The ‘Current Trends in Biomedicine' workshop on the role of RNA structures in the translation of viral and cellular RNAs took place between 27 and 29 October 2008, in Baeza, Spain, and was organized by E. Martínez-Salas, G. Belsham & J. Gómez.
Glossary
Introduction
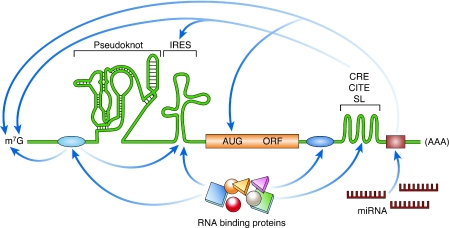
Since the elucidation of the genetic code approximately 40 years ago, the role of RNA in crucial biological processes has gained increasing attention, and is now one of the fastest developing areas in molecular biology and biochemistry. The advances made in structural studies of transfer RNA (tRNA), ribozymes, riboswitches, the ribosome and viral IRES elements in particular have shown the importance of secondary and tertiary structures in RNA biology, and have significantly changed our understanding of the translational control of gene expression (Fig 1). The World Heritage town of Baeza in Andalucia, Spain, hosted the ‘Current Trends in Biomedicine' workshop on the role of RNA structures in the translation of viral and cellular RNAs. About 45 participants met in the beautiful buildings of the Sede Antonio Machado at the Universidad Internacional de Andalucía.
Figure 1.
Schematic illustration of discreet RNA regulatory elements. The translation of an mRNA is regulated by diverse mechanisms that involve both structural and non-structural RNA elements, as well as interactions with RNA-binding proteins. IRESes are found in the 5′ UTR and promote cap-independent translation. Pseudoknots can be located in the 5′ UTR, the 3′ UTR, or the coding region, and their localization influences their effect on translation; for example, initiation, frameshifting and termination. 3′ UTR structural elements such as CREs, CITEs and SLs often function through long-range RNA interactions. The miRNA target sites are located in the 3′ UTR. The canonical mRNA features of the cap (m7G) and poly(A) are targets of several regulatory interactions. The RNA-binding protein-binding sites are shown as blue ovals, and the miRNA-binding site is shown as a brown rectangle. AUG, initiation codon; CITE, cap-independent translational enhancer; CRE, cis-acting replication element; IRES, internal ribosome entry site; m7G, 7-methyl-guanosine; miRNA, microRNA; mRNA, messenger RNA; ORF, open-reading frame; SL, stem loop; UTR, untranslated region.
RNA structure
Despite extensive efforts, accurate modelling of the two-dimensional and three-dimensional structures of RNA, and the search for consensus structural motifs remain challenging. In the opening session of the workshop, E. Westhof (Strasbourg, France) laid the foundations for extracting rules for RNA architecture so that bioinformatics approaches can be used to search for new non-coding RNAs. He characterized 12 families of geometrical interactions that occur between nucleic-acid base pairs; besides the standard Watson–Crick (WC) base pairing, additional non-WC pairings have an important role in RNA structure. Therefore, RNA architecture can be viewed as the result of the assembly of preformed double-stranded helices defined by the WC base pairs and RNA motifs that are maintained by the non-WC base pairs. These interactions are less dependent on the primary RNA sequence, and might explain the molecular neutrality observed in RNA sequences and structures throughout evolution, making the search for non-coding RNAs even more complex.
M. Holcik (Ottawa, ON, Canada) summarized the bioinformatics efforts made by his group to identify new cellular IRES elements based on structural similarities to known cellular IRESes. The surprising conclusion of this presentation was that, unlike viral IRESes, cellular IRESes probably cannot be defined by common structural elements, but rather by short motifs and binding sites for protein factors (Baird et al, 2007). By contrast, the function of pseudoknots—which are well-characterized RNA structural elements—is intimately linked to RNA structure. I. Brierley (Cambridge, UK) spoke about the roles of pseudoknots in translation. Initially discovered in plant virus genomes, pseudoknots are widespread in their functions and locations (Brierley et al, 2008). For example, the pseudoknots found in the 5′ UTRs of messenger RNA (mRNA) act in translation initiation, whereas those located in coding regions regulate frameshifting, termination-codon readthrough, bacterial transfer mRNA rescue, elongation and termination. The pseudoknots located at the 3′ UTR have a role in the switch between translation and replication of viral RNAs. For example, the IBV pseudoknot located at the 1a–1b overlap induces a –1 frameshift and is required for the expression of RNA-dependent RNA polymerases. The purification of pseudoknot-stalled complexes from RRL and cryo-electron microscopy studies of the ribosomal–mRNA complex revealed that the pseudoknot interacts with the ribosome to block the mRNA-entry channel, which might prevent its unwinding, effectively ‘jamming up' the translation of the mRNA and inducing the ribosome to pause. What leads to this resistance to mechanical unwinding is now under investigation.
