | R | C | 13 | L | 25 | 5 | 1 | 0 |
| 10 | F | 83 | L | I | 0.5 | - | 12 | 0 | 8 | 20 |
| 11 | M | 52 | L | A | 3 | R | 5 | 0 | 15 | 5 |
| 12 | F | 47 | L | C | 45 | R | 16 | 8 | 25 | 5 |
| 13 | M | 39 | R | A | 0.25 | L | 28 | 35 | 0 | 5 |
| 14 | M | 21 | L | C | 21 | R | 15 | 0 | 25 | 10 |
| 15 | F | 36 | L | C | 36 | R | 25 | 12 | 50 | 9 |
| 16 | M | 28 | R | C | 28 | L | 12 | 15 | 0 | 15 |
| 17 | F | 15 | R | C | 15 | L | 12 | 20 | 0 | 7 |
Note: F = female. M = male. L = left. R = right. I = Idiopathic. C= Congenital. A= Acquired
Figure 2.
Hess screen test for Subject 2 shows pre-operative left incomitant hypertropia, but excellent post-operative alignment after undergone ipsilesional inferior oblique weakening and contralesional inferior rectus recession. Right column shows positions of the right eye during left eye fixation of targets at ±15° and ±30° horizontal and vertical eccentricities, while the left column shows positions of the left eye during right eye fixations of the same targets.
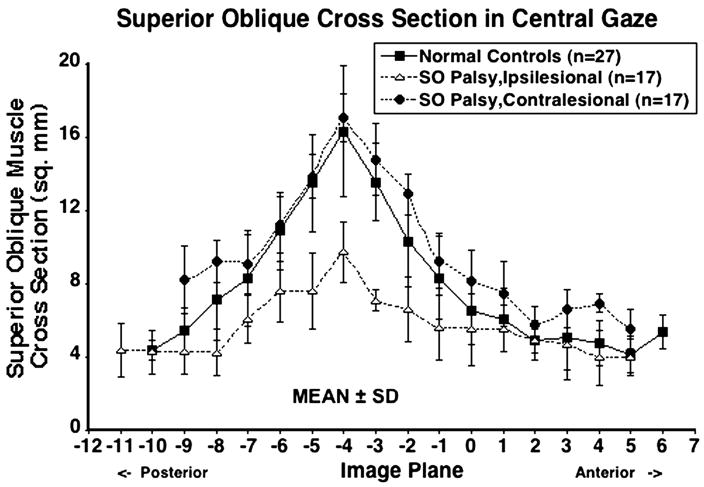
As shown in Fig. 3, SO cross-sectional area could be determined over a wide anteroposterior extent for both normal and subjects with SO palsy. SO cross-sections were maximal in midorbit 8 mm posterior to the globe–optic nerve junction, and were smaller anteriorly and posteriorly. Mean ipsilesional, contralesional and normal maximum SO cross-sections were 7.5 ± 4.6 mm2, 16.5 ± 3.1mm2 and 16.4 ± 3.6mm2, respectively (Table 2). The 95% confidence interval for mean maximum normal SO cross-sectional area was 15.0–17.9 mm2, so all palsied SO muscles were smaller than the lower confidence limit of normal. Ipsilesional SO cross-sections averaged significantly smaller than both the normal and contralesional SO throughout the midorbit (P < 0.0001), but not near the origin and tendon (Fig. 3).
Figure 3.
Mean superior oblique (SO) cross-sectional area in central gaze for 27 normal control muscles, 17 ipsilesional, and 17 contralesional SO muscles. Cross-sections are plotted as functions of 2-mm thickness image plane number, referenced to image plane 0 at the globe–optic nerve junction in central gaze. SD-standard deviation. Ipsilesional SO cross-sections were significantly smaller than both normal and contralesional SO throughout the mid-orbit (P < 0.0001). There was no significant difference between contralesional and normal SO cross-sections.
