Abstract
Bacterial Tat systems export folded proteins, including FeS proteins such as NrfC and NapG, which acquire their cofactors before translocation. NrfC and NapG are proofread by the Tat pathway, and misfolded examples are degraded after interaction with the translocon. Here, we identify TatD as a crucial component of this quality control system in Escherichia coli. NrfC/NapG variants lacking FeS centres are rapidly degraded in wild-type cells but stable in a ΔtatD strain. The precursor of another substrate, FhuD, is also transiently detected in wild-type cells but stable in the ΔtatD strain. Surprisingly, these substrates are stable in ΔtatD cells that overexpress TatD, and export of the non-mutated precursors is inhibited. We propose that TatD is part of a quality control system that is intimately linked to the Tat export pathway, and that the overexpression of TatD leads to an imbalance between the two systems such that both Tat-initiated turnover and export are prevented.
Keywords: FeS protein, protein transport, TatD, twin arginine, Tat
Introduction
The Tat system operates in the chloroplast thylakoid membrane and in the plasma membranes of most free living bacteria (reviewed in Robinson & Bolhuis, 2004; Müller, 2005). Unusually, it is able to transport substrates in a fully folded state, and some of the best characterized Tat substrates include periplasmic proteins containing any of a range of redox cofactors, including FeS clusters and molybdopterin centres (Berks, 1996; Santini et al, 1998; Sargent et al, 1998; Weiner et al, 1998). In Gram-negative bacteria, the Tat system is minimally composed of TatABC subunits; TatE also participates in Escherichia coli, but this seems to be a TatA paralogue of minor importance (Sargent et al, 1999). The tatABC genes are present in an operon together with a fourth gene, tatD, but TatD has been shown to be a cytoplasmic protein that is not involved in translocation (Wexler et al, 2000).
The role of the Tat system in the export of folded, cofactor-containing proteins necessitates a means of ensuring that substrates have cofactors in place when transported across the membrane. Several studies have investigated this requirement for ‘proofreading' of substrate conformation. The molybdoproteins TorA and DmsD, for example, are synthesized together with specific chaperone molecules (TorD and DmsD), which seem to bind to the signal peptide and prevent export until folding and assembly are completed (Oresnik et al, 2001; Sargent, 2007). Similar roles have been proposed for the Ralstonia eutropha HoxO and HoxQ proteins, which interact with the HoxK subunit of the membrane-bound NiFe hydrogenase (Schubert et al, 2007).
Recently, we examined the Tat-dependent export of two predicted FeS proteins, NrfC and NapG, in E. coli. These proteins are part of the CooF family of iron–sulphur proteins, all of which are involved in anaerobic bacterial metabolism (reviewed in Cole, 1996). They contain 16 conserved cysteine residues involved in 4 separate [4Fe–4S] clusters, and we have shown that mutagenesis of even a single FeS centre was sufficient to block the export of NrfC or NapG. Interestingly, it was found that mutated substrates were rapidly degraded, but only after interaction with the Tat translocon. Apparently, this quality control system involves extensive interaction with the TatABC system—and probably partial translocation—at which point the substrate is somehow rejected and tagged for degradation (Matos et al, 2008). Here, we show that TatD, although not required for Tat-dependent export, is an integral component of this linked quality control system. We also show TatD-dependent degradation of a non-FeS precursor protein, pre-FhuD, and provide evidence that the quality control system is intimately linked to the Tat translocation system.
Results
TatABC support NrfC/NapG export but not turnover
Mutations in the FeS centres of NrfC and NapG lead to rapid degradation of the proteins, but only in cells with an active Tat system (Matos et al, 2008). TatABC subunits were shown to be essential for their degradation, suggesting that turnover occurs only after interaction with the Tat translocon. In follow-up studies, we expressed the tatABC operon in a tat-null mutant strain (ΔtatABCDE), expecting that co-expression of tatABC from a compatible plasmid would restore both the export of protein and the turnover of NrfC/NapG. The tatDE gene products were assumed to be irrelevant because TatE is a relatively unimportant TatA paralogue and TatD was not believed to be involved in the Tat export pathway (Sargent et al, 1999; Wexler et al, 2000).
