Abstract
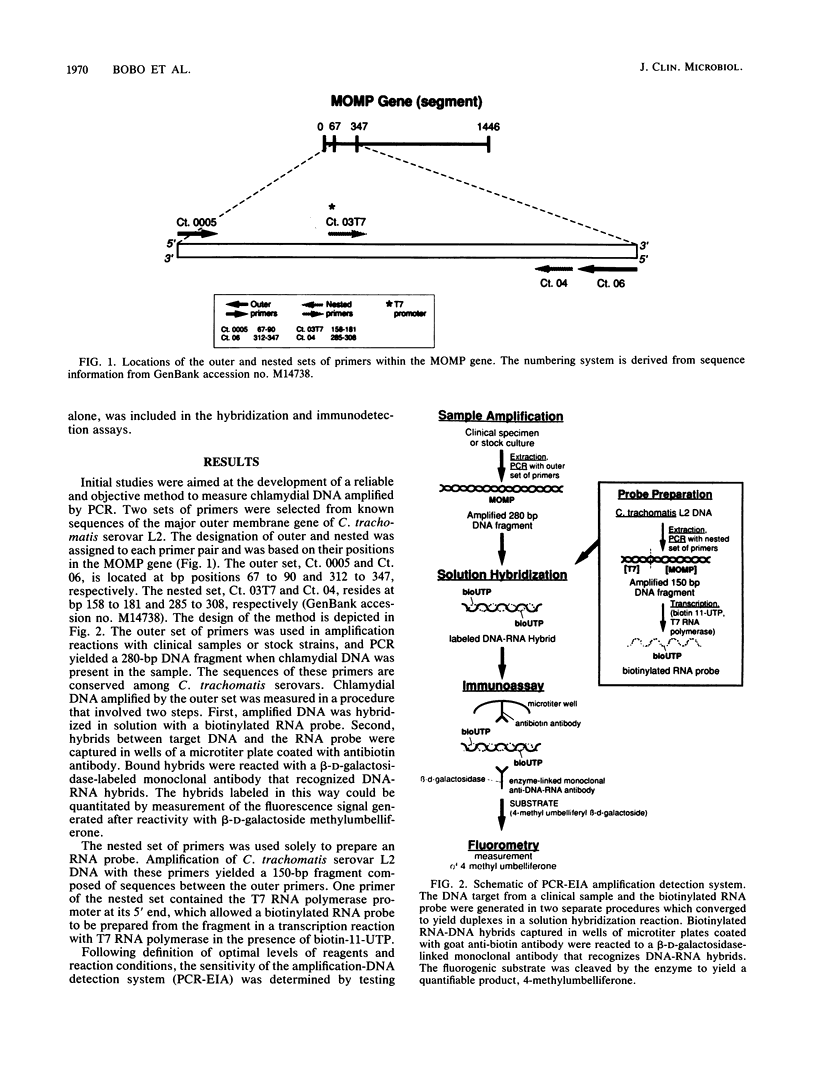
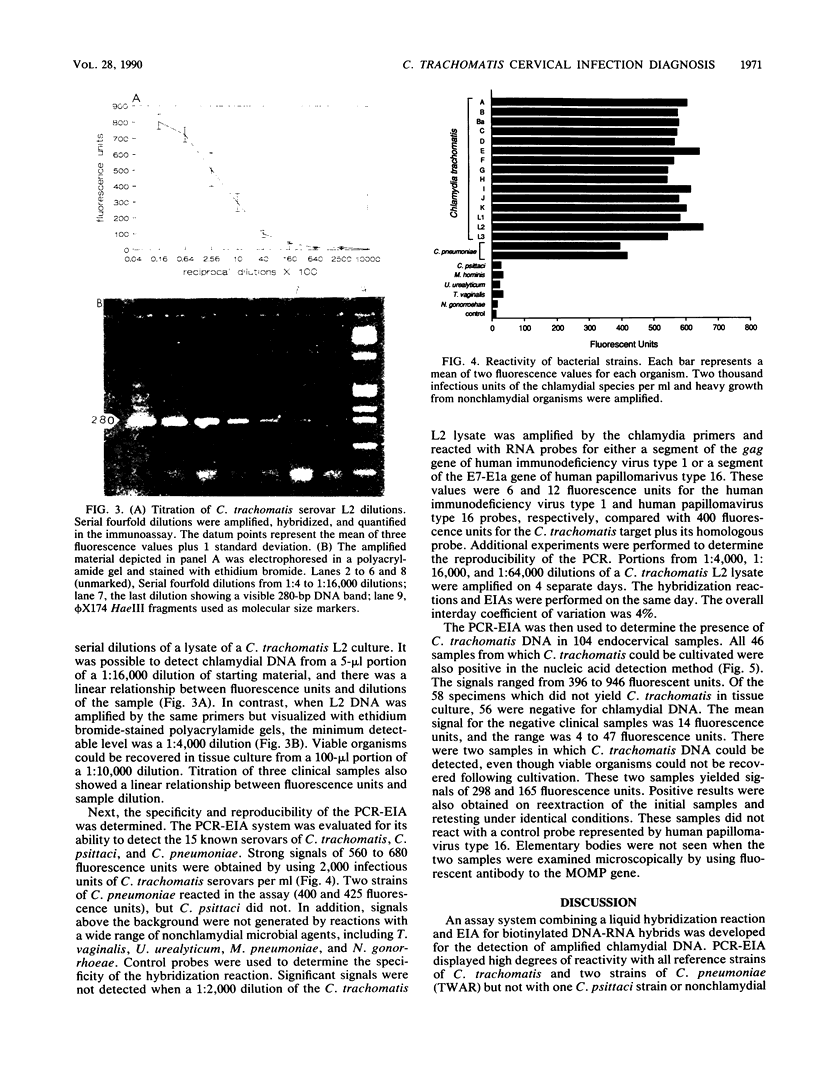
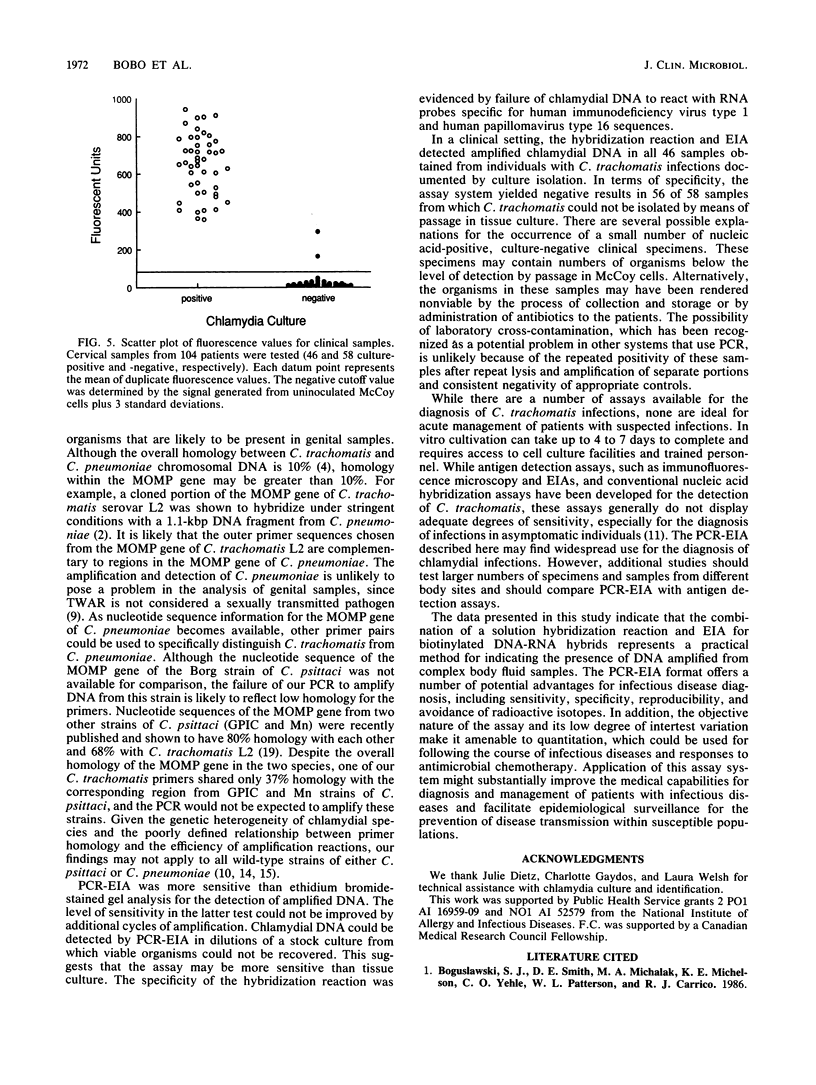
A sensitive and specific system for detection of amplified Chlamydia trachomatis DNA from cervical specimens by fluorometric quantitation in an enzyme immunoassay (EIA) format (polymerase chain reaction [PCR]-EIA) is described. The primers selected for PCR-amplified DNA were from the 15 serovars of C. trachomatis and two strains of Chlamydia pneumoniae (TWAR). One strain of Chlamydia psittaci (Borg) was not amplified. One hundred four previously cultured cervical specimens were evaluated. Forty-six culture-positive specimens containing from 1+ to 4+ inclusion bodies were all positive by PCR-EIA. Of 58 culture-negative specimens, 2 were repeatedly positive and were nonreactive with control probes. This assay system represents a sensitive and specific combination of technologies for the quantitative detection of C. trachomatis DNA directly from a body fluid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell L. A., Kuo C. C., Grayston J. T. Characterization of the new Chlamydia agent, TWAR, as a unique organism by restriction endonuclease analysis and DNA-DNA hybridization. J Clin Microbiol. 1987 Oct;25(10):1911–1916. doi: 10.1128/jcm.25.10.1911-1916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlée F., Bobo L., Mayur K., Yolken R. H., Viscidi R. P. Immunodetection of DNA with biotinylated RNA probes: a study of reactivity of a monoclonal antibody to DNA-RNA hybrids. Anal Biochem. 