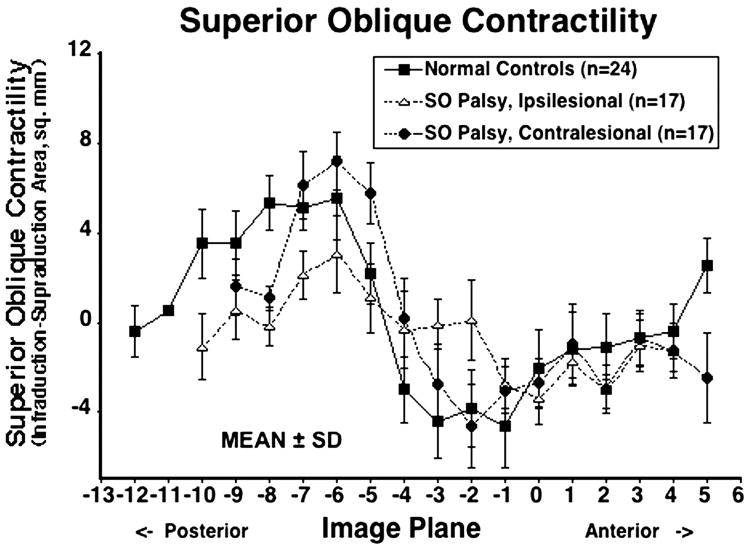
Mean maximum contractility for ipsilesional, contralesional and normal SO muscles was 2.7± 2.0 mm2, 6.8 ±2.9 mm2, and 5.5 ± 1.8 mm2, respectively (Table 2). The contractile change in SO cross-section from supraduction to infraduction is plotted for each image plane in Fig. 4, which demonstrates that ipsilesional SO contractility was significantly lower than that of contralesional and normal SO contractility (P < 0.0001). Although contralesional SO cross-section and contractility were slightly larger than normal, neither maximum contralesional SO central area (P=0.965) nor contractility (P=0.107) significantly differed from normal.
Figure 4.
Contractile change in superior oblique (SO) cross-sectional area from infraduction to supraduction for 24 normal control muscles, 17 ipsilesional and 17 contralesional SO muscles. SD-standard deviation. Maximal contractile change for normal muscles was in image plane −6, 12 mm posterior to the globe–optic nerve junction. Ipsilesional SO contractility was significantly subnormal (P < 0.0001), but contralesional contractility did not significantly differ from normal controls.
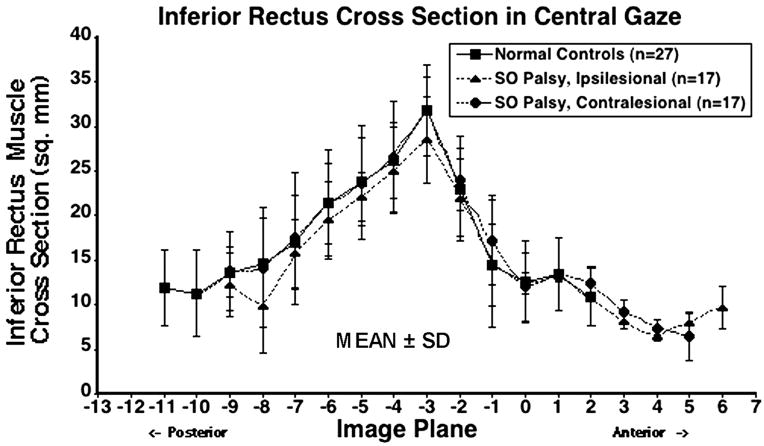
Following the approach of Miller23 and Demer et al24, IR cross-section was determined from contiguous quasi-coronal images over a wide anteroposterior extent for both normal subjects and subjects with SO palsy (Fig. 5). The IR was small at its origin and progressively enlarged as it coursed temporally in a straight line. The IR cross-section was maximal in mid orbit, 6 mm posterior to the globe–optic nerve junction, and was smaller anteriorly and posteriorly. The mean ipsilesional, contralesional and normal IR cross-sections were 28.5 ± 3.5 mm2, 31.9 ± 3.8 mm2 and 31.8 ± 5.8 mm2, respectively (Table 3). Ipsilesional IR cross-section was significantly smaller than contralesional (P < 0.001) and was slightly, though significantly smaller than normal (P = 0.043). Contralesional IR cross-section was slightly but not significantly larger than normal (P = 0.875).