The experiments involved expression of pre-NrfC and two mutated forms—M1 and M2, with mutations in FeS centers—in E. coli MC4100 cells. M1 and M2 are described in detail by Matos et al (2008). Expression of the NrfC proteins was initiated using the arabinose-inducible pBAD24 plasmid, and the expression profile for non-mutated pre-NrfC is shown in Fig 1. The left-hand panels show expression in wild-type MC4100 cells, whereas the right-hand panels show expression in ΔtatABCDE cells that co-express tatABC from a compatible plasmid. In wild-type cells, expression of pre-NrfC is apparent after about 2 h of induction, whereas the mature protein is observed thereafter; mature NrfC is localized in the periplasm as expected (supplementary information online). When M1 and M2 are expressed in wild-type cells, it is apparent that these proteins are subjected to rapid and near-complete degradation. These data closely resemble the results obtained by Matos et al (2008).
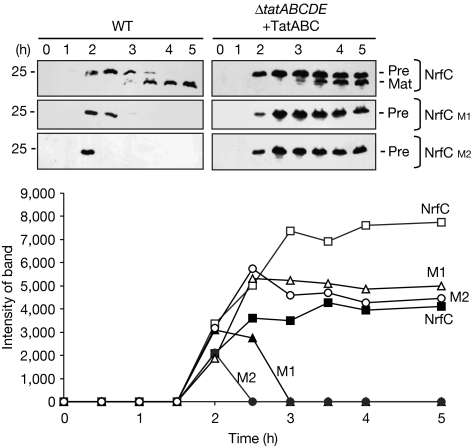
Figure 1.
TatABC subunits support export and maturation of pre-NrfC mutants in ΔtatABCDE cells, but not their turnover. Pre-NrfC and the M1 and M2 variants were expressed from the arabinose-inducible pBAD24 plasmid in wild-type (WT) MC4100 cells or from the ΔtatABCDE cells expressing TatABC from the pEXT22 plasmid as indicated. Cells were analysed at times (in hours) after induction with arabinose, and immunoblotted using antibodies to the 6-His tag on pre-NrfC. Precursor and mature forms are indicated (Pre and Mat), and mobility of a 25-kDa marker is shown. NrfC bands were quantified by densitometry and are shown in the graph, in which data for pre-NrfC, M1 and M2 are indicated after expression in wild-type cells (filled symbols) or in ΔtatABCDE cells expressing TatABC (open symbols).
When the NrfC forms are expressed in ΔtatABCDE cells that co-express TatABC (right-hand panels), mature NrfC again accumulates during the expression of non-mutated pre-NrfC, showing that the Tat system is active as expected. However, M1 and M2 are highly stable, indicating that the turnover system is compromised. Importantly, the precursor form of non-mutated NrfC (‘pre') is also stable throughout this period, whereas it is observed only transiently in wild-type cells.
The quantified data are illustrated graphically in Fig 1, and a crucial point is that the total levels of non-mutated NrfC—mature and precursor proteins—are significantly higher than those in wild-type cells. In summary, the TatABC proteins support efficient export of NrfC in a ΔtatABCDE background, but the mutated substrates are highly stable, which means that the degradation pathway is effectively blocked. As ΔtatABCDE cells lack only the tatDE genes under these conditions, the data suggest a crucial role for one of these proteins. TatE has already been shown to exhibit a TatA-type function, although a role in quality control cannot be excluded (Matos et al, 2008), so we focused attention on TatD.
