Abstract
Investigating the mechanisms controlling the asymmetric division of neocortical progenitors that generate neurones in the mammalian brain is crucial for understanding the abnormalities of cortical development. Partitioning of fate determinants is a key instructive step and components of the apical junctional complex (adherens junctions), including the polarity proteins PAR3 and aPKC as well as adhesion molecules such as N-cadherin, have been proposed to be candidate determinants. In this study, however, we found no correlation between the partitioning of N-cadherin and fate determination. Rather, we show that adherens junctions comprise three membrane domains, and that during asymmetrical division these are split such that both daughters retain the adhesive proteins that control cell position, but only one daughter inherits the polarity proteins along with the apical membrane. This provides a molecular explanation as to how both daughters remain anchored to the ventricular surface after mitosis, while adopting different fates.
Introduction
Neural stem cells (NSCs) are able to self-renew and are multipotent. Before the onset of neurogenesis (embryonic day (E)11 in the mouse), they undergo symmetric proliferative divisions, with one NSC giving rise to two daughter stem cells to increase the size of the progenitor pool. As neurogenesis proceeds (E11–E17), they switch to an asymmetric neurogenic mode of division to generate one stem cell along with one cell committed to the neuronal lineage. In the dorsal telencephalon of mouse embryos, NSCs reside at the ventricular zone and show an elongated shape, with their processes attached to both the ventricular and pial surfaces. During mitosis, the cell body moves to the ventricular surface (a process known as interkinetic nuclear migration), where cytokinesis occurs, but the cells remain attached to both the basal and apical surfaces. When cytokinesis is complete the cell body moves away from the ventricular surface, but both daughter cells remain attached to the ventricle through their apical process for some hours before the neuronal precursor loses apical contact and migrates out of the ventricular zone (Miyata et al, 2001; Noctor et al, 2004). The significance of retaining apical attachment is still unclear, but it might allow cell–cell interactions between the two daughter cells, that may instruct their fate (Miyata, 2007).
In vertebrates, NSCs of the dorsal telencephalon divide along the apico-basal axis (Kosodo et al, 2004; Konno et al, 2008). The distribution during division of the fate-determining factors confined within or close to the apical plasma membrane is also considered to be important in determining the fate of the daughter cell (Kosodo et al, 2004; Gotz & Huttner, 2005). The sub-apical junctional complexes—that is, adherens junctions (AJs)—seem to be partitioned along with the apical plasma membrane, as evidenced by the distribution during division of the AJ-enriched polarity protein PAR3 (Kosodo et al, 2004). As a result, AJ components have been considered to provide a mechanism for localization of fate determinants and regulation of their inheritance (Gotz & Huttner, 2005). However, such a model in which AJs are unequally inherited during asymmetric division cannot explain how the daughter cell that has not inherited any AJ retains its apical attachment. Here, we explain this discrepancy by showing the presence of distinct domains within the AJs that separate adhesion and putative fate-determining molecules, which are inherited differentially during cell division.
Results And Discussion
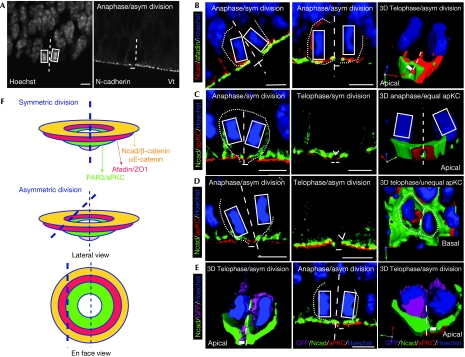
Planar asymmetry of N-cadherin distribution in NSCs
N-cadherin is a crucial structural component of neuroepithelial AJs (Kadowaki et al, 2007). By stabilizing the Delta ligand of Notch receptors at the plasma membrane (Mizuhara et al, 2005) and by controlling the cytoplasmic pools of β- and αE-catenin, which are molecules implicated in Wnt and Shh signalling (Chenn & Walsh, 2002; Lien et al, 2006), N-cadherin might regulate the balance between symmetrical and asymmetrical division. A role for N-cadherin in fate determination would predict an appropriate partitioning of the protein in the two daughter cells on mitosis—that is, equal inheritance in symmetrical divisions but unequal inheritance in asymmetrical divisions. Therefore, we examined the distribution of N-cadherin in SOX2-positive neocortical progenitors (Fig 1; supplementary Fig S1A online). We observed that the protein accumulates apically at sites of contact between NSCs undergoing mitosis and the processes of neighbouring cells (Fig 1A; Chenn et al, 1998). This accumulation represents AJs at the most apical end of the lateral plasma membrane, as evidenced by partial colocalization with actin filaments organized in a belt next to the ventricle. In addition, the protein is present in a broader distribution within the lateral membrane. Closer examination of dividing cells rounded up next to the ventricle showed that N-cadherin forms an apical crescent, but remains absent from the apical plasma membrane identified with the apical marker prominin 1 (Fig 1B). Within this crescent, we observed a lateral gradient in the distribution of N-cadherin in some dividing cells (Figs 1B and 2A), showing planar asymmetry in these cells.