Figure 5.
Mean inferior rectus (IR) cross-sectional area in central gaze for 27 normal control muscles, 17 ipsilesional, and 17 contralesional IR muscles. Cross-sections are plotted as functions of 2-mm thickness image plane number, referenced to image plane 0 at the globe–optic nerve junction in central gaze. SD=standard deviation. Ipsilesional IR cross-section was significantly smaller than contralesional (P < 0.001) and was slightly, though significantly, smaller than normal (P = 0.043). Contralesional IR cross-section was slightly but not significantly larger than normal (P = 0.875).
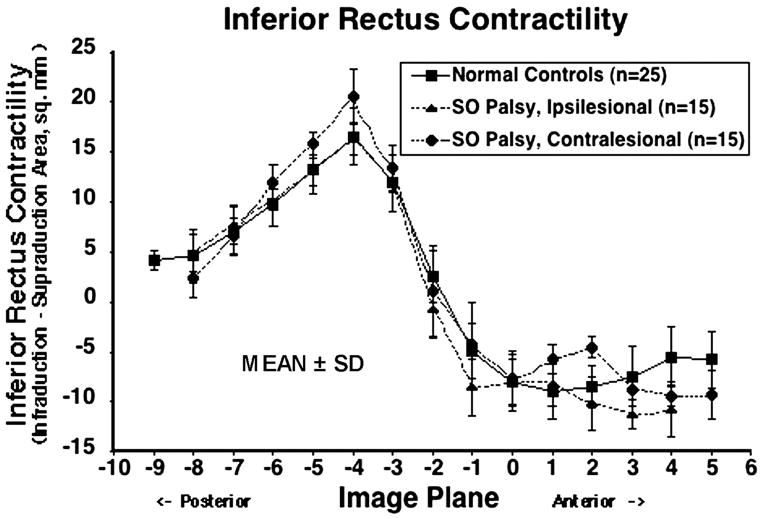
The contractile change in IR cross-section from supraduction to infraduction is plotted for each image plane in Fig. 6. Maximal contractile change for normal muscles was in image plane −4, 8 mm posterior to the globe–optic nerve junction, reflecting the corresponding posterior movements of the points of maximum cross-section from image plane −3, 6 mm posterior to the globe–optic nerve junction as the IR contracts in infraduction. 23 Ipsilesional IR contractility was significantly less than contralesional (P = 0.005) but did not significantly differ from normal controls (P=0.827). Contralesional contractility was slightly though significantly greater than normal (P = 0.012, Table 3).
Figure 6.
Contractile change in inferior rectus (IR) cross-sectional area from infraduction to supraduction for 25 normal control muscles, 15 ipsilesional and 15 contralesional SO muscles. SD = standard deviation. Ipsilesional IR contractility was significantly less than contralesional (P = 0.005) but did not significantly differ from normal controls. Contralesional contractility was slightly though significantly greater than normal (P = 0.012).
The 95% confidence interval for maximum normal IR cross-sectional area was 29.5 to 34.1 mm2. The 95% confidence interval for maximum normal IR contractility was 14.6 to 18.5 mm2. Cross sections and contractility of the contralesional IR were analyzed according to presentations of SO palsy. For the contralesional IR, maximum cross-section in presumed congenital cases was 28.9 ± 4.1 mm2, and 28.1 ± 3.3 mm2 in acquired cases. Maximum contralesional IR contractility in presumed congenital and acquired cases was 18.5 ± 4.6 mm2 and 15.5 ± 2.7 mm2, respectively. Neither difference was significant (P > 0.05).