HCV IRES: a paradigm to study structured RNAs
The HCV 5′ UTR—which is one of the most studied RNA structures—contains an IRES and discrete domains I–VI. J. Gomez (Granada, Spain) presented data describing a conformational change in the HCV 5′ UTR. Domain I is a 43-nucleotide motif that enhances viral replication and is recognized by the liver-specific microRNA miR122. Gomez showed that the left-hand side of domain IV anneals with a complementary sequence in domain I to form a long-range annealing (LRA) RNA that presents the HCV IRES in a circular form, which is a structure confirmed by double-stranded RNA-dependent RNase III probing. Further analysis suggested that the binding of miR122 to this LRA RNA might induce a conformational change in the LRA, which promotes translation.
P. Lukavsky (Cambridge, UK) presented the nuclear magnetic resonance structure of the HCV IRES domain II and discussed the function of individual HCV IRES domains during ribosome assembly. Domain III alone can function in the assembly of 48S complexes on the IRES, whereas a new role for domain II downstream of 80S-complex assembly was demonstrated by showing that it promotes eIF5-induced GTP hydrolysis and eIF2/GDP release from the 48S complex (Locker et al, 2007). L. Easton (Cambridge, UK) spoke about her work on the pseudoknot region of the HCV-like PTV-1 IRES. This group has identified a conserved tertiary interaction between domains IIIe and IIIf of the pseudoknot, which involves a base-pair match that is conserved in all HCV-like IRESes. Mutation of the C of the GACA loop resulted in loss of IRES activity, whereas a compensatory mutation of the G residue in the stem restored the activity, suggesting that this interaction is important for IRES function. As this region has also been shown to interact with sequences in the 3′ UTR, the function of both of these interactions remains to be resolved. L. Roberts (Surrey, UK) highlighted the recent discovery of an HCV-like IRES within the genome of the picornavirus SVV that closely resembles the CSFV IRES. However, this work led her to suggest that the conserved structures that are thought to exist within the CSFV IRES—that is, domain IIId2—might not have any role in SVV IRES function.
RNA–protein interactions in structured RNA
The binding of RNA-binding proteins to RNAs is crucial for the interpretation of the regulatory signals that are encoded by the RNA structure or sequence. The members of one such class of RNA-binding proteins are the ITAFs, which facilitate the function of both cellular and viral IRESes (Baird et al, 2006). Although many ITAFs have been identified, the search for new or common factors is clearly required to understand fully the mechanism of IRES action. E. Martinez-Salas (Madrid, Spain) presented the approach used by her group for analysing ITAFs that are associated with various viral IRESes—that is, HCV and FMDV—and comparing different domains within the same IRES. By using a proteomics approach, Martinez-Salas and colleagues identified several new proteins that interact with domains 3 and 5 of the FMDV IRES, and domain III of the HCV IRES (Pacheco et al, 2008). Functional analysis showed that the knockdown of Gemin 5—the only protein that binds to both FMDV and HCV IRESes—not only enhanced the activity of these IRESes, but also affected cap-dependent translation from a dicistronic mRNA. These results suggest that Gemin 5 is a repressor of both IRES-dependent translation and cap-dependent translation, possibly through an interaction with eIF4E (Pacheco et al, 2009).
D. Ostareck (Halle, Germany) described a riboproteomics approach to search for HCV ITAFs. Using an in vitro-based translation system he has shown a 3′ UTR-dependent enhancement of HCV IRES activity, which was the result of increased 48S-complex formation on the IRES when the 3′ UTR was present. To identify factors involved in this process, Ostareck used a tobramycin aptamer-affinity technique, and identified IGF2BP1 as binding to both the 5′ and 3′ UTRs of the HCV RNA. Work from his laboratory also showed that IGF2BP1 knockout in Huh-7 cells decreased IRES activity and therefore HCV translation, but had no effect on replication, suggesting a specific function of IGF2BP1 in the process of HCV RNA translation.