Figure 1.
Apical enrichment of N-cadherin. (A) Lateral and en face confocal views of N-cadherin (Ncad) immunolabelled E14 cortex show an apical-to-basal gradient of the protein distribution. (B) High magnifications of apical mitotic cells. Left panels: note the interruption in N-cadherin staining indicated by a thick line (cadherin hole), which identifies the prominin 1 (Prom1)-enriched apical plasma membrane. Right panels: differential accumulation of N-cadherin in the lateral membranes can be observed. The differences in staining intensity are highlighted with a rainbow look up table LUT (red colour represents the highest intensity and blue colour the lowest intensity). Scale bars, 5 μm in lateral views, and 10 μm in en face views.
Figure 2.
N-cadherin partitioning is not related to fate. (A) High magnifications of an apically dividing neural stem cell (NSC) in anaphase representative of equal (left panels) and unequal (right panels) N-cadherin (Ncad) partitioning. The cleavage plane (dashed line) bisects the two sets of condensed chromatin (rectangles) and is drawn to predict the membrane territory inherited by each daughter cell. Confocal stacks of 2 μm depth, corresponding three-dimensional (3D) reconstructed views and calculated ratios (see below) are provided in each case. (B) Comparisons of proteins accumulated in each presumptive territory are expressed as ratios of fluorescence intensities, the distribution of which are presented in the graph for populations of embryonic day (E)10 and E14 dorsal telencephalon. (C) High magnifications of anaphasic cells from E14 TIS21-GFP+/− brains representative of N-cadherin partitioning in relation to green fluorescent protein (GFP) expression. The N-cadherin fluorescence intensity ratios are plotted against GFP expression for both proliferative (GFP negative) or neurogenic (GFP positive) division.
No link between cadherin distribution and cell fate
As most of the mitotic cells show a vertical cleavage plane (73 out of 84 cells; supplementary Fig S2 online), the planar asymmetry in N-cadherin distribution predicts that two daughter cells might inherit different amounts of the protein at the time of division (Fig 2). To test this, we compared the fraction of N-cadherin accumulated in the lateral membranes of the two daughter cells at the end of mitosis by measuring the ratio of their fluorescence intensities (Fig 2B; supplementary information online). We found that in nearly half of the divisions, one of the two daughter cells inherits a larger fraction of the protein (at least 1.5-fold more protein in almost 50% of cases, median value 1.4, n=30 at E10 and n=85 at E14; Fig 2A,B). We ruled out the possibility that this is only a consequence of oblique cleavage planes, as most of the divisions with differential partitioning of N-cadherin (ratios equal to or higher than 1.5) were vertical (n=33 out of 41) rather than oblique (n=7 out of 41; supplementary Fig S2 online).