Discussion
SO palsy is commonly diagnosed by strabismologists, 25 and has traditionally been defined indirectly on the basis of clinical observations of ocular versions and measurements of binocular misalignment. 1–4 This method is likely to be reliable in cases of the acute onset of SO palsy in a previously normal person who has experience a plausibly causative event such as neurosurgery or head trauma. However, the clinical presentation and findings may sometimes be misleading for the diagnosis of chronic or idiopathic SO palsy, particularly since the mechanical state of the SO muscle belly itself is inaccessible to direct study without use of MRI or computed tomography (CT). 26 Masquerading mechanical abnormalities including rectus pulley heterotopy10,11 and pulley instability may mimic clinical features of SO palsy. 12, 13 Central neural adaptations may also mimic features of SO palsy, including the head tilt response. For example, normal people who wear a vertical prism for only a short time develop a vertical phoria that changes with head tilt in a manner resembling SO paresis.27
Recent histological evidence shows in a primate model that the SO muscle develops severe atrophy within one year following after trochlear denervation, and that this atrophy can be demonstrated by MRI (Demer JL, Poukens V, Ying H, Shan X, et al. Effects of acute trochlear denervation on primate superior oblique muscle: Differential sparing of orbital layer. Paper presented at ARVO annual meeting, Apr 30, 2008; Fort Lauderdale). This basic observation supports use of high-resolution MRI as an objective, direct method for confirmation of SO palsy by imaging SO muscle size. Demonstration of subnormal SO size and contractility may be considered as securing the unequivocal diagnosis of SO palsy,6,28 and excluding other masquerading abnormalities. 6, 10–12, 28–30 While abnormalities of the SO tendon or its scleral insertion might occur in cases where the size and contractility of the SO belly might appear normal by MRI, the MRI evidence in such a hypothetical situation would lead to a false negative diagnosis of SO palsy. While not every palsied SO muscle would necessarily be hypoplastic, it does seem safe to presume that every hypoplastic SO muscle is palsied. The present sort of MRI evidence of SO atrophy and hypocontractility is sufficient for a reliable and unequivocal diagnosis of SO palsy, 6, 10–12, 28–30 with no likelihood of a false positive diagnosis. In the present study, the masquerading abnormalities were avoided by the strict diagnostic requirement of quantitative MRI demonstration of ipsilesional SO size less than the normal 95% confidence limit for orthotropic subjects, as well as clinical findings classically typical of SO palsy. These two standards reliably avoid false positive diagnoses of SO palsy in the current study.
Data of the current study are consistent with the previous report by Kono et al 28 that both contralesional SO cross section and contractility were slightly greater than in normal subjects. While it may not enlarge significantly, it seems clear that the contralesional SO at least does not undergo adaptive size reduction in pathologic ipsilesional SO weakness. The mechanism for this phenomenon is unclear, but it may be quite informative to indicate that not all chronic changes mediated by the brain are positive for helping restoring binocularity.
Biomechanical modeling consistently suggests that hypertropia observed in SO palsy is not due merely to ipsilesional SO weakness.14–16 Surgical weakening of the ipsilesional IO is not always sufficient treatment for hypertropia in SO palsy. Contralesional IR recession has been proven useful, especially when central gaze hypertropia exceeds 15 Δ.17 It might have been thought that contralesional IR recession is effective because the normally acting yoke IR muscle is weakened to match the paretic contralesional SO. Our study surprisingly indicates that the contralesional IR is hypertrophic and hypercontractile compared to the ipsilesional IR. This inference is secure, because in the current study each eye was scanned during central gaze fixation by the scanned eye, so instantaneous eye position did not influence apparent muscle size. The relatively smaller IR ipsilesional to SO palsy was not anticipated before the study. One might have guessed that the ipsilesional IR might have had to work harder in the presence of a weak atrophic SO, and would thus hypertrophy. On the contrary, we observed the ipsilesional IR to be smaller relative to contralesional and control IR muscles, a relationship that would worsen the vertical misalignment.
