The development of the mammalian brain requires a large expansion of the neural cell-progenitor pool followed by terminal differentiation. One of the most general and fundamental mechanisms that regulates the conversion of undifferentiated neural stem cells and progenitor cells to differentiated neurons and glia is the conversion of the orientation of mitotic cleavage, which leads to a change from symmetric to asymmetric cell division. Proliferating neural stem cells divide symmetrically during early mammalian neurogenesis to increase the progenitor pool, whereas both symmetric (proliferative) and asymmetric (neurogenic) mitosis occur later in neurogenesis to generate neural stem cells and post-mitotic neurons or glia, respectively (Noctor et al, 2004; Götz & Huttner, 2005). During cell division at the apical (ventricular) surface of the brain, the proliferating neuroepithelial cells and the radial glial progenitors have a distinct apico-basal polarity and an elongated radial morphology (Kosodo et al, 2004; Götz & Huttner, 2005). The disruption of mitotic spindle orientation during neurogenesis results in proliferation and cell-fate defects, supporting the importance of controlling symmetric and asymmetric division to determine cell fate. For example, loss of the G-protein-regulator complex LGN (Leu–Gly–Asn repeat-enriched protein)/AGS3 (activator of G-protein signalling 3)—also known as Gpsm2 (G-protein-signalling modulator 2)/Gpsm3—or the loss or reduction of LIS1 (Lissencephaly 1), which is a noncatalytic subunit of the platelet-activating factor acetylhydrolase Ib, results in randomized spindle orientation during apico-basal division and causes changes in the cell-fate decisions of radial glial progenitors (Konno et al, 2008; Yingling et al, 2008). Although the importance of symmetric and asymmetric cleavage in the maintenance and differentiation of neural progenitors is well understood, the cell fate-determining factors that are symmetrically or asymmetrically distributed during cleavage and regulate the daughter cell-fate decision after mitosis are unknown. Some interesting candidates are distributed asymmetrically at the plasma membrane, including the polarity proteins of the PAR (‘partitioning defective')/aPKC (atypical protein kinase C) complex, adherens junctional proteins of the cadherin/catenin complex, and zonular functional proteins such as ZO1 (zonula occludens 1), Numb and Notch-1 receptor (for a review, see Gotz & Huttner 2005).
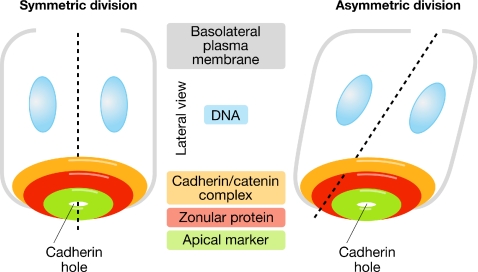
In this issue of EMBO reports, Marthiens and ffrench-Constant provide strong support for the idea that the distribution of the polarity proteins is a crucial determining factor that differentiates symmetric and asymmetric cell divisions; during asymmetric divisions, both daughter cells inherit adhesive proteins, whereas only one daughter cell inherits the polarity proteins (Marthiens & ffrench-Constant, 2009). Marthiens and ffrench-Constant determine the precise positioning of these apically localized polarity and adhesion membrane proteins at the apical surface of the embryonic brain by collecting lateral or en face stacks of confocal images, and use a novel three-dimensional model to visualize the patterns of localization and the spatial relationship between these proteins. By using the localization of actin filaments along the apico-basal axis as a guide, the authors identify three functionally distinct microdomains—which contain Ncad (N-cadherin)/βcat (β-catenin), Afadin/ZO1 or PAR3/aPKC—and determine their precise location in the polarized cell. The polarity proteins were found to be located in the most apical domain, the two junctional proteins—ZO1 and Afadin—were positioned medially, and the adherens junctional proteins—such as Ncad and βcat—were in a slightly more basal position (Fig 1). Surprisingly, only the PAR3/aPKC polarity complex was asymmetrically distributed in the daughter cells after asymmetric cell division. Therefore, Marthiens and ffrench-Constant propose that the orientation of the spindle axis results in an equal inheritance of each of these three domains during symmetric cell division, and conversion of the plane of division that leads to asymmetric division results in the unequal distribution of the polarity complex, thereby separating the adhesive and polarity domains (Fig 1). According to this model, post-mitotic neuronal precursor cells that delaminate from the ventricular zone should not contain apical membrane polarity proteins. The aPKCζ/λ complex has recently been shown to be located in the apical surface of neural stem cells—but not post-mitotic cells—in the developing avian neural tube (Ghosh et al, 2008), which supports the Marthiens and ffrench-Constant model.
Figure 1.
Ring structure models of the partitioning of three distinct adherens junctional microdomains during the division of a neural stem cell. The Marthiens and ffrench-Constant (2009) model proposes that the adherens junctional proteins are partitioned between the cadherin hole and the cadherin/catenin complex microdomains. All three domains are distributed to each cell during symmetric division (left). By contrast, during asymmetric cell division (right