A. Willis (Nottingham, UK) described the identification of PSF, GRSF, YB-1 and P54nrb as ITAFs for the Myc family of IRESes. Investigation of the requirements of the Myc IRESes for the canonical initiation factors eIF4G, eIF4A, PABP, eIF3 and eIF2 showed that N-Myc and c-Myc IRESes required the middle fragment of eIF4G, eIF4A and eIF2. They were dependent on eIF3 for function, although it seemed that this factor was not recruited by eIF4G. By contrast, the L-Myc IRES required eIF4F and an interaction of eIF3 with eIF4G. In addition, the expression of some ITAFs was induced during TRAIL-induced apoptosis, suggesting a possible role for IRES function during cellular stress (Spriggs et al, 2009). However, the fact that many IRES-containing mRNAs—as well as being associated with polysomes—are translationally repressed during normal growth conditions and only become derepressed during apoptosis, complicates this issue.
I. Shatsky (Moscow, Russia) challenged the prevalent view that the long and structured 5′ UTRs found in some cellular mRNAs interfere with efficient cap-dependent translation. He presented data showing that translation mediated by several cellular IRESes such as c-Myc, hsp70 and Apaf-1, is strongly dependent on the presence of an m7G-cap structure, challenging the idea that these are indeed IRESes capable of cap-independent initiation. To explain how mRNAs can be differentially regulated under stress conditions, he offered an alternative view in which individual 5′ UTRs have different m7G-cap requirements and are resistant to the inhibition of eIF4E activity to a varying extent. According to this hypothesis, the variable cap-dependence is determined, at least in part, by specific features of the 5′ UTR and its associated RNA-binding proteins. G. Belsham (Lindholm, Denmark) offered a similar conclusion in his discussion of the characteristics of HCV-like picornavirus IRESes. Intriguingly, although mutant forms of these IRESes cannot support the internal initiation of translation from a bicistronic mRNA, these highly structured 5′ UTRs do not inhibit efficient cap-dependent translation from monocistronic mRNAs. Furthermore, he presented evidence that this cap-dependent translation is dependent on intact eIF4G and active eIF4A. These data suggest that structured 5′ UTRs (lacking IRES activity) could evolve into a functional IRES element within a capped mRNA without inhibiting cap-dependent translation (Belsham et al, 2008).
F. Gebauer (Barcelona, Spain) reported on the translational regulation of dosage compensation in Drosophila. MSL-2 is the limiting component of the DCC of D. melanogaster, which is responsible for increased transcription of the single male X chromosome. The repression of msl-2 mRNA translation in females is required to prevent the lethal assembly of the DCC onto both X chromosomes, and constitutes a model for translational control by RNA-binding proteins (Hentze et al, 2007). msl-2 repression in females requires the interaction of the female-specific SXL protein with the co-repressor UNR. UNR is required to prevent DCC formation in females, whereas UNR-mutant males also showed decreased DCC formation and X-chromatin alteration. In addition, the overexpression of UNR preferentially kills males. These data suggest that UNR performs opposing sex-specific functions in males and females: in the latter, UNR inhibits DCC formation by repressing msl-2 mRNA translation; whereas in the former, UNR promotes DCC formation by a mechanism that is independent of known DCC components (Patalano et al, 2009). These presentations emphasize the fact that many common RNA-binding proteins are recruited by distinct RNA elements for various biological processes, and highlight the progress being made in defining specific functions for these factors.
Switch from translation to replication of viral genomes
C. Romero-López (Armilla, Spain) discussed new findings on how the switch between translation and replication of viral RNA is mediated during HCV infection. She presented evidence that the 5′ and 3′ UTRs interact during replication. By using in silico predictions and in vitro analysis, Romero-López and colleagues found that this interaction requires domain IIId of the IRES, and SLII and SLIII of the 3′ UTR, as well as the CRE found at the 3′ end of the genome, suggesting the formation of an important high-order structure. Their data indicate that a molecular ‘bridge' is formed that might connect the two ends of the genome, and somehow modulate the switch between translation and replication during infection. Such switches have been documented extensively in picornaviruses and other positive-strand viruses. J. Díez (Barcelona, Spain) presented her new data on the role of the cellular decapping activator complex LSm1-7 in the BMV life cycle. A correlation was found between LSm1-7 binding to specific sequences in the BMV genome and the regulation of viral translation and replication. Therefore, it seems that the BMV has hijacked a cellular pathway of RNA degradation to promote viral propagation.