1989 Aug 15;181(1):96–105. doi: 10.1016/0003-2697(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Dallas P. B., Flanagan J. L., Nightingale B. N., Morris B. J. Polymerase chain reaction for fast, nonradioactive detection of high- and low-risk papillomavirus types in routine cervical specimens and in biopsies. J Med Virol. 1989 Feb;27(2):105–111. doi: 10.1002/jmv.1890270207. [DOI] [PubMed] [Google Scholar]
- Dutilh B., Bébéar C., Rodriguez P., Vekris A., Bonnet J., Garret M. Specific amplification of a DNA sequence common to all Chlamydia trachomatis serovars using the polymerase chain reaction. Res Microbiol. 1989 Jan;140(1):7–16. doi: 10.1016/0923-2508(89)90053-3. [DOI] [PubMed] [Google Scholar]
- Engelke D. R., Hoener P. A., Collins F. S. Direct sequencing of enzymatically amplified human genomic DNA. Proc Natl Acad Sci U S A. 1988 Jan;85(2):544–548. doi: 10.1073/pnas.85.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa D. T., Lee M. H., Wolinsky S. M., Sano K., Morales F., Kwok S., Sninsky J. J., Nishanian P. G., Giorgi J., Fahey J. L. Human immunodeficiency virus type 1 infection in homosexual men who remain seronegative for prolonged periods. N Engl J Med. 1989 Jun 1;320(22):1458–1462. doi: 10.1056/NEJM198906013202205. [DOI] [PubMed] [Google Scholar]
- Li D. K., Daling J. R., Wang S. P., Grayston J. T. Evidence that Chlamydia pneumoniae, strain TWAR, is not sexually transmitted. J Infect Dis. 1989 Aug;160(2):328–331. doi: 10.1093/infdis/160.2.328. [DOI] [PubMed] [Google Scholar]
- Mack D. H., Sninsky J. J. A sensitive method for the identification of uncharacterized viruses related to known virus groups: hepadnavirus model system. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6977–6981. doi: 10.1073/pnas.85.18.