How do we explain the relative changes in the ipsilesional and contralesional IR muscles that perversely increase the hypertropia in SO palsy? One possibility is that size differences reflect represent neurologically mediated trophic changes that paradoxically increase the vertical deviation and worsen binocular misalignment. Such a paradox would have been highly implausible except for the recent observation of paradoxical, counterproductive alignment changes developing over time in a monkey model of acute, acquired SO palsy due to experimental trochlear denervation SO palsy.31 In monkeys who experience only monocular viewing following acute trochlear denervation, the vertical phoria due to acute SO palsy is small and decreases over time; however, when diplopic binocular viewing is restored, ipsilesional hypertropia increases and changes in comitance.31 In the primate SO palsy models, vertical misalignment increased even in abducted infraduction, the position in which there had been a small decrease in vertical misalignment during the immediate postlesion period of monocular patching. Changes over time in binocular alignment are difficult to explain except as neural adaptations that paradoxically increase the angle of strabismus. The changes in the ipsi- and contralesional IR muscles observed here in humans with SO palsy may be among the neurally-mediated effects that increase the vertical deviation in chronic SO palsy, and make it more noncomitant, when diplopic binocular vision is permitted. These neurologically mediated effects on IR size and contractility presumably serve some mysterious physiologic goal different from restoration of binocular fusion, which they clearly impair. Elucidation of the basis of these neurally mediated effects may significantly advance the understanding of the pathogenesis of strabismus generally.
Footnotes
Conflict of Interest: No conflicting relationship exists for any author.
Commercial Interest: None
Meeting Presentation: Presented at the Association for Research in Vision and Ophthalmology Annual Meeting, May, 2007
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knapp P. Diagnosis and surgical treatment of hypertropia. Am Orthop J. 1971;21:29–37. [PubMed] [Google Scholar]
- 2.Knapp P. Classification and treatment of superior oblique palsy. Am Orthop J. 1974;24:8–22. [PubMed] [Google Scholar]
- 3.Ellis FD, Helveston EM. Superior oblique palsy: diagnosis and classification. Int Ophthalmol Clin. 1976;16:127–35. [PubMed] [Google Scholar]
- 4.Flanders M, Draper J. Superior oblique palsy: diagnosis and treatment. Can J Ophthalmol. 1990;25:17–24. [PubMed] [Google Scholar]
- 5.Horton JC, Tsai RK, Truwit CL, Hoyt WF. Magnetic resonance imaging of superior oblique muscle atrophy in acquired trochlear nerve palsy [letter] Am J Ophthalmol. 1990;110:315–6. doi: 10.1016/s0002-9394(14)76358-5. [DOI] [PubMed] [Google Scholar]
- 6.Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995;36:906–13. [PubMed] [Google Scholar]
- 7.Ozkan S, Aribal ME, Sener EC, et al. Magnetic resonance imaging in evaluation of congenital and acquired superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1997;34:29–34. doi: 10.3928/0191-3913-19970101-07. [DOI] [PubMed] [Google Scholar]
- 8.Sato M, Yagasaki T, Kora T, Awaya S. Comparison of the muscle volume between congenital and acquired superior oblique palsies by magnetic resonance imaging. Jpn J Ophthalmol. 1998;42:466–70. doi: 10.1016/s0021-5155(98)00044-6. [DOI] [PubMed] [Google Scholar]
- 9.Sato M. Magnetic resonance imaging and tendon anomaly associated with congenital superior oblique palsy. Am J Ophthalmol. 1999;127:379–87. doi: 10.1016/s0002-9394(98)00329-8. [DOI] [PubMed] [Google Scholar]
- 10.Clark RA, Miller JM, Rosenbaum AL, et al. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 11.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G, editor. Advances in Strabismology. Buren, Netherlands: Aeolus Press; 1999. p. 92. [Google Scholar]
