Abstract
Background
The Male Specific Lethal (MSL) complex is enriched on the single X chromosome in male Drosophila cells and functions to upregulate X-linked gene expression and equalize X-linked gene dosage with XX females. The zinc finger protein Zn72D is required for productive splicing of the maleless (mle) transcript, which encodes an essential subunit of the MSL complex. In the absence of Zn72D, MLE levels are decreased, and as a result, the MSL complex no longer localizes to the X chromosome and dosage compensation is disrupted. To understand the molecular basis of Zn72D function, we identified proteins that interact with Zn72D.
Results
Among several proteins that associate with Zn72D, we found the DEAD box helicase Belle (Bel). Simultaneous knockdown of Zn72D and bel restored MSL complex localization to the X chromosome and dosage compensation. MLE protein was restored to 70% of wild-type levels, although the level of productively spliced mle transcript was still four-fold lower than in wild-type cells. The increase in production of MLE protein relative to the amount of correctly spliced mle mRNA could not be attributed to an alteration in MLE stability.
Conclusion
These data indicate that Zn72D and Bel work together to control mle splicing and protein levels. Thus Zn72D and Bel may be factors that coordinate splicing and translational regulation.
Background
There is increasing evidence linking the processes involved in gene expression, such as transcription, splicing, mRNA localization, and translation. For example, splicing factors, such as hnRNP proteins and the serine/arginine-rich (SR) protein SF2/ASF, can remain associated with mature transcripts, shuttle to the cytoplasm, and regulate translation [1-4]. For the Drosophila oskar transcript, splicing of the first intron is required for proper localization of the transcript to the posterior end of the oocyte, where it is locally translated [5]. Correct localization of this transcript also depends on the exon junction complex components Y14 and Mago nashi [6-8]. Despite evidence for coordination of splicing, mRNA localization, and translation, the mechanisms underlying this coordination remain poorly characterized.
We previously demonstrated that the Drosophila zinc finger protein Zn72D is required for proper splicing of the maleless (mle) transcript. The MLE protein is an essential component of the Male Specific Lethal (MSL) complex (also known as the Dosage Compensation Complex). The MSL complex is enriched on the sole X chromosome in male cells, where it upregulates gene expression two-fold to equalize gene dosage between XY males and XX females [9]. Zn72D is necessary for productive splicing of mle mRNA. Without Zn72D, the majority of mle transcripts preferentially retain part of the second intron, which contains in-frame stop codons, and therefore MLE protein is not produced at normal levels [10]. As a result, the MSL complex does not localize to the X chromosome and dosage compensation does not occur.
To further explore the role of Zn72D, we used mass spectrometry to identify proteins that interact with HA-tagged Zn72D. The majority of proteins that co-immunoprecipitate (co-IP) with Zn72D are involved in some aspect of RNA metabolism. One Zn72D-associated protein is the DEAD box helicase Bel, which is implicated in translational regulation [11]. Since Bel associated with Zn72D, we asked whether Bel played a role in regulating mle gene expression. Depletion of bel alone did not significantly affect the level of MLE or localization of the MSL complex. However, simultaneous knockdown of Zn72D and bel rescued X chromosome localization of the MSL complex. The level of MLE protein was restored to ~70% of wild-type in the double knockdown, even though the level of productively spliced mle transcripts was still four-fold lower than in wild-type cells. These data indicate that Zn72D and Bel work together to control mle splicing and protein levels and suggest that Zn72D and Bel may target spliced mRNAs for localized, regulated translation in the cytoplasm.
Results and discussion
Identification of proteins that interact with Zn72D
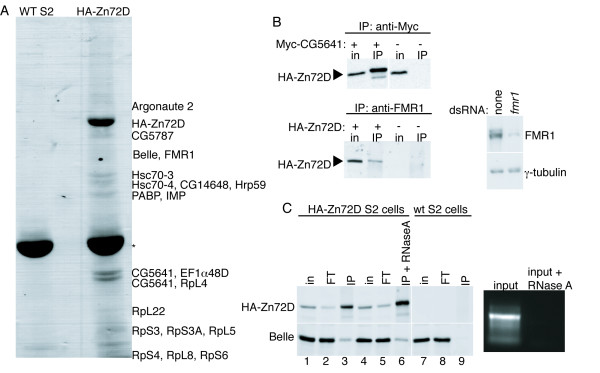
To gain additional insight into the molecular functions of Zn72D, we carried out tandem mass spectrometric analysis to identify proteins that physically interact with HA-tagged Zn72D in Drosophila S2 cells. Proteins that co-IP with anti-HA antibody in S2 cells expressing HA-Zn72D but not in wild-type S2 cells include: Bel, Elongation factor 1α48D (EF1α48D), Fragile × Mental Retardation protein (FMR1), Hrp59, insulin growth factor II mRNA-binding protein (IMP), Argonaute 2 (Ago2), Poly A Binding Protein (PABP), Heat shock cognate proteins 3 and 4 (Hsc70-3 and -4), several ribosomal proteins, and three proteins of unknown function, CG5787, CG14648, and CG5641 (Figure 1A and Table 1). Zn72D was previously shown to interact with eight proteins in a large-scale two-hybrid protein-protein interaction analysis [12]. Among these proteins, the only one identified as a Zn72D-interacting protein in our assay was CG5641, which has a 2'5'-oligoadenylate synthase motif and a DZF domain of unknown function also found in Zn72D. We confirmed that CG5641 and two additional proteins, FMR1 and Bel, co-IP with HA-Zn72D by western blotting (Figure 1B and 1C). Zn72D and four of the Zn72D-interacting proteins, Bel, FMR1, CG5641, and Hrp59, display a common pattern of localization during stages 13–16 of embryonic development [13-15], suggesting the possibility that the interaction of these proteins may play a role in regulation of central nervous system development.
Figure 1.