TatD is essential for turnover of NapG/NrfC mutants
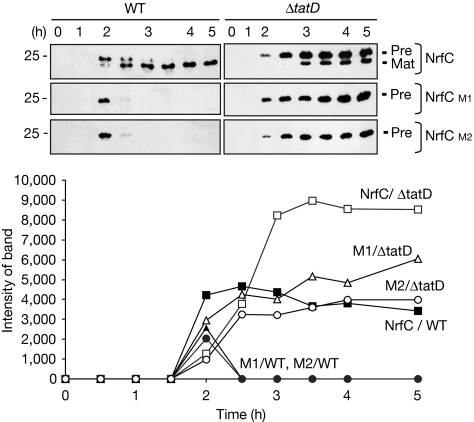
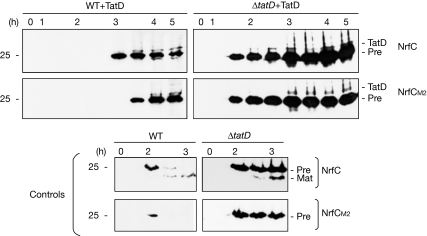
We studied the turnover of NrfC in a ΔtatD strain (Wexler et al, 2000). Fig 2 shows a direct comparison of the induction, maturation and turnover of NrfC/M1/M2 in wild-type and ΔtatD cells. Non-mutated pre-NrfC is exported with typical kinetics in the ΔtatD strain, in terms of the appearance of mature-sized NrfC. However, although the M1 and M2 forms are degraded with typically rapid kinetics in wild-type cells, they are fully stable in ΔtatD cells. This indicates a crucial role for TatD in the turnover of the mutated NrfC proteins––the first evidence that TatD is associated with the Tat pathway. The precursor form of non-mutated NrfC is also stable in the ΔtatD cells, indicating the role of TatD in the turnover of this protein.
Figure 2.
FeS mutant forms of NrfC are stable in ΔtatD cells. Pre-NrfC, M1 and M2 were expressed in wild-type (WT) and ΔtatD cells. Cells were analysed at times (in hours) after the induction of synthesis with arabinose as in Fig 1, and the graph shows the amounts of protein present (from quantitative immunoblotting) over time. The data for the expression of M1 and M2 in wild-type cells are denoted by filled triangles and circles, respectively, and data for other samples are indicated on the graph. Immunoblots are shown with precursor and mature forms (Pre and Mat).
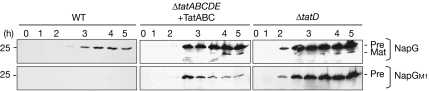
We tested whether a second FeS substrate, NapG, is similarly stabilized in the absence of TatD, having previously shown that a mutant—M1, affected in one FeS center—is unstable in wild-type cells but stable in ΔtatABCDE cells (Matos et al, 2008). We expressed NapG and NapG-M1 in wild-type cells, ΔtatABCDE cells co-expressing TatABC and ΔtatD cells (Fig 3). As found by Matos et al (2008), pre-NapG accumulates in wild-type cells, whereas the M1 mutant is barely detectable at any time point and clearly rapidly degraded. By contrast, both pre-NapG and M1 are stable in ΔtatABCDE cells that express TatABC, with the M1 variant now almost as stable as non-mutated NapG. Thus, TatD is essential for the rapid turnover of the M1 variant. Interestingly, both pre-NapG and M1 are even more stable in ΔtatD cells, with higher signals apparent on the immunoblots. It is unclear why this is the case, as both cell types have an active Tat system and lack TatD. One possibility is an indirect effect that originates from the higher translocation activity of ΔtatABCDE cells that also express TatABC from a multi-copy plasmid. If the mutated versions undergo partial translocation at a higher rate, they might become destabilized and degraded to a greater extent by other nonspecific proteases.
Figure 3.
Mutated pre-NapG lacking an FeS cluster is rapidly degraded in wild-type cells but stable in ΔtatABCDE cells expressing TatABC or in ΔtatD cells. Pre-NapG was expressed from the pBAD24 vector in WT cells, ΔtatABCDE cells that co-express TatABC from the pEXT22 plasmid, and ΔtatD cells as indicated. Samples were analysed at times (in hours) after the addition of arabinose to induce synthesis. A mutated version (preNapG-M1) lacking an FeS ligand was analysed simultaneously. Mobilities of precursor and mature NapG (Pre and Mat, respectively) and a 25-kDa marker are shown. WT, wild type.