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D. M., Atta A. I., Setti S. K. Detection of toxigenic Escherichia coli using biotin-labelled DNA probes following enzymatic amplification of the heat labile toxin gene. Mol Cell Probes. 1988 Mar;2(1):47–57. doi: 10.1016/0890-8508(88)90043-6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shih A., Misra R., Rush M. G. Detection of multiple, novel reverse transcriptase coding sequences in human nucleic acids: relation to primate retroviruses. J Virol. 1989 Jan;63(1):64–75. doi: 10.1128/jvi.63.1.64-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer R., Tautz D. Minimal homology requirements for PCR primers. Nucleic Acids Res. 1989 Aug 25;17(16):6749–6749. doi: 10.1093/nar/17.16.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm W. E. Diagnosis of Chlamydia trachomatis genitourinary infections. Ann Intern Med. 1988 May;108(5):710–717. doi: 10.7326/0003-4819-108-5-710. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Mullenbach G., Sanchez-Pescador R., Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986 Dec;168(3):1277–1282. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich P. P., Bhat R. A., Seto B., Mack D., Sninsky J., Vyas G. N. Enzymatic amplification of hepatitis B virus DNA in serum compared with infectivity testing in chimpanzees. J Infect Dis. 1989 Jul;160(1):37–43. doi: 10.1093/infdis/160.1.37. [DOI] [PubMed] [Google Scholar]
- Zhang Y. X., Morrison S. G., Caldwell H. D., Baehr W. Cloning and sequence analysis of the major outer membrane protein genes of two Chlamydia psittaci strains. Infect Immun. 1989 May;57(5):1621–1625. doi: 10.1128/iai.57.5.1621-1625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]