Translation and miRNA
miRNAs, which are approximately 21–23 nucleotide-long regulators of gene expression, base pair to target mRNAs and either cause mRNA cleavage or repress translation (Pillai et al, 2007). However, the mechanism by which miRNAs repress translation has not been fully elucidated. M. Hentze (Heidelberg, Germany) described the development of a cell-free system to study miRNA-mediated translational repression (Thermann & Hentze, 2007). miRNAs target the recruitment of the 40S ribosomal subunit to the mRNA in a cap-dependent manner. Hentze reported the generation of new synthetic cap analogues that do not attenuate cap-dependent translation and augment miRNA-mediated translational repression. Furthermore, he showed that miRNAs cause the deadenylation of mRNAs and that artificial removal of the poly(A) tail reduces miRNA-mediated translational repression. These data suggest a two-hit model for miRNA-mediated translational repression in which the RISC targets the cap complex, which is enhanced by deadenylation.
M. Bushell (Nottingham, UK) suggested a link between the promoter sequence that is used to transcribe the mRNA and the mechanism of miRNA-mediated translational regulation in the cytoplasm. Previous studies have identified two mutually exclusive mechanisms of miRNA-mediated translational repression at either the initiation or the post-initiation step of translation. Bushell showed that the mode of repression is dependent on the promoter that is used to transcribe the mRNA containing the miRNA-target sequence. These data suggest that the mode of translational repression is determined by the intrinsic properties and co-transcriptional events associated with the promoter because no correlation with the 5′ UTR was found and the translational repression of mRNAs that are transfected directly into the cytoplasm occurs only at the initiation step (Kong et al, 2008). Both presentations clarified some of the outstanding issues in understanding how miRNAs affect translation of their target mRNAs.
Targeting translation in disease
The recruitment of the ribosome during the initiation of translation is rate limiting and is a target for regulation in various conditions such as viral infection and many cancers (Mathews et al, 2007). It has been known for several years that increased levels of eIF4E are associated with the transformation of cells and that decreasing the level of eIF4E can reverse the malignant phenotype. Similarly, increased levels of phosphorylated 4E-BP1 have been associated with advanced disease and poor prognosis in human tumours. J. Pelletier (Montreal, QC, Canada) described a screen based on in vitro translation in Krebs cell extracts to search for new compounds that inhibit eIF4E/4F activity and cap-dependent translation. Of 150,000 compounds, several have been shown to inhibit eIF4F activity specifically; for example, hippuristanol, pateamine and silvestrol interfere with eIF4A function (Bordeleau et al, 2006). Hippuristanol decreases the ability of eIF4A to bind to RNA, whereas the other two compounds stimulate binding to RNA, thereby depleting the levels of eIF4A in the eIF4F complex (Lindqvist et al, 2008). Importantly, silvestrol inhibits translation in a mouse tumour model and maintains a tumour-free state for longer periods than the use of standard treatment alone. Finally, two new inhibitors that lead to the inhibition of cap-dependent translation by blocking the interactions of eIF4GI and eIF4GII with eIF4E, and eIF4E with 4E-BP1, respectively, have been reported. It has been shown, however, that HCV IRES-driven translation is relatively unaffected and that no effect is seen on the CrPV IGR IRES. The prospect of using such inhibitors in cancer treatment is exciting because eIF4E and the pathways that regulate its activity sit at a convergence point of many pathways that are dysregulated in cancers.
N. Sonenberg (Montreal, QC, Canada) reported on the translational control of innate immunity by IRF-7. By using mice and MEFs lacking the translational repressors 4E-BP1 and 4E-BP2, Sonenberg showed that there is enhanced production of type-I interferon in the absence of 4E-BPs, which correlates with the suppression of viral replication and significant resistance to viral infection. This response is a result of the translational upregulation of the IRF-7 mRNA, which encodes the master regulator of the type-I interferon response. Notably, the 5′ UTR of IRF-7 mRNA is highly structured and evolutionarily conserved, and although it does not contain an IRES or any other discernable regulatory element, it is the genetic determinant of the translational regulation of IRF-7 (Colina et al, 2008).
S. Vagner (Toulouse, France) investigated the post-transcriptional events in a metastatic model of breast cancer. His group focused on ITAFs, alternative splicing and miRNAs, as they can regulate groups of genes that are equivalent to transcriptional operons. Vagner and colleagues showed that the expression of a single miRNA is sufficient to convert a non-metastatic cell line into a metastatic cell line. In addition, he identified hnRNP A1 as an independent prognostic marker for relapse-free survival in breast cancer. Interestingly, the forced expression of a cytoplasmic variant of hnRNP A1 was sufficient to increase the invasive properties of several breast tumour cell lines and altered the polysomal association of some mRNAs, suggesting that hnRNP A1 regulates the translation of one or several target genes involved in metastatic progression.