Identification of proteins that co-immunoprecipitate with Zn72D. (A) IPs were performed with an anti-HA antibody in wild-type (wt) S2 cells and in S2 cells expressing HA-Zn72D. Proteins that co-IP with HA-Zn72D were identified by mass spectrometry. (B) (Top) HA-Zn72D co-IPs with Myc-CG5641 when cells expressing HA-Zn72D were transiently transfected with a vector expressing Myc-CG5641 (left 2 lanes). IPs were performed using an anti-Myc antibody, and immunoblotting was performed with an HA antibody. Right 2 lanes are controls in which no Myc-CG5641 was transfected. (Bottom left) HA-Zn72D co-IPs with FMR1. IPs were performed using an anti-FMR1 antibody and immunoblotting was performed to detect HA. IPs were performed in cells that did (2 left lanes) and did not (2 right lanes) express HA-Zn72D. (Bottom right) The FMR1 antibody specifically recognizes FMR1 protein. S2 cell extracts from untreated and FMR1 knockdown cells were probed with anti-FMR1 and anti-γ-tubulin. (C) The interaction between Zn72D and Bel is RNA-independent. IPs using anti-HA antibody were performed on S2 cells expressing HA-Zn72D, in the absence (lane 3) or presence (lane 6) of RNase A. Lane 9 is a control IP performed with an anti-HA antibody using S2 cells not expressing HA-Zn72D. HA and Bel were detected using immunoblotting. The ethidium bromide stained gel to the right shows equivalent amounts of input extract loaded per lane, either untreated or treated with RNase A. For parts B and C, "in" indicates 2% of the input into each IP and "FT" indicates 2% of flow-through from each IP.
Table 1.
Identification of Zn72D-interacting proteins by mass spectrometry
| Annotation Symbol | Name | Number of Peptides | % Coverage |
| CG7349 | Argonaute 2 | 6 | 7 |
| CG5215 | HA-Zn72D | 44 | 43 |
| CG5787 | CG5787 | 6 | 5 |
| CG9748 | Belle | 4 | 5 |
| CG6203 | FMR1 | 2 | 3 |
| CG4147 | Hsc70-3 | 28 | 41 |
| CG4264 | Hsc70-4 | 16 | 26 |
| CG14648 | CG14648 | 8 | 14 |
| CG9393 | Hrp59 | 2 | 3 |
| CG5119 | PABP | 11 | 25 |
| CG1691 | IMP | 2 | 3 |
| CG5641 | CG5641 | 21 | 49 |
| CG8280 | EF1α48D | 6 | 12 |
| CG5641 | CG5641 | 25 | 55 |
| CG5502 | RpL4 | 3 | 6 |
| CG7434 | RpL22 | 3 | 16 |
| CG6779 | RpS3 | 7 | 23 |
| CG2168 | RpS3A | 6 | 20 |
| CG17489 | RpL5 | 4 | 18 |
| CG11276 | RpS4 | 18 | 64 |
| CG1263 | RpL8 | 4 | 17 |
| CG10944 | RpS6 | 3 | 10 |
While Zn72D is implicated regulating splicing of mle mRNA [10], the only Zn72D-interacting protein with a known role in splicing is Hrp59 [16,17]. Hrp59 depletion did not phenocopy Zn72D knockdown (data not shown), suggesting that Zn72D is unlikely to regulate mle splicing solely through Hrp59. The majority of the Zn72D-interacting proteins are involved or implicated in other aspects of RNA metabolism, including transport/nucleocytoplasmic shuttling of RNAs, RNA binding, RNA localization, translation, and RNA interference [18,11-26]. These data suggest that the mle mRNA splicing regulation by Zn72D may be indirect.
Simultaneous knockdown of Zn72D and bel restored MSL complex localization to the X chromosome and X-linked gene expression
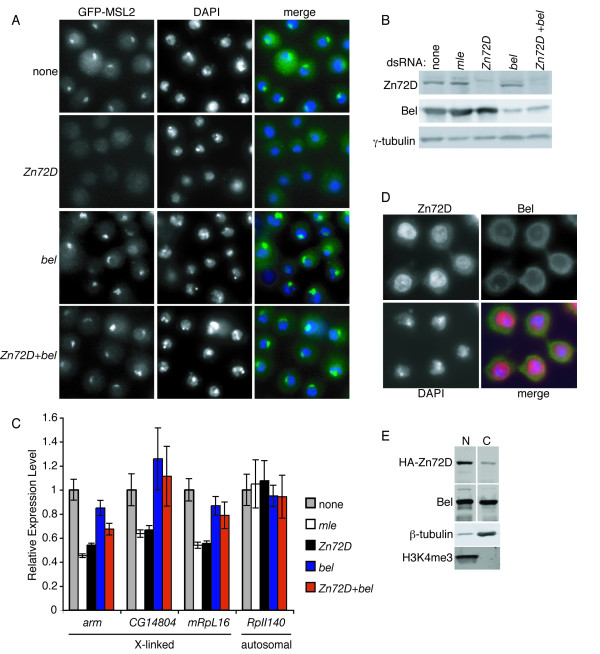
We previously showed that Zn72D is necessary for generating productively spliced mle mRNA and assembly of the MSL complex on the X chromosome [10]. We tested whether knockdown of any protein that co-IPed with Zn72D would phenocopy Zn72D knockdown. None of these proteins were necessary for MSL complex localization, as assayed by accumulation of GFP-MSL2 on the X chromosome in S2 cells (Figure 2A and data not shown). Depletion of pAbp, RpL4, RpS3, RpS3A, RpS4, and RpS6 resulted in cell death, consistent with an essential role for these proteins. Argonaute 2 knockdown caused upregulation of GFP-MSL2, as did knockdown of another protein that regulates RNAi, Dicer-2 (data not shown). Upregulation of endogenous MSL2 was not observed when either Argonaute 2 or Dicer-2 was knocked down (data not shown), suggesting that upregulation of GFP-MSL2 was due to disruption of the Argonaute 2/Dicer-2 RNAi pathway. We also knocked down each protein that co-IPed with Zn72D in combination with Zn72D knockdown. We found that for one co-knockdown, Zn72D and bel, MSL complex localization to the X chromosome was restored (Figure 2A). Zn72D knockdown was equally effective alone or in combination with bel knockdown, as assayed by western blotting (Figure 2B). This suggests that knockdown of bel in cells depleted of Zn72D allows for production of sufficient MLE protein for assembly of the MSL complex.