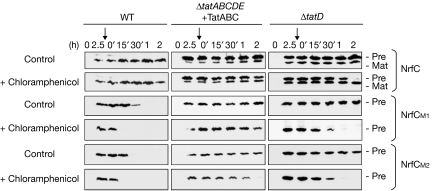
Does the absence of TatD specifically affect the stability of these Tat substrates, or is there also an effect on their synthesis? Although plasmid-encoded, the synthesis of these substrates could be affected in ΔtatD cells for unknown reasons. To investigate this question, we analysed the stability of NrfC, M1 and M2 after the addition of chloramphenicol to block protein synthesis (Fig 4). After induction for 2.5 h with arabinose, pre-NrfC and the M1/M2 variants are all detectable in wild-type cells, ΔtatD cells and ΔtatABCDE cells that co-express TatABC. In the absence of chloramphenicol (‘control' panels), the data resemble those shown above, with pre-NrfC, M1 and M2 all transiently detected in wild-type cells, but relatively stable in ΔtatD cells and ΔtatABCDE cells co-expressing TatABC. After the 2.5-h time point, the M1 and M2 forms are still present after a further 15 min in wild-type cells, but they are virtually undetectable after 30 min. After the addition of chloramphenicol at the 2.5-h time point (as denoted by vertical arrows), the proteins are extremely short-lived in wild-type cells, and undetectable even at the 15 min chase point (in fact, we found that neither M1 nor M2 can be detected even 3 min after the addition of chloramphenicol to wild-type cells; data not shown). When expressed in ΔtatD cells under identical conditions, Fig 4 shows that these proteins are expressed at similar levels, with no evidence for enhanced rates of synthesis. After the addition of chloramphenicol, both M1 and M2 are present at high levels after a 15-min chase and both are detected in the 30-min chase sample. The proteins are thus far more stable in the absence of TatD. It is, however, noteworthy that M1 and M2 are degraded within 60 min under these conditions, and we speculate that the addition of chloramphenicol might affect the stability of substrates in other ways, perhaps by blocking the synthesis of other factors that might otherwise be induced.
Figure 4.
Stability of NrfC is enhanced in ΔtatD cells. Pre-NrfC and the M1/M2 variants were expressed in WT cells, ΔtatABCDE cells co-expressing TatABC, or ΔtatD cells as indicated. After induction for 2.5 h, samples were analysed immediately (0′) or at times thereafter as indicated. Samples were analysed in parallel from control cells or from cells to which chloramphenicol was added at the 2.5-h time point (denoted by the vertical arrow). WT, wild type.
Overexpressed TatD interferes with NrfC export
We expressed TatD constitutively from the pEXT22 plasmid in both wild-type and ΔtatD cells to test whether the degradation of the NrfC forms was restored in ΔtatD cells. In fact, unexpected results were obtained, as shown in Fig 5. In ΔtatD cells, plasmid-expressed TatD does not restore turnover of pre-NrfC or of the M2 mutant, with both forms stable throughout the time course. However, a crucial point is that the export of non-mutated pre-NrfC is inhibited in these cells; the precursor protein is present in a stable form, but mature-sized NrfC is not detected. When expressed in wild-type cells, the plasmid-expressed TatD again fails to promote the degradation of pre-NrfC or of the M2 form. The export of NrfC is again blocked, but one difference (compared with the data obtained in ΔtatD cells) is that the induction of NrfC/M2 is slowed down in wild-type cells, with these proteins being observed only after 3–3.5 h of induction. In control tests using wild-type cells and ΔtatD cells that do not co-express TatD (lower panels), non-mutated pre-NrfC is exported and processed in both cases. Thus, the data show that overexpression of TatD affects both the export and quality control functions of the Tat pathway. One possible explanation is that it is present at concentrations that perturb the interactions involved (see below), and we have attempted to test this by expressing TatD at different levels. However, expression using the pBAD24 plasmid, with various arabinose concentrations, yielded the same result, as did expression from the pEXT22 plasmid in either the presence or absence of the IPTG inducer (data not shown). However, it is probable that TatD was expressed at relatively high levels under all of these conditions, in comparison with wild-type levels, and further tests are required to assess rigorously the effects of directly complementing the ΔtatD mutation.
Figure 5.