C. Florentz (Strasbourg, France) discussed data linking mutations in mitochondrial tRNAs to human neurodegenerative disorders. tRNAs are essential components of the translational machinery that deliver amino acids to the translating ribosome. Although the structural features of tRNAs are well studied and are conserved across species, mitochondrial tRNAs deviate markedly from this conservation. Detailed structural investigations are in progress in order to understand the primary molecular impacts of more than 130 mutations that are found in genes for mitochondrial tRNAs and linked to severe human pathologies.
In plants, susceptibility to virus infection is often linked to the translation-initiation factors eIF4E and eIF4G (Robaglia & Caranta, 2006), although the underlying basis for this is poorly understood. V. Truniger (Murcia, Spain) presented data showing that a sequence in the 3′ UTR of the MNSV genome is responsible for overcoming eIF4E-mediated resistance in plants by functioning as a 3′ CITE. The 3′ CITE interacts with sequences in the 5′ UTR to increase cap-independent translational activity. The Truniger model suggests that inefficient interaction between eIF4E of the resistant plant and the 3′ CITE of an avirulent isolate of MNSV prevents efficient formation of the translation-initiation complexes on the viral RNAs and, ultimately, leads to resistance of the melon to MNSV infection. Targeting new mechanisms of translation initiation used by pathogenic viruses is also an attractive idea for antiviral therapy.
J. Toulmé (Bordeaux, France) used a genetic suppressor-element strategy to identify sequences that are able to inhibit HCV replication. This involved screening randomized HCV genetic elements within a retroviral library, and resulted in the discovery of a 20-amino-acid peptide corresponding to part of the NS5A sequence, named GE4, that displays inhibitory properties. GE4 specifically inhibited HCV translation and replication in Huh-7 cells, indicating that it might be used as an HCV translational inhibitor (Jaffrelo et al, 2008). These presentations clearly illustrated the link between the dysregulation of translation and disease states, and provided a glimpse into the possible therapeutic exploitation of the components of the translational machinery to develop cures for many disorders or viral infections.
Concluding remarks
Although great progress has been made in elucidating the role of RNA structures in the regulation of translation, many questions remain. This meeting provided an excellent opportunity to discuss emerging concepts in the field, including the importance of translational control in disease states and the idea of using translation initiation as a mechanism to control gene expression. The meeting also highlighted the importance of RNA structures, the role of the interaction between 5′ and 3′ UTRs of mRNAs in translational regulation, and the link between translation and replication in viruses. The intellectually stimulating meeting was complemented by outstanding food and wine, the picturesque setting of the Universidad Internacional de Andalucía and the warm Andalucian hospitality.
4E-BP eukaryotic initiation factor 4E-binding protein
Apaf-1 apoptotic protease-activating factor-1
BMV brome mosaic virus
CITE cap-independent translational enhancer
c-Myc myelocytomatosis viral related oncogene
CRE cis-replication element
CrP cricket paralysis virus
CSFV classical swine fever virus
DCC dosage-compensation complex
eIF eukaryotic initiation factor
FMDV foot and mouth disease virus
GE4 genetic element 4
GRSF G-rich sequence factor
HCV hepatitis C virus
hnRNP A1 heterogeneous nuclear ribonucleoprotein A1
Hsp70 heat-shock protein 70
Huh-7 human hepatoma cell line
IBV infectious bronchitis virus
IGF2BP1 insulin-like growth factor-II messenger RNA-binding protein 1
IGR intergenic region
IRES internal ribosome entry site
IRF-7 interferon-regulatory factor 7
ITAF internal ribosome entry site trans-acting factor
L-myc myelocytomatosis viral related oncogene, lung carcinoma derived
LSm1-7 like Sm protein 1-7
m7G 7-methyl-guanosine
MEFs mouse embryonic fibroblasts
miR122 micro RNA 122
MNSV melon necrotic spot virus
MSL-2 male-specific lethal 2
N-myc myelocytomatosis viral related oncogene, neuroblastoma derived
NS5A non-structural 5A
P54nrb 54 kD nuclear RNA-binding protein
PABP poly(A)-binding protein
PSF polypyrimidine tract-binding protein-associated splicing factor
PTV-1 porcine teschovirus-1
RISC RNA-induced silencing complex
RRL rabbit reticulocyte lysate
SL stem loop
SVV Seneca Valley virus
SXL sex lethal
TRAIL tumour necrosis factor-related apoptosis-inducing ligand
UNR upstream of N-ras
UTR untranslated region
YB-1 Y-box binding protein 1

Lisa Roberts

Martin Holcik
Acknowledgments
We thank E. Martínez-Salas, G. Belsham and J. Gómez for organizing the conference, and the Universidad Internacional de Andalucía (UNIA) and the Instituto de Salud Carlos III (ISCIII) for financial support. We apologize to those speakers whose work could not be mentioned owing to space constraints.