Figure 2.
Co-knockdown of Zn72D and bel restores MSL localization and X-linked gene expression. (A) GFP-MSL2 expressing S2 cells were either untreated or treated with Zn72D, bel, or Zn72D+bel dsRNAs. The left panels show GFP fluorescence (green), the middle panels show DAPI staining to highlight the nucleus (blue), and right panels show the merged image. (B) Western blots with anti-Zn72D (top), anti-Bel (middle), or anti-γ-tubulin (bottom) as a loading control, in knockdowns indicated above. (C) qRT-PCR for the X-linked genes arm, CG14804, and mRpL16 and the autosomal gene RpII140 was done after S2 cells were untreated (gray bars), or treated with dsRNAs to knockdown mle (white), Zn72D (black), bel (blue), or Zn72D+bel (red). Samples were normalized first to rp49 and then to the untreated sample, setting untreated to 1. The error bars represent the average of three independent experiments, with qPCR performed in triplicate, and error was determined using standard error propagation methods. (D) Zn72D and Bel are both present in the cytoplasm. Cells were stained with antibodies to HA (red), Bel (green), and DAPI (blue) to delineate the nucleus. The nucleolar staining in the anti-Bel image was the result of antibody cross-reactivity, as when bel is knocked down, the nucleolar staining remained while the Bel staining decreased (data not shown). (E) Zn72D and Bel are present in both nuclear and cytoplasmic extracts. The cytoplasmic extract is enriched for β-tubulin and depleted for H3K4me3, whereas the nuclear extract is depleted for β-tubulin and enriched for H3K4me3.
Zn72D is required for proper X-linked gene expression in males [10]. We asked whether, in addition to restoring MSL complex localization to the X chromosome, combined knockdown of Zn72D and Bel returned X-linked gene expression to normal. S2 cells were either untreated or treated with dsRNAs against mle, Zn72D, bel, or Zn72D+bel, and levels of three X-linked transcripts and one control autosomal transcript were assayed by quantitative reverse transcription PCR (qRT-PCR). Expression of the autosomal gene RpII140 was unaffected by any of the knockdowns. The levels of the X-linked transcripts arm, CG14804, and mRpL16 were decreased by approximately twofold upon knockdown of mle or Zn72D (Figure 2C), consistent with a defect in dosage compensation. Knockdown of bel had little to no affect on X-linked gene expression (Figure 2C). Co-knockdown of Zn72D and bel restored X-linked gene expression of CG14804 and RpL16 and partially restored expression of arm (Figure 2C). Therefore, in addition to restoring MSL complex localization to the X chromosome, depletion of both Zn72D and bel either completely or partially restored proper X-linked gene expression.
Since our data suggest that Bel and Zn72D physically interact and both function in regulation of mle expression, we examined whether these proteins co-localize. While Zn72D is predominantly nuclear, it can also be detected in the cytoplasm, and Bel is detected in both the nucleus and cytoplasm (Figure 2D and 2E). Thus, Zn72D and Bel may interact in the nucleus, cytoplasm, or both.
Since both Bel, a DEAD box helicase, and Zn72D are potential RNA binding proteins, we examined whether their interaction was RNA-dependent. We assayed co-IP of these proteins in the presence or absence of RNase A (Figure 1C). Bel and HA-Zn72D co-IPed whether or not RNase A was added to the extract, indicating that the interaction between Zn72D and Bel is independent of RNA. More HA-Zn72D was immunoprecipitated from RNase A treated extracts than from untreated extracts, suggesting that a fraction of Zn72D may be present in RNA containing complexes and released upon RNase A treatment. Because these co-immunoprecipitations are not quantitative, it is possible that a fraction of the Zn72D that interacts with Bel is in an RNA containing complex.
Co-knockdown of Zn72D and bel partially rescues mle mRNA splicing
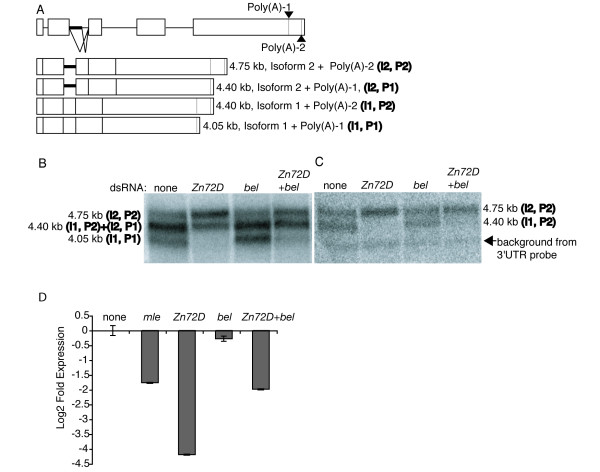
We next examined whether depletion of bel rescues the mle mRNA splicing defect that occurs upon Zn72D knockdown. Northern blot analysis of wild-type whole cell RNA with a cDNA probe showed three bands, which represent the two spliced forms (I1 and I2, with I1 indicating the productively spliced transcripts and I2 indicating the aberrantly spliced transcripts) combined with two polyadenylation sites (P1 and P2) (Figure 3A and 3B). Because the two splice sites are approximately the same distance apart as the two polyadenylation sites, the middle band represents a mixture of two isoforms (I1, P2 and I2, P1), while the upper and lower bands each represent a single isoform (I2, P2 and I1, P1 respectively). The Zn72D knockdown cells showed a considerable decrease in the intensity of the lower and middle bands (Figure 3B). Northern blot analysis using a 3'UTR probe, which detects only the P2 isoforms, showed that the decrease in intensity of the middle band was due to altered spliced site usage and not altered polyadenylation (Figure 3C). qRT-PCR, employing a primer set that specifically amplifies productively spliced mle transcripts [10], showed a 16-fold decrease in correctly spliced mle mRNA in Zn72D knockdown cells relative to wild-type cells (Figure 3D). Thus, there is a substantial decrease in levels of productively spliced mle transcripts upon Zn72D depletion, as previously reported [10].