Overexpression of TatD leads to enhanced stability of NrfC and inhibition of export. His-tagged TatD was expressed in wild-type (WT) and ΔtatD cells together with pre-NrfC or the M2 mutant. Samples were immunoblotted for NrfC at times (in hours) after the induction of NrfC synthesis. Mobility of the His-tagged TatD is also indicated. Control panel: the same cells were analysed without the overexpression of TatD. Mat, matured; Pre, precursor.
TatD promotes turnover of a non-FeS substrate, FhuD
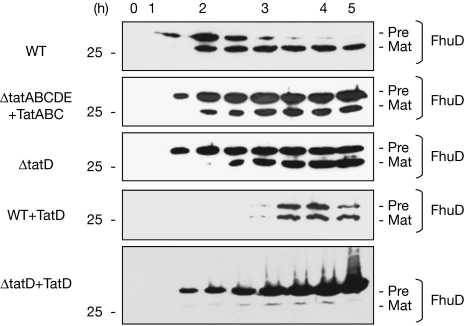
To understand further the role of TatD, we examined its influence on FhuD, a 30-kDa Tat substrate that does not contain cofactors (Ize et al, 2004). Pre-FhuD was expressed from the pBAD24 plasmid and, in a typical time course study in wild-type cells (Fig 6, top panel), pre-FhuD is detected after 2 h of induction, after which its levels decrease rapidly and the mature protein becomes the predominant form thereafter. Control fractionation tests confirmed that the mature form is in the periplasm (data not shown). In ΔtatD cells, and in ΔtatABCDE cells that co-express TatABC, FhuD is exported with normal kinetics but the precursor form becomes much more stable. These data closely resemble those obtained with non-mutated pre-NrfC, and they similarly point to a role for TatD in degrading wild-type pre-protein molecules that have failed to be exported.
Figure 6.
TatD directs the rapid turnover of a non-FeS Tat substrate, pre-FhuD. Pre-FhuD was expressed from the pBAD24 plasmid in Wt, ΔtatD cells and ΔtatABCDE cells expressing TatABC. Samples were also analysed from the same cell types expressing pre-FhuD and TatD from the pEXT22 plasmid.
Overexpression of TatD has interesting effects on the export and stability of FhuD. When expressed in ΔtatD cells, pre-FhuD accumulates and its export is effectively blocked. The effects of co-expressing TatD thus resemble those observed with pre-NrfC as shown in Fig 5: the (presumably) enhanced levels of TatD have the dual effect of inhibiting export while failing to promote the turnover of the precursor protein. When expressed in wild-type cells, however, a rather different picture emerges. First, the appearance of the pre-protein is delayed until the 3- to 3.5-h time point, and the levels of FhuD are decreased, as found when TatD was expressed in wild-type cells expressing pre-NrfC. However, export is not blocked in this case, with some mature-sized exported protein detected at later time points. In summary, the presence of wild-type levels of TatD supports the rapid turnover of pre-FhuD molecules that are not exported in wild-type cells, but the overexpression of TatD affects its ability to support pre-FhuD degradation while simultaneously blocking the export of the protein in ΔtatD cells.
Discussion
It is crucial for the Tat system to sense when complex proteins are fully ready for export, and several studies have investigated these issues with various redox proteins (Oresnik et al, 2001; Sargent, 2007; Schubert et al, 2007). Our studies on NrfC and NapG have shown that the Tat pathway recognizes when these proteins are misfolded or unable to assemble a full complement of FeS centres, and export is blocked (Matos et al, 2008). The study has also identified an additional and unexpected facet of NrfC/NapG export, in that the Tat system itself initiates the degradation of misfolded forms.
Here, we have identified TatD as a central element of this quality control system. This is surprising because TatD is not required for Tat export activity and the presence of the tatD gene in the tat operon has been regarded as fortuitous (Wexler et al, 2000). We found that TatD is not required for the export of NrfC, but is essential for the rapid turnover of mutated NrfC and NapG. These proteins are rapidly degraded in wild-type cells but highly stable in the ΔtatD strain.