- 12.Oh SY, Clark RA, Velez F, et al. Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci. 2002;43:2169–78. [PubMed] [Google Scholar]
- 13.Pirouzian A, Goldberg RA, Demer JL. Inferior rectus pulley hindrance: Orbital imaging mechanism of restrictive hypertropia following lower lid surgery. J AAPOS. 2004;8:338–44. doi: 10.1016/j.jaapos.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Miller JM, Robinson DA. A model of the mechanics of binocular alignment. Comput Biomed Res. 1984;17:436–70. doi: 10.1016/0010-4809(84)90012-0. [DOI] [PubMed] [Google Scholar]
- 15.Robinson DA. Bielschowsky head-tilt test--II. Quantitative mechanics of the Bielschowsky head-tilt test. Vision Res. 1985;25:1983–8. doi: 10.1016/0042-6989(85)90023-9. [DOI] [PubMed] [Google Scholar]
- 16.Clark RA, Miller JM, Demer JL. Displacement of the medial rectus pulley in superior oblique palsy. Invest Ophthalmol Vis Sci. 1998;39:207–12. [PubMed] [Google Scholar]
- 17.Helveston EM, Mora JS, Lipsky SN, et al. Surgical treatment of superior oblique palsy. Trans Am Ophthalmol Soc. 1996;94:315–28. [PMC free article] [PubMed] [Google Scholar]
- 18.Clark RA, Rosenbaum AL, Demer JL. Magnetic resonance imaging after surgical transposition defines the anteroposterior location of the rectus muscle pulleys. J AAPOS. 1999;3:9–14. doi: 10.1016/s1091-8531(99)70088-1. [DOI] [PubMed] [Google Scholar]
- 19.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–97. [PubMed] [Google Scholar]
- 20.Kono R, Clark RA, Demer JL. Active pulleys: magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–88. [PubMed] [Google Scholar]
- 21.Oh SY, Poukens V, Cohen MS, Demer JL. Structure-function correlation of laminar vascularity in human rectus extraocular muscles. Invest Ophthalmol Vis Sci. 2001;42:17–22. [PubMed] [Google Scholar]
- 22.Velez FG, Clark RA, Demer JL. Facial asymmetry in superior oblique palsy and pulley heterotopy. J AAPOS. 2000;4:233–9. doi: 10.1067/mpa.2000.105277. [DOI] [PubMed] [Google Scholar]
- 23.Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–40. doi: 10.1016/0042-6989(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 24.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, Lampert R, editors. Clinical Strabismus Management: Principles and Techniques. Philadelphia: WB Saunders; 1999. p. 85. [Google Scholar]
- 25.Plager DA. Superior oblique palsy and superior oblique myokymia. In: Rosenbaum AL, Santiago AP, Lampert R, editors. Clinical Strabismus Management: Principles and Techniques. Philadelphia: WB Saunders; 1999. p. 219. [Google Scholar]
- 26.Kushner BJ. Errors in the three-step test in the diagnosis of vertical strabismus. Ophthalmol. 1989;96:127–32. doi: 10.1016/s0161-6420(89)32933-2. [DOI] [PubMed] [Google Scholar]
- 27.Guyton DL. Ocular torsion reveals the mechanisms of cyclovertical strabismus The Weisenfeld Lecture. Invest Ophthalmol Vis Sci. 2008;49:847–57. doi: 10.1167/iovs.07-0739. [DOI] [PubMed] [Google Scholar]
- 28.Kono R, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior oblique muscle in superior oblique palsy. Ophthalmol. 2003;110:1219–29. doi: 10.1016/S0161-6420(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 29.Demer JL. Clarity of words and thoughts about strabismus. Am J Ophthalmol. 2001;132:757–9. doi: 10.1016/s0002-9394(01)01099-6. [DOI] [PubMed] [Google Scholar]
- 30.Demer JL, Miller MJ, Koo EY, et al. True versus masquerading superior oblique palsies: muscle mechanisms revealed by magnetic resonance imaging. In: Lennerstrand G, editor. Update on Strabismus and Pediatric Ophthalmology. Boca Raton, FL: CRC Press; 1995. p. 305. [Google Scholar]
- 31.Shan XY, Tian J, Ying HS, et al. Acute superior oblique palsy in monkeys: I Changes in static eye alignment. Invest Ophthalmol Vis Sci. 2007;48:2602–11. doi: 10.1167/iovs.06-1316. [DOI] [PubMed] [Google Scholar]