Figure 3.
Co-knockdown of Zn72D and bel partially restores productively spliced mle mRNA levels. (A) Depicted is the mle gene, which contains 5 exons (white boxes) and 4 introns (black horizontal lines). Poly(A)-1 and -2 are depicted as gray vertical lines within the fifth exon. (Bottom) Four mle transcript isoforms differ in retention of part of intron 2 (Isoforms I1 and I2) and usage of the upstream or downstream poly(A) sites (P1 and P2). The partially retained intron is shown as a thicker horizontal black line. (B) Northern analysis with RNA collected from S2 cells untreated or treated with dsRNAs to Zn72D, bel, or Zn72D+bel. The blot was probed with a probe antisense to mle cDNA. The top band corresponds to isoform I2, P2; the middle band corresponds to both isoforms I1, P2 and I2, P1; and lower band corresponds to isoform I1, P1. In the absence of Zn72D, only I2 isoforms remain. Upon Zn72D+bel knockdown, there are predominantly I2 isoforms but a subtle increase in I1 isoforms compared to Zn72D knockdown. (C) The blot on the left was stripped and reprobed with a probe located between the two poly(A) sites. This probe only recognizes the P2 isoforms. There are similar slight increases in the level of correctly spliced I1 isoforms when comparing the left and right blots, indicating no difference in recovery of the two isoforms of mle mRNA that use alternative poly(A) sites. (D) S2 cells were untreated or treated with mle, Zn72D, bel, or Zn72D+bel dsRNAs and qRT-PCR was performed with primers to the mle transcript that specifically amplify isoform 1. Samples were normalized first to rp49 and then to the untreated sample, setting untreated to 1. The error bars represent the average of four independent experiments, with qPCR performed in triplicate, and error was determined using standard error propagation methods.
Cells depleted for bel showed a similar distribution of mle mRNA isoforms as wild type cells (Figure 3B and 3C). Levels of productively spliced mle mRNA were slightly decreased upon knockdown of bel (Figure 3D). Thus bel knockdown alone does not significantly affect production of productively spliced mle mRNA. The combined knockdown of Zn72D+bel showed a four-fold decrease in the abundance of productively spliced mle mRNA isoforms (Figure 3B and 3C), a decrease that was not as great as that observed upon depletion of Zn72D alone (Fig. 3D). A similar result was observed when Zn72D and bel were knocked down in the female Drosophila Kc cell line, indicating that the effect on spliced mle transcript levels was not the result of improper dosage compensation in males (data not shown). Thus, there is some recovery of splicing of the mle transcript when Zn72D and bel are co-depleted, compared to knockdown of Zn72D alone, suggesting that bel may inhibit productive splicing of mle mRNA. However, bel knockdown alone does not increase the amount of productively spliced mle mRNA, indicating that bel depletion must be combined with Zn72D depletion for bel depletion to effect mle splicing. These data suggest that Bel and Zn72D may function together in regulation of mle mRNA processing.
Zn72D and Bel regulate MLE protein levels
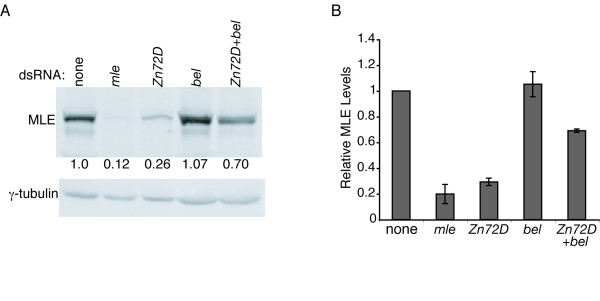
Since co-knockdown of Zn72D and bel resulted in restoration of MSL complex localization to the X chromosome, we expected that more MLE protein would be produced than when only Zn72D was knocked down. Quantitative western blots for MLE protein were performed on S2 cell extracts in which mle, Zn72D, bel or Zn72D+bel had been knocked down (Figure 4A and 4B). In the absence of Zn72D, the level of MLE protein decreased. Upon knockdown of bel, MLE protein levels were unaffected. When Zn72D and bel were knocked down together, MLE protein levels were restored to 70% of wild-type levels. This 70% of MLE protein is produced from 4-fold less productively spliced mle mRNA than is present in wild-type cells.
Figure 4.
Co-knockdown of Zn72D and bel restored MLE protein levels to 70% of normal levels. (A) One representative quantitative western blot for MLE protein upon knockdown of mle, zn72d, bel, or zn72d+bel. (B) MLE levels were quantitated by first normalizing to the amount of γ-tubulin and then averaging the numbers obtained from the 3 experiments with 2 technical replicates per experiment. Standard deviation was found for the average of each experiment.
As high levels of protein are produced from a comparably small amount of mle transcript in the absence of Zn72D and bel, this suggests that perhaps the mle transcript is translated more efficiently or that MLE protein stability is increased in double knockdown cells. MLE protein levels did not change appreciably after treating cells for 8 hours with cycloheximide, suggesting that MLE is not an unstable protein (Additional File 1). In addition, MLE half-life was not affected by co-knockdown of Zn72D and bel, providing further evidence that the combined knockdown of Zn72D and bel does not affect the abundance of MLE protein by increasing its stability (data not shown). Therefore, these results suggest that co-knockdown of Zn72D and bel may release mle mRNA from translational inhibition, such that high levels of MLE protein are produced in spite of the decreased levels of productively spliced mle transcripts. This effect on translation could be through direct translational regulation or through regulation of cytoplasmic localization of mle transcripts, thus influencing translation efficiency.