Interestingly, non-mutated pre-NrfC is also subjected to targeted degradation. The precursor protein is unstable and almost undetectable at later time points in wild-type cells, but stable in the ΔtatD strain, remaining at high levels over several hours. Pre-NapG is similarly much more stable in the absence of TatD. We conclude that, in wild-type cells, a proportion of pre-NrfC and pre-NapG molecules are rapidly exported, whereas another population fails to be exported and is targeted for degradation by the TatD-dependent quality control system. The same applies to the precursor form of FhuD, another Tat substrate that does not carry a cofactor (Ize et al, 2004), suggesting that TatD is also crucial in the quality control of other non-FeS Tat substrates.
TatD is a soluble protein (Wexler et al, 2000) and is unlikely to be a permanent component of the Tat translocon. At present, we have no information on its mode of action; the ΔtatD strain has no clear phenotype (Wexler et al, 2000), and although studies on the purified protein showed that it has DNase activity, it seems unlikely that this DNase activity is involved in this particular quality control pathway. Presumably, TatD has other activities and it will be important to determine how, and at which point, it is involved in the degradation of NrfC/NapG/FhuD.
Overexpression of TatD does not restore the turnover of these Tat substrates in the ΔtatD strain, and has the dual effects of rendering these Tat substrates more stable in wild-type cells while blocking the export of both NrfC and FhuD in ΔtatD cells. These data provide a crucial initial indication that this quality control system is intimately linked to the Tat pathway. We believe that overexpression of TatD might cause an imbalance between the two pathways, with the artificially high levels of TatD perhaps sequestering crucial components of the Tat system or associated factors. In this manner, the substrates might be prevented from interacting with the Tat translocon, thereby preventing both translocon-initiated degradation and export. Clearly, it will be important to understand the precise role of TatD in this scheme and to determine whether it has a more global role in the quality control of other Tat substrates.
Methods
Bacterial strains, plasmids and growth conditions. E. coli strain MC4100 was the parental strain (Casadaban & Cohen, 1979); ΔtatD (TDD1) and ΔtatABCDE have been described previously (Wexler et al, 2000). NrfC and NapG were expressed as detailed by Matos et al (2008). Arabinose-resistant derivatives of MC4100 and ΔtatABCDE were used as described previously (Guzman et al, 1995; Bolhuis et al, 2000). The strain ΔtatD was made arabinose resistant as described previously (Englesberg et al, 1962). E. coli was grown aerobically at 37 °C in Luria–Bertoni broth (Bolhuis et al, 2000). Medium supplements were used at the following concentrations: ampicillin (100 μg/ml) and kanamycin (50 μg/ml).
Cell fractionation. Starter cultures (5 ml) were grown overnight and used to inoculate 50 ml of Luria–Bertoni broth (with appropriate antibiotic selection and 100 μM arabinose to induce the expression of NrfC and 200 μM arabinose to induce the expression of NapG) to a starting OD600 of 0.1. Cells were grown to the mid-exponential phase before fractionation as described by Randall & Hardy (1986). Spheroplasts were lysed by sonication, and intact cells and cellular debris were removed by centrifugation (5 min at 10,000g). Membranes were separated from the cytoplasmic fraction by centrifugation (30 min at 250,000g). Equal amounts of each fraction were separated by 15% SDS–polyacrylamide gel electrophoresis and immunoblotted with His antibodies (Invitrogen, Paisley, UK). As a secondary antibody, horseradish peroxidase anti-mouse immunoglobulin G conjugate was used and detected by ECL detection system (Amersham Pharmacia Biotech, Little Chalfont, UK). ImageQuant software (Molecular Dynamics, Little Chalfont, UK) was used for quantitative analysis of immunoblots.
Time course during the induction of arabinose and chloramphenicol treatment. Starter cultures (5 ml) were grown overnight and used to inoculate 50 ml of Luria–Bertoni broth with 100 μM arabinose added to induce the expression of NrfC and FhuD, and 200 μM to induce NapG. Cells were grown at 37 °C and samples were taken over 5 h after the induction of arabinose.