This differential partitioning of N-cadherin was observed at all stages examined (E10 and E14), irrespective of the predominant mode of division (Fig 2B). To investigate any correlation with the mode of division (as would be expected if the hypothesis that N-cadherin has a role in fate determination is correct), we plotted the relative inheritance of the protein against the predicted fate of the daughter cells (Fig 2C; supplementary Fig S3 online). Cell fate was predicted by following the partitioning of the apical plasma membrane (Kosodo et al, 2004; supplementary Fig S3 online) or the presence of green fluorescent protein (GFP) expression in NSCs committed to neurogenic divisions using the TIS21-GFP knock-in mouse embryos (Haubensak et al, 2004; Fig 2C). In both cases, we found no difference in the distribution of N-cadherin ratios between the various types of division, as indicated by the absence of statistically significant differences between median values of 1.3 (n=29) and 1.6 (n=23) when prediction is made by inheritance of the apical membrane (supplementary Fig S3 online), or between median values of 1.5 (n=34) and 1.3 (n=27) when prediction is made by the TIS21-driven GFP expression (Fig 2C). Cells arising from symmetrical and asymmetrical divisions are therefore equally likely to inherit different amounts of N-cadherin, from which we conclude that the differential inheritance of N-cadherin observed in some dividing cells is not related to the fate of the daughter cells.
Organization of AJs in membrane domains
Previous observations that the polarity protein PAR3 is inherited by only one of the two daughter cells in asymmetric divisions have suggested the hypothesis that unequal AJ partitioning determines their fate (Kosodo et al, 2004). However, we find no correlation between N-cadherin distribution and fate. In addition, the distribution of the zona occludens protein ZO1, as followed by time-lapse imaging, shows that this component of the AJ is also inherited by the two daughter cells in most cases (Konno et al, 2008). One possible explanation for this discrepancy regarding N-cadherin and ZO1 being inherited by both daughter cells but PAR3 by only one daughter cell could be the differential partitioning of junctional proteins during division. Therefore, we examined the micro-organization of AJs by investigating a panel of proteins already resolved by electron microscopy to be junctional proteins (Aaku-Saraste et al, 1996; Manabe et al, 2002; Takekuni et al, 2003). These were two junctional proteins, ZO1 (part of the AJ rather than tight junctions in the neuroepithelium; Aaku-Saraste et al, 1996) and afadin (the cytoplasmic partner of nectin adhesion molecules), and the two PAR complex polarity proteins PAR3 and aPKC. As expected for proteins concentrated at the AJ, their distribution appears as a discrete line along the ventricle (supplementary Fig S1B online) and they accumulate at the most apical end of the lateral plasma membrane, where they form ring-like structures (Fig 3; supplementary Fig S4 online). N-cadherin was perfectly colocalized with β-catenin (supplementary Fig S4B online) and was used as a general marker for all neuroepithelial cadherins. The organization of actin filaments in a belt at the apical pole of polarized cells is commonly used to position the AJ, and consequently was used as a reference point to position the various membrane proteins along the apico-basal axis (Fig 3A; supplementary Fig S4A online). This helped to identify three membrane domains within the AJ: ZO1 and afadin were positioned centrally, the apical part was enriched in PAR3/aPKC and the basal part in N-cadherin (Fig 4F). This configuration was confirmed when N-cadherin was used as the point of reference instead of actin (Fig 3B; supplementary Fig S4B online), as we observed a partial colocalization of N-cadherin with the afadin/ZO1 rings and an absence of colocalization with the PAR3/aPKC rings. As the PAR3/aPKC-enriched domain occupies the most apical position, we tested and ruled out the possibility that it belongs to the apical plasma membrane rather than the AJ by showing no overlap with the apical marker prominin 1 (Fig 3A). Notably, the organization of the AJ was found to be identical irrespective of the brain region, developmental stage or cell-cycle phase (data not shown). The apico-lateral plasma membrane stratification that we report suggests that neuroepithelial AJs show a higher degree of organization than described previously (Manabe et al, 2002; Ghosh et al, 2008). Studies on other epithelial tissues have revealed two subdivisions of AJs, with PAR3 co-localized with cadherins but distinct from aPKC (Afonso & Henrique, 2006). Our work reveals that cadherin complexes can be separated from the two polarity proteins and suggests the existence of at least three functionally distinct microdomains within the AJ structure.
Figure 3.