The combined knockdown of Zn72D and bel resulted in increased levels of MLE protein from decreased amounts of productively spliced mle mRNA. Knockdown of bel alone did not have an appreciable affect on mle expression at either the mRNA or protein level. Together these data suggest that Bel may function, via its interaction with Zn72D, to regulate translation or transport of mle mRNA. There is considerable evidence implicating Bel in translational regulation: 1) Bel colocalizes at the oocyte posterior with the translational regulator and DEAD box helicase Vasa, and Vasa is required for the proper localization of Bel, thus implicating Bel as having a role in regulating local translation [11]. 2) bel knockdown in S2 cells reduced the amount of β-gal produced from an inducible LacZ transgene, without altering the abundance of LacZ mRNA [27]. 3) Bel is homologous to and can functionally substitute for the DEAD box helicase Ded1p protein in yeast, which is required for translation [28,11]. Biochemical and genetic evidence suggest that Ded1p plays a role, not only in translation, but also in splicing [29-31]. 4) Like Ded1p, the human Bel homologue, the DEAD box helicase DDX3, is a translational regulator that is also associated with the spliceosome [27,32-34]. DDX3 acts either as a translational repressor or activator, depending on the target mRNA [35,27,33]. These data have led to a model in which DDX3/Ded1p is loaded onto mRNA in the nucleus and then functions in translation in the cytoplasm [36]. This is particularly interesting, since Bel interacts with Zn72D, which regulates mle mRNA splicing. By associating with factors that regulate splicing, DDX3 and Bel may be recruited to specific transcripts to regulate their translation.
In addition to its role as a translational regulator, DDX3 is also implicated as a factor important for mRNA export and in localizing mRNAs for translation [37,35,39]. Recently, it was demonstrated that knockdown of bel does not affect nuclear export of bulk mRNA [27]. We found that Bel does not regulate nuclear export of mle transcripts, as we found that mle transcripts accumulated in the cytoplasm when bel was knocked down and when Zn72D and bel were knocked down together (Additional File 2). However, it is possible that Bel affects localization of mle mRNA within the cytoplasm to regulate translation. This would be consistent with Bel being specifically localized with Vasa at the posterior of the oocyte, where transcripts are deposited for localized translation.
The aforementioned data implicate Bel and its homologues in regulating gene expression at the level of mRNA localization and translation. Knockdown of bel by itself had no significant effect on MLE protein levels (Figure 4). Only when Bel depletion was combined with Zn72D depletion was an effect on MLE protein evident. This suggests two possibilities. First, that Bel is a weak suppressor of mle mRNA translation (either directly or via regulating cytoplasmic localization), such that when mle mRNA levels are high, Bel's effect on MLE protein levels is not significant. Alternatively, it may be that Zn72D blocks Bel activity on mle transcripts, and that these two proteins work together to finely tune production of MLE protein.
Why is it important to regulate MLE protein levels? MLE localizes to all chromosomes and throughout the nucleus when overexpressed [40,10]. Incorrect MLE expression is detrimental to the development of the fly, because heat shock over-expression of transgenic MLE protein results in male and female lethality [40]. It is possible that translational repression by Zn72D and Bel is one mechanism by which levels of MLE protein are tightly controlled. Expression of a transgenic mle cDNA in S2 cells resulted in overproduction of MLE protein; however, inclusion of the first two introns in the same transgene reduced the amount of MLE protein produced from the transgene [10]. This suggests that perhaps recruitment of Zn72D to the mle transcript has the effect of not only productively splicing the transcript but also targeting it for translational regulation.
Like Zn72D, its human homologue ZFR is also found mainly in the nucleus, with a subset in the cytoplasm. Cytoplasmic ZFR colocalizes in neuronal granules with Staufen2, a protein involved in mRNA transport and localization. ZFR interacts with and is required for cytoplasmic localization of the Staufen262 isoform [41]. As neuronal granules are involved in translational regulation and localization of mRNAs, these data suggest that ZFR may have a role regulating cytoplasmic localization of mRNAs. If Zn72D has a similar function in flies, it has the potential to regulate gene expression at two steps. Zn72D may first promote productive splicing of mRNAs and then later affect their cytoplasmic localization, which in turn may impact translation.
Conclusion
We identified several proteins that interact with Zn72D, including the DEAD box helicase Bel. Co-knockdown of both bel and Zn72D restores the MSL complex localization to the X chromosome and dosage compensation of X-linked genes that was lost in the absence of Zn72D. In addition, we found that co-knockdown of Zn72D and bel resulted in restoration of MLE protein levels to 70% of wild-type levels, despite a four-fold reduction in properly spliced mle mRNA. These data implicate Zn72D and bel as being factors that target spliced mRNAs for localized, regulated translation in the cytoplasm.
Methods
Cell Culture and Generation of Stable Cell Lines
S2 cells were grown in Schneider's media plus 10% fetal bovine serum, penicillin and streptomycin. Cells were maintained according to the Invitrogen Drosophila Expression System Protocol. S2 cell lines expressing GFP-MSL2 and HA-Zn72D were described previously [10]. pAM-CG5641 (Myc-CG5641) was cloned using the Invitrogen Gateway system. 20 μg of the plasmid plus 1 μg pCoBlast (Invitrogen) was transfected into S2 cells using the protocol described in the Invitrogen Drosophila Expression System Protocol and selected with 15 μg/mL Blasticidin S HCl.