The chloramphenicol treatment was carried out in cells induced as described above, with the difference that after 2.5 h of induction, chloramphenicol at a concentration of 100 μg/ml was added to the cells to stop protein synthesis but not to the control. Proteins were separated on a 15% SDS–polyacrylamide gel and immunoblotted as above.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Acknowledgments
This study was supported by a grant from the Biotechnology and Biological Sciences Research Council and by grant LSHG-CT-2004-005257 from the European Union to C.R.
Footnotes
The authors declare that they have no conflict of interest.
References
- Berks BC (1996) A common export pathway for proteins binding complex redox cofactors? Mol Microbiol 22: 393–404 [DOI] [PubMed] [Google Scholar]
- Bolhuis A, Bogsch EG, Robinson C (2000) Subunit interactions in the twin-arginine translocase complex of Escherichia coli. FEBS Lett 472: 88–92 [DOI] [PubMed] [Google Scholar]
- Casadaban MJ, Cohen SN (1979) Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci USA 76: 4530–4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J (1996) Nitrate reduction to ammonia by enteric bacteria: redundancy, or a strategy for survival during oxygen starvation? FEMS Microbiol Lett 136: 1–11 [DOI] [PubMed] [Google Scholar]
- Englesberg E, Anderson RL, Weinberg R, Lee N, Hoffee P, Huttenhauer G, Boyer H (1962) L-Arabinose-sensitive, L-ribulose 5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J Bacteriol 84: 137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177: 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ize B, Porcelli I, Lucchini S, Hinton JC, Berks BC, Palmer T (2004) Novel phenotypes of Escherichia coli tat mutants revealed by global gene expression and phenotypic analysis. J Biol Chem 279: 47543–47554 [DOI] [PubMed] [Google Scholar]
- Matos CFRO, Robinson C, Di Cola A (2008) The Tat system proofreads FeS protein substrates and directly initiates the disposal of rejected molecules. EMBO J 27: 2055–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M (2005) Twin-arginine specific export in Escherichia coli. Res Microbiol 156: 131–136 [DOI] [PubMed] [Google Scholar]
- Oresnik IJ, Ladner CL, Turner RJ (2001) Identification of a twin-arginine leader-binding protein. Mol Microbiol 40: 323–331 [DOI] [PubMed] [Google Scholar]
- Randall LL, Hardy SLS (1986) Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding in E coli. Cell 46: 921–928 [DOI] [PubMed] [Google Scholar]
- Robinson C, Bolhuis A (2004) Tat-dependent protein targeting in prokaryotes and chloroplasts. Biochim Biophys Acta 1694: 135–147 [DOI] [PubMed] [Google Scholar]
- Santini CL, Ize B, Chanal A, Müller M, Giordano G, Wu LF (1998) A novel sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J 17: 101–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent F (2007) The twin-arginine transport system: moving folded proteins across membranes. Biochem Soc Trans 35: 835–847 [DOI] [PubMed] [Google Scholar]
- Sargent F, Bogsch EG, Stanley NR, Wexler M, Robinson C, Berks BC, Palmer T (1998) Overlapping functions of components of a bacterial Sec-independent protein export pathway. EMBO J 17: 3640–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent F, Stanley NR, Berks BC, Palmer T (1999) Sec-independent protein translocation in Escherichia coli. A distinct and pivotal role for the TatB protein. J Biol Chem 274: 36073–36082 [DOI] [PubMed] [Google Scholar]
- Schubert T, Lenz O, Krause E, Volkmer R, Friedrich B (2007) Chaperones specific for the membrane-bound [NiFe]-hydrogenase interact with the Tat signal peptide of the small subunit precursor in Ralstonia eutropha H16. Mol Microbiol 66: 453–467 [DOI] [PubMed] [Google Scholar]
- Weiner JH, Bilous PT, Shaw GM, Lubitz SP, Frost L, Thomas GH, Cole JA, Turner RJ (1998) A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell 93: 93–101 [DOI] [PubMed] [Google Scholar]
- Wexler M, Sargent F, Jack RL, Stanley NR, Bogsch EG, Robinson C, Berks BC, Palmer T (2000) TatD Is a cytoplasmic protein with DNase activity. No requirement for TatD family proteins in Sec-independent protein export. J Biol Chem 275: 16717–16722 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information