Stratification of adherens junctions. (A) The relative positioning of adherens junction (AJ) markers to actin was examined from lateral (top panels) and en face (bottom panel) views in embryonic day (E)10–E14 dorsal telencephalon. The N-cadherin (Ncad)/actin en face view shows both xy and xz perspectives, with the z-axis orientated from basal to apical. The graphs represent the fluorescence-staining intensities of indicated markers along the z-axis for a given xy position and were drawn from en face view stacks. Dashed lines drawn at the peak of staining intensity for each marker help the comparison. The absence of colocalization between aPKC and prominin 1 (Prom) is shown in the far top-right panel. (B) Lateral and three-dimensional reconstructed en face views show the relative positioning of AJ markers to N-cadherin. Scale bars, 5 μm for lateral views and 10 μm for en face views.
Figure 4.
Adherens junctions can be split by the cleavage plane. (A) Example of a cell in anaphase, in which the cleavage plane is deduced by drawing a line (dashed line) bisecting the two sets of condensed chromatin (white rectangles); prediction of the type of division is made by distribution of the N-cadherin hole (thick line) in the two daughter cells. (B) Partitioning of afadin in anaphasic cells, in which the cadherin hole is bisected (left) or bypassed (middle) by the cleavage plane. The shape of each cell is revealed by N-cadherin staining and indicated by a dotted line. The right panel shows a snapshot from a three-dimensional (3D)-confocal z-stack reconstructed movie, with afadin partitioned in both daughter cells on bypass of the cadherin hole. In this telophasic cell, the cleavage furrow (dashed line) has already fused with the lateral membrane. (C) Partitioning of aPKC in an anaphasic (left) or a telophasic cell (middle), in which the cadherin hole is bisected by the cleavage plane (dashed line in anaphase). For the telophasic cell, the apically ingressing cleavage furrow is indicated by an open arrowhead. The right panel shows a 3D snapshot from an apical perspective with aPKC partitioned in both daughter cells. The cleavage plane is deduced from the orientation of the two sets of chromosomes in the first focal plane (white rectangles). (D) Partitioning of aPKC in an anaphasic (left) or a telophasic cell (middle), in which the cadherin hole is bypassed by the cleavage plane (dashed line in anaphase and open arrowhead in telophase). The right panel shows a 3D snapshot from a basal perspective, with the aPKC ring inherited by one of the two daughter cells (cell shapes indicated by a dotted line) after fusion of the cleavage furrow (indicated by a dashed line). (E) An example of an asymmetric neurogenic division is provided in the left panel. The 3D snapshot shows a telophasic cell expressing TIS21-GFP (green fluorescent protein), indicative of one of the daughter cells committing to become a neuron, with bypass of the apical membrane (the thick line filling the cadherin hole). The middle and right panels show 2D and 3D views of unequal partitioning of aPKC along with the apical membrane in a GFP-positive neurogenic division. (F) Model of adherens junction microdomain partitioning at the time of neural stem cell (NSC) division. Scale bars, 5 μm. asym, asymmetric; Ncad, N-cadherin; sym, symmetric; vt, ventricle.
Differential inheritance of AJ domains on division
The unequal distribution of these microdomains could provide an explanation as to how AJ components could be differentially inherited on asymmetric division. Therefore, we examined the partitioning of these microdomains in the progeny of NSCs (Fig 4). This partitioning was evaluated in anaphasic cells by predicting the orientation of the cleavage plane (Fig 4A; supplementary information online), and in telophasic cells based on fusion of the cleavage furrow ingressing from basal to apical with the apico-lateral membrane (supplementary information online; Kosodo et al, 2004). The aPKC domain was inherited by both daughter cells on symmetric division when the apical membrane was bisected by the cleavage plane (27 out of 27 symmetric divisions; Fig 4C; supplementary 3D movie 2 online) and by only one of the two daughter cells on asymmetric division when the apical membrane was bypassed (26 out of 29 asymmetric divisions; Fig 4D; supplementary 3D movie 3 online). From these observations, we conclude that the aPKC domain always partitions with the apical membrane. In addition, we showed that differential inheritance of aPKC by the progeny is usually observed when one of the two daughter cells detaches from the ventricle and loses its polarity, as predicted by the TIS21 promoter-driven GFP expression identifying neurogenic divisions (11 cells with unequal aPKC out of 12 GFP positive; Fig 4E; supplementary 3D movies 4 and 5 online; Attardo et al, 2008). By