Co-immunoprecipitation
S2 cells from a 10 cm dish were washed with 1×PBS and lysed in lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% TritonX-100, supplemented with protease inhibitors and RNasin [Promega]). For Co-IPs that were performed in the presence of RNase A, RNAsin was left out of the buffer and RNAse A was added at a concentration of 0.1 mg/mL after sonication. The lysate was sonicated three times, 10 seconds each (on ice for one minute in between pulses) on setting 3, constant duty cycle on a Branson sonicator. The extract was clarified by centrifugation at 14,000 rpm for 15 min. at 4 degrees C. At least 600 ug extract was added to the 10 μL of Dynal Dynabeads (Invitrogen), precaptured with the appropriate antibody. Beads and extracts were rotated overnight at 4 degrees C and then washed 3 times with lysis buffer. Proteins were boiled off the beads in 1× sample buffer. (2× sample buffer: 8.3% glycerol, 1.25% SDS, 0.1 M Tris-HCl pH 6.7, 0.083 mg/mL bromophenol blue, 50 μL/mL 2-mercaptoethanol.) For large scale IPs for mass spectrometry, 45 mg of clarified S2 cell extract was added to 250 μL protein G Dynal Dynabeads preincubated with 25 μL HA.11 antibody (Covance). The proteins boiled off the beads were loaded on a 7.8% SDS-PAGE gel and which was later stained with G-250 coomassie blue.
Cellular fractionation
Nuclear and cytoplasmic extracts were prepared as described in [42] (nuclear) and in [43] (cytoplasmic).
On-line Capillary LC-MS and LC-MS-MS Analysis
Affinity-purified Zn72D-containing samples were separated by SDS-PAGE gel, in-gel digested and analyzed by LC-MS and LC-MS-MS as described previously [44]. Briefly, 1 μl aliquot of the digestion mixture was injected into an Ultimate capillary LC system via a FAMOS Autosampler (LC Packings, Sunnyvale, CA), and separated by a 75 μm × 15 cm reverse-phase capillary column at a flow rate of ~330 nL/min. The HPLC eluent was connected directly to the micro-ion electrospray source of a QSTAR Pulsar QqTOF mass spectrometer (Applied Biosystem/MDS Sciex, Foster City, CA). Typical performance characteristics were > 8000 resolution with 30 ppm mass measurement accuracy in both MS and CID mode. LC-MS data were acquired in an information-dependent acquisition mode, cycling between 1-s MS acquisition followed by 3-s low energy CID data acquisition. The centroided peak lists of the CID spectra were searched against the National Center for Biotechnology Information (NCBI) Drosophila melanogaster protein database using Batch-Tag, a program in the in-house version of the University of California San Francisco ProteinProspector package. The CID spectra were further inspected manually. Protein hits with more than two confident MSMS spectra are reported in the table.
Quantitative western blotting
S2 cells +/- dsRNA treatment for 6 days were counted, spun down, and lysed in 1× Sample buffer (2× sample buffer: 8.3% glycerol, 1.25% SDS, 0.1 M Tris-HCl pH 6.7, 0.083 mg/mL bromophenol blue, 50 μL/mL 2-mercaptoethanol) at 5 × 104 cells/μL. Samples were boiled for 5 minutes and spun down at 14,000 rpm at 4 degrees C for 20 minutes. 15 μL lysate was loaded per lane of a 7.8% gel. Gels were transferred to nitrocellulose, blocked with 1% nonfat dry milk/0.05% Tween/1×PBS and probed overnight at 4 degrees with mouse anti-HA antibody at a 1:2000 dilution (HA.11, Covance), guinea pig anti-MLE antibody at 1:500 (gift from John Lucchesi), mouse anti γ-tubulin at 1:1000 (GTU-88, Sigma), mouse anti β-tubulin at 1:1000 (Developmental Studies Hybridoma Bank), rabbit anti-Bel at 1:2500 (gift from Paul Lasko), and chicken anti-Zn72D serum at 1:200 [10]. Donkey anti-guinea pig Cy3 (1:1000), anti-mouse Cy3 (1:500), anti-rabbit (1:1000), and anti-chicken (1:500) (Jackson ImmunoResearch), were used as a secondary antibodies, detected using a Typhoon 9400 instrument and quantitated using Quantity One software (Bio-Rad). MLE levels were normalized to γ-tubulin.
RNAi
dsRNAs were added to S2 cells (~15 μg/mL) in 50% conditioned/50% fresh Schneider's media. The following primers were used to produce PCR products, which were used in T7 in-vitro transcription reactions to produce dsRNA:
mle(s): GGGCGGGTTTATGGCTTCGTACTCTAGCACC,
mle(as): GGGCGGGTAAGTTAAGCCAGTTGTCAACGC,
bel(s): GGGCGGGTGTCTGGACTTGAATGGCGGC,
bel(as): GGGCGGGTCGTTGTAGTTGTCCTCGAAACGTC,
Zn72D(s): GGGCGGGTGTTGAACTTACAATCGCACAGC,
Zn72D(as): GGGCGGGTACTGGTATCAGCGTAAGATGGG,
Zn72D3'UTR(s): GGGCGGGTGCGGCGAGAATAGGTTATATAC,
Zn72D3'UTR(as): GGGCGGGTCCGCTTCGTTCTAGTATTTGTG.
qRT-PCR
qRT-PCR on whole cell RNA was performed as described previously[10]. The primers used are described below:
mle QF1: CGGAACACGCTAGGAGCTTT, QR1: TGAGCGCCGGCACAT;
rp49 QF3: GCCGTAATTGTCGTTTTTGG, QR3: CGAACAGCGCACGGACTA;
mRpL16 QF2: TCAACACAGCCGGTCTTAAGTAT, QR2: GGCTGCTCCACATTCTGGTA; arm-F GCTGCTGAACGATGAGGATCA, arm-R: CCAAAGCGGCTACCATCTGA; CG14804-F: CTGAGCACAAGACGGCAGAG, CG14804-R:GAGGGTCACGTTCACCTTGC;
RpII140-F: CACAATGGCGGCGGTT, RpII140-R: ACGCAGATGTTCAGGCAGAGT [45].
Northern blotting
Northern blots were performed with a NorthernMax Kit (Ambion) and BrightStar-Plus membrane (Ambion). 10 μg of RNA of whole cell extracts from S2 cells treated +/- Zn72D dsRNA for 6 days was loaded per lane. Blots were probed with a labeled DNA probe antisense to the full-length mle cDNA or to the 3'UTR of mle between the polyadenlyation sites, and exposed to a phosphor screen overnight.
Authors' contributions
KAW and FC carried out the experiments. KW conceived the study and drafted the manuscript. BP participated in the study design and helped to draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Supplemental Fig. 1. Over 8 hours of cycloheximide (chx) treatment, MLE protein levels do not increase.
Supplemental Fig. 2. mle mRNA is present in the cytoplasm when bel and Zn72D+bel are knocked down.
Acknowledgments
Acknowledgements
We thank Tony Shermoen for his continual support with the RNAi library and Brian Margolin for help with statistical analysis. We greatly appreciate the generosity of Paul Lasko for providing the Bel antibody and John Lucchesi for providing the MLE antibody. We thank Thomas Fazzio and Morgan Royce-Tolland for critical reading of this manuscript. This work was supported by the ARCS Foundation (K.A.W.) and the NIH (B.P).
Contributor Information
Kathleen A Worringer, Email: kworringer@gladstone.ucsf.edu.
Feixia Chu, Email: fvy2@unh.edu.
Barbara Panning, Email: barbara.panning@ucsf.edu.
References
- Huang Y, Gattoni R, Stevenin J, Steitz JA. SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol Cell. 2003;11:837–843. doi: 10.1016/S1097-2765(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Steitz JA. Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol Cell. 2001;7:899–905. doi: 10.1016/S1097-2765(01)00233-7. [DOI] [PubMed] [Google Scholar]
- Sanford JR, Gray NK, Beckmann K, Caceres JF. A novel role for shuttling SR proteins in mRNA translation. Genes Dev. 2004;18:755–768. doi: 10.1101/gad.286404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres JF, Screaton GR, Krainer AR. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev. 1998;12:55–66. doi: 10.1101/gad.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachet O, Ephrussi A. Splicing of oskar RNA in the nucleus is coupled to its cytoplasmic localization. Nature. 2004;428:959–963. doi: 10.1038/nature02521. [DOI] [PubMed] [Google Scholar]
- Hachet O, Ephrussi A. Drosophila Y14 shuttles to the posterior of the oocyte and is required for oskar mRNA transport. Curr Biol. 2001;11:1666–1674. doi: 10.1016/S0960-9822(01)00508-5. [DOI] [PubMed] [Google Scholar]
- Mohr SE, Dillon ST, Boswell RE. The RNA-binding protein Tsunagi interacts with Mago Nashi to establish polarity and localize oskar mRNA during Drosophila oogenesis. Genes Dev. 2001;15:2886–2899. doi: 10.1101/gad.927001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmark PA, Boswell RE. The mago nashi locus encodes an essential product required for germ plasm assembly in Drosophila. Development. 1994;120:1303–1313. doi: 10.1242/dev.120.5.1303. [DOI] [PubMed] [Google Scholar]
- Lucchesi JC, Kelly WG, Panning B. Chromatin remodeling in dosage compensation. Annu Rev Genet. 2005;39:615–651. doi: 10.1146/annurev.genet.39.073003.094210. [DOI] [PubMed] [Google Scholar]
- Worringer KA, Panning B. Zinc finger protein Zn72D promotes productive splicing of the maleless transcript. Mol Cell Biol. 2007;27:8760–8769. doi: 10.1128/MCB.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone O, Deuring R, Bock R, Linder P, Fuller MT, Lasko P. Belle is a Drosophila DEAD-box protein required for viability and in the germ line. Dev Biol. 2005;277:92–101. doi: 10.1016/j.ydbio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao YL, Ooi CE, Godwin B, Vitols E, et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- FlyExpress An image-matching web-tool for finding genes with overlapping patterns of expression in Drosophila embryos http://www.flyexpress.net
- Wan L, Dockendorff TC, Jongens TA, Dreyfuss G. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile × mental retardation protein. Mol Cell Biol. 2000;20:8536–8547. doi: 10.1128/MCB.20.22.8536-8547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomancak P, Berman BP, Beaton A, Weiszmann R, Kwan E, Hartenstein V, Celniker SE, Rubin GM. Global analysis of patterns of gene expression during Drosophila embryogenesis. Genome Biol. 2007;8:R145. doi: 10.1186/gb-2007-8-7-r145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase ME, Yalamanchili P, Visa N. The Drosophila heterogeneous nuclear ribonucleoprotein M protein, HRP59, regulates alternative splicing and controls the production of its own mRNA. J Biol Chem. 2006;281:39135–39141. doi: 10.1074/jbc.M604235200. [DOI] [PubMed] [Google Scholar]
- Kiesler E, Hase ME, Brodin D, Visa N. Hrp59, an hnRNP M protein in Chironomus and Drosophila, binds to exonic splicing enhancers and is required for expression of a subset of mRNAs. J Cell Biol. 2005;168:1013–1025. doi: 10.1083/jcb.200407173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner S, Lum L, Kim M, Paro R, Beachy PA, Green R. A genomewide screen for components of the RNAi pathway in Drosophila cultured cells. Proc Natl Acad Sci USA. 2006;103:11880–11885. doi: 10.1073/pnas.0605210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovemann B, Richter S, Walldorf U, Cziepluch C. Two genes encode related cytoplasmic elongation factors 1 alpha (EF-1 alpha) in Drosophila melanogaster with continuous and stage specific expression. Nucleic Acids Res. 1988;16:3175–3194. doi: 10.1093/nar/16.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J. Elongation factor 1 alpha, translation and the cytoskeleton. Trends Biochem Sci. 1995;20:169–170. doi: 10.1016/S0968-0004(00)88998-7. [DOI] [PubMed] [Google Scholar]