References
- Baird SD, Turcotte M, Korneluk RG, Holcik M (2006) Searching for IRES. RNA 12: 1755–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird SD, Lewis SM, Turcotte M, Holcik M (2007) A search for structurally similar cellular internal ribosome entry sites. Nucleic Acids Res 35: 4664–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham GJ, Nielsen I, Normann P, Royall E, Roberts LO (2008) Monocistronic mRNAs containing defective hepatitis C virus-like picornavirus internal ribosome entry site elements in their 5′ untranslated regions are efficiently translated in cells by a cap-dependent mechanism. RNA 14: 1671–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeleau ME, Mori A, Oberer M, Lindqvist L, Chard LS, Higa T, Belsham GJ, Wagner G, Tanaka J, Pelletier J (2006) Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat Chem Biol 2: 213–220 [DOI] [PubMed] [Google Scholar]
- Brierley I, Gilbert RJ, Pennell S (2008) RNA pseudoknots and the regulation of protein synthesis. Biochem Soc Trans 36: 684–689 [DOI] [PubMed] [Google Scholar]
- Colina R et al. (2008) Translational control of the innate immune response through IRF-7. Nature 452: 323–328 [DOI] [PubMed] [Google Scholar]
- Hentze MW, Gebauer F, Preiss T (2007) cis-regulatory sequences and trans-acting factors in translational control. In Translational Control in Biology and Medicine, MB Mathews, N Sonenberg, JWB Hershey (Eds), pp 269–298. New York, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Jaffrelo L, Chabas S, Reigadas S, Pflieger A, Wychowski C, Rumi J, Ventura M, Toulme JJ, Staedel C (2008) A functional selection of viral genetic elements in cultured cells to identify hepatitis C virus RNA translation inhibitors. Nucleic Acids Res 36: e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YW et al. (2008) The mechanism of micro-RNA-mediated translation repression is determined by the promoter of the target gene. Proc Natl Acad Sci USA 105: 8866–8871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist L et al. (2008) Selective pharmacological targeting of a DEAD box RNA helicase. PLoS ONE 3: e1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker N, Easton LE, Lukavsky PJ (2007) HCV and CSFV IRES domain II mediate eIF2 release during 80S ribosome assembly. EMBO J 25: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews MB, Sonenberg N, Hershey JWB (2007) Origins and principles of translational control. In Translational Control in Biology and Medicine, MB Mathews, N Sonenberg, JWB Hershey (Eds), pp 1–40. Cold Spring Harbor, New York, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Pacheco A, Reigadas S, Martinez-Salas E (2008) Riboproteomic analysis of polypeptides interacting with the internal ribosome-entry site element of foot-and-mouth disease viral RNA. Proteomics 8: 4782–4790 [DOI] [PubMed] [Google Scholar]
- Pacheco A, Lopez de Quinto S, Ramajo J, Fernandez N, Martinez-Salas E (2009) A novel role for Gemin5 in mRNA translation. Nucleic Acids Res 37: 582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patalano S, Mihailovich M, Belacortu Y, Paricio N, Gebauer F (2009) Dual sex-specific functions of Drosophila Upstream of N-ras in the control of X chromosome dosage compensation. Development 136: 689–698 [DOI] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Filipowicz W (2007) Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol 17: 118–126 [DOI] [PubMed] [Google Scholar]
- Robaglia C, Caranta C (2006) Translation initiation factors: a weak link in plant RNA virus infection. Trends Plant Sci 11: 40–45 [DOI] [PubMed] [Google Scholar]
- Spriggs KA et al. (2009) Canonical initiation factor requirements of the Myc family of internal ribosome entry segments. Mol Cell Biol 29: 1565–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermann R, Hentze MW (2007) Drosophila miR2 induces pseudo-polysomes and inhibits translation initiation. Nature 447: 875–878 [DOI] [PubMed] [Google Scholar]