contrast, we observed that in most of the divisions, the afadin central domain was inherited by both daughter cells (28 out of 30 cells), with only a small fraction showing a clear bypass of this domain (2 out of 30 cells; Fig 4B; supplementary 3D movie 1 online). These respective distributions of afadin and aPKC in the progeny at the time of division indicate that the AJ microdomains can be differentially inherited. Taken together, our observations therefore provide new evidence that the AJ is split, on asymmetric division, between the polarity and the adhesive microdomains (Fig 4F). We propose that the inheritance of adhesion molecules by both daughter cells, whatever the mode of division, will allow the retention of their apical processes next to the ventricle after mitosis is complete. This is not to say that the AJs will remain normal in the process that loses polarity proteins after asymmetrical division; indeed, given the proposed role of polarity proteins in the maintenance of AJs (Imai et al, 2006; Afonso & Henrique, 2006; Cappello et al, 2006; Ghosh et al, 2008), the splitting away of these proteins from the adhesion molecules would be predicted to destabilize the AJ and might be responsible for the later detachment of the apical processes from the ventricle. In support of this, aPKC has not been detected in those apical processes involving detachment from the ventricle (Ghosh et al, 2008), and we observe that aPKC is inherited by the daughter stem cell whose apical membrane remains in contact with the ventricle.
Our model requires that the orientation of the cleavage plane is tightly regulated to split the adhesive and polarity domains. It follows that abnormal cleavage planes will increase the probability that one daughter cell does not inherit adhesion molecules and prematurely loses its apical attachment. In agreement with this, recent studies have described mispositioning of the progeny as a result of random cleavage planes (Morin et al, 2007; Konno et al, 2008; Yingling et al, 2008). The consequences of this abnormal cell positioning in neurogenesis are crucial questions that remain to be answered.
Methods
Immunohistochemistry. Pregnant CD-1 mice were purchased from a UK supplier. Central nervous system embryonic tissues were harvested in accordance with the Animals (Scientific Procedures) Act 1986. Tissues were fixed with 4% paraformaldehyde overnight at 4°C and incubated in 30% sucrose before cryostat sectioning. Paraformaldehyde-fixed brains collected from E14 heterozygous embryos of the knock-in mouse line expressing GFP under the control of the TIS21 promoter were generously provided by W. Huttner.
Sections were treated with a blocking buffer containing Triton X100 detergent (0.5–1%) and bovine serum albumin (3%) for 1 h. Primary antibodies (supplementary Table 1 online) were incubated overnight in the blocking buffer without detergent at 20°C. Appropriate AlexaFluor dye conjugated secondary antibodies (Molecular Probes, Invitrogen Ltd, Paisley, UK) were incubated for 1–2 h along with Hoechst for nuclei staining. Images were captured with a Leica SP1/SP2 or a Zeiss confocal.
Prediction of cleavage plane positioning. Cleavage plane orientation was determined according to the procedure described by Kosodo et al (2004), and is detailed in the supplementary information online section.
Partitioning of N-cadherin and AJ microdomains. To quantify the amount of N-cadherin fluorescence in each presumptive membrane territory (Fig 2), the 1.5–2.5-μm depth stack of 3–5 consecutive confocal sections showing the same chromosome morphologies was projected as a sum of pixels and the fluorescence intensity evaluated in the sum-projected image with the integrated density function of the Image J software. The comparison of fluorescence intensities in the two presumptive membrane territories was expressed as the ratio of the sum of pixel values—that is, integrated density. The distribution of ratios was depicted for each population and the medians calculated to compare the distributions in different populations. Statistical analysis was carried out using the nonparametric Mann–Whitney U test.
For determination of AJ domains inheritance (Fig 4), the focal plane for which the ‘cadherin hole' was the largest was considered for further analysis. The 3D reconstructed views provided in Figs 2 and 4 were built from lateral or en face views of z-stacks using the Volocity software.
Positioning of AJ rings. The relative positioning of AJ rings was evaluated using en face stacks of confocal images acquired at 0.12-μm intervals. Fluorescence intensity profile analyses along the z-axis were drawn using the Leica confocal software. 3D reconstructions were performed with stacks of images at 0.2-μm intervals acquired on cryosections of 30 μm depth using Imaris software (Bitplane, Zurich, Switzerland).