- Costa A, Wang Y, Dockendorff TC, Erdjument-Bromage H, Tempst P, Schedl P, Jongens TA. The Drosophila fragile × protein functions as a negative regulator in the orb autoregulatory pathway. Dev Cell. 2005;8:331–342. doi: 10.1016/j.devcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Geng C, Macdonald PM. Imp associates with squid and Hrp48 and contributes to localized expression of gurken in the oocyte. Mol Cell Biol. 2006;26:9508–9516. doi: 10.1128/MCB.01136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka A, Siomi MC, Siomi H. A Drosophila fragile × protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002;16:2497–2508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangus DA, Evans MC, Jacobson A. Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 2003;4:223. doi: 10.1186/gb-2003-4-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Bogert BA, Li W, Su K, Lee A, Gao FB. The fragile X-related gene affects the crawling behavior of Drosophila larvae by regulating the mRNA level of the DEG/ENaC protein pickpocket1. Curr Biol. 2004;14:1025–1034. doi: 10.1016/j.cub.2004.05.055. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- Lee CS, Dias AP, Jedrychowski M, Patel AH, Hsu JL, Reed R. Human DDX3 functions in translation and interacts with the translation initiation factor eIF3. Nucleic Acids Res. 2008;36:4708–4718. doi: 10.1093/nar/gkn454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang RY, Weaver PL, Liu Z, Chang TH. Requirement of the DEAD-Box protein ded1p for messenger RNA translation. Science. 1997;275:1468–1471. doi: 10.1126/science.275.5305.1468. [DOI] [PubMed] [Google Scholar]
- Burckin T, Nagel R, Mandel-Gutfreund Y, Shiue L, Clark TA, Chong JL, Chang TH, Squazzo S, Hartzog G, Ares M., Jr Exploring functional relationships between components of the gene expression machinery. Nature structural & molecular biology. 2005;12:175–182. doi: 10.1038/nsmb891. [DOI] [PubMed] [Google Scholar]
- Jamieson DJ, Rahe B, Pringle J, Beggs JD. A suppressor of a yeast splicing mutation (prp8-1) encodes a putative ATP-dependent RNA helicase. Nature. 1991;349:715–717. doi: 10.1038/349715a0. [DOI] [PubMed] [Google Scholar]
- Stevens SW, Ryan DE, Ge HY, Moore RE, Young MK, Lee TD, Abelson J. Composition and functional characterization of the yeast spliceosomal penta-snRNP. Mol Cell. 2002;9:31–44. doi: 10.1016/S1097-2765(02)00436-7. [DOI] [PubMed] [Google Scholar]
- Merz C, Urlaub H, Will CL, Luhrmann R. Protein composition of human mRNPs spliced in vitro and differential requirements for mRNP protein recruitment. Rna. 2007;13:116–128. doi: 10.1261/rna.336807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JW, Tsai TY, Chao CH, Wu Lee YH. Candidate tumor suppressor DDX3 RNA helicase specifically represses cap-dependent translation by acting as an eIF4E inhibitory protein. Oncogene. 2008;27:700–714. doi: 10.1038/sj.onc.1210687. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Licklider LJ, Gygi SP, Reed R. Comprehensive proteomic analysis of the human spliceosome. Nature. 2002;419:182–185. doi: 10.1038/nature01031. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lee YH, Tarn WY. The DEAD-box RNA helicase DDX3 associates with export messenger ribonucleoproteins as well as tip-associated protein and participates in translational control. Mol Biol Cell. 2008;19:3847–3858. doi: 10.1091/mbc.E07-12-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashchekin D, Zhao J, Visa N, Daneholt B. A novel Ded1-like RNA helicase interacts with the Y-box protein ctYB-1 in nuclear mRNP particles and in polysomes. J Biol Chem. 2006;281:14263–14272. doi: 10.1074/jbc.M600262200. [DOI] [PubMed] [Google Scholar]
- Ishaq M, Hu J, Wu X, Fu Q, Yang Y, Liu Q, Guo D. Knockdown of Cellular RNA Helicase DDX3 by Short Hairpin RNAs Suppresses HIV-1 Viral Replication Without Inducing Apoptosis. Mol Biotechnol. 2008 doi: 10.1007/s12033-008-9040-0. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell. 2004;119:381–392. doi: 10.1016/j.cell.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Richter L, Bone JR, Kuroda MI. RNA-dependent association of the Drosophila maleless protein with the male X chromosome. Genes Cells. 1996;1:325–336. doi: 10.1046/j.1365-2443.1996.26027.x. [DOI] [PubMed] [Google Scholar]
- Elvira G, Massie B, DesGroseillers L. The zinc-finger protein ZFR is critical for Staufen 2 isoform specific nucleocytoplasmic shuttling in neurons. J Neurochem. 2006;96:105–117. doi: 10.1111/j.1471-4159.2005.03523.x. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold A, Teixeira L, Izaurralde E. Genome-wide analysis of nuclear mRNA export pathways in Drosophila. Embo J. 2003;22:2472–2483. doi: 10.1093/emboj/cdg233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F, Nusinow DA, Chalkley RJ, Plath K, Panning B, Burlingame AL. Mapping post-translational modifications of the histone variant MacroH2A1 using tandem mass spectrometry. Mol Cell Proteomics. 2006;5:194–203. doi: 10.1074/mcp.M500285-MCP200. [DOI] [PubMed] [Google Scholar]
- Straub T, Gilfillan GD, Maier VK, Becker PB. The Drosophila MSL complex activates the transcription of target genes. Genes Dev. 2005;19:2284–2288. doi: 10.1101/gad.1343105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Over 8 hours of cycloheximide (chx) treatment, MLE protein levels do not increase.
Supplemental Fig. 2. mle mRNA is present in the cytoplasm when bel and Zn72D+bel are knocked down.