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary 3D movie 1
Supplementary 3D movie 2
Supplementary 3D movie 3
Supplementary 3D movie 4
Supplementary 3D movie 5
Supplementary Information
Acknowledgments
We are grateful to the members of C.ff.-C. and G. Woods labs for their helpful comments and critical reading of the paper. We thank I. Kazanis for stimulating discussions during this work. We are particularly grateful to W. Huttner for kindly providing the TIS21-GFP mouse tissues and for his helpful comments on this work. We thank the Cambridge Anatomy Department and the Cambridge Institute for Medical Research microscopy facilities for their invaluable help. V.M. was funded by a long-term European Molecular Biology Organization fellowship and by a Biotechnology and Biological Sciences Research Council project grant to C.ff.-C. and V.M.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aaku-Saraste E, Hellwig A, Huttner WB (1996) Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure—remodeling of the neuroepithelium prior to neurogenesis. Dev Biol 180: 664–679 [DOI] [PubMed] [Google Scholar]
- Afonso C, Henrique D (2006) PAR3 acts as a molecular organizer to define the apical domain of chick neuroepithelial cells. J Cell Sci 119: 4293–4304 [DOI] [PubMed] [Google Scholar]
- Attardo A, Calegari F, Haubensak W, Wilsch-Brauninger M, Huttner WB (2008) Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS ONE 3: e2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello S et al. (2006) The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci 9: 1099–1107 [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA (2002) Regulation of cerebral cortical size by control of cell cycle exit in neuronal precursors. Science 297: 365–369 [DOI] [PubMed] [Google Scholar]
- Chenn A, Zhang YA, Chang BT, McConnell SK (1998) Intrinsic polarity of mammalian neuroepithelial cells. Mol Cell Neurosci 11: 183–193 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Marquardt T, Thaler JP, Carter N, Andrews SE, Pfaff SL, Hunter T (2008) Instructive role of aPKCzeta subcellular localization in the assembly of adherens junctions in neural progenitors. Proc Natl Acad Sci USA 105: 335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6: 777–788 [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB (2004) Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA 101: 3196–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S (2006) Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development 133: 1735–1744 [DOI] [PubMed] [Google Scholar]
- Kadowaki M, Nakamura S, Machon O, Krauss S, Radice GL, Takeichi M (2007) N-cadherin mediates cortical organization in the mouse brain. Dev Biol 304: 22–33 [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F (2008) Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol 10: 93–101 [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB (2004) Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J 23: 2314–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V (2006) αE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science 311: 1609–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe N, Hirai S, Imai F, Nakanishi H, Takai Y, Ohno S (2002) Association of ASIP/mPAR-3 with adherens junctions of mouse neuroepithelial cells. Dev Dyn 225: 61–69 [DOI] [PubMed] [Google Scholar]
- Miyata T (2007) Asymmetric cell division during brain morphogenesis. Prog Mol Subcell Biol 45: 121–142 [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M (2001) Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31: 727–741 [DOI] [PubMed] [Google Scholar]
- Mizuhara E, Nakatani T, Minaki Y, Sakamoto Y, Ono Y, Takai Y (2005) MAGI1 recruits Dll1 to cadherin-based adherens junctions and stabilizes it on the cell surface. J Biol Chem 280: 26499–26507 [DOI] [PubMed] [Google Scholar]
- Morin X, Jaouen F, Durbec P (2007) Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat Neurosci 10: 1440–1448 [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR (2004) Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 7: 136–144 [DOI] [PubMed] [Google Scholar]
- Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, Mondens M, Takai Y (2003) Direct binding of cell polarity protein Par3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem 278: 5497–5500 [DOI] [PubMed] [Google Scholar]
- Yingling J, Youn YH, Darling D, Toyo-Oka K, Pramparo T, Hirotsune S, Wynshaw-Boris A (2008) Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell 132: 474–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 3D movie 1
Supplementary 3D movie 2
Supplementary 3D movie 3
Supplementary 3D movie 4
Supplementary 3D movie 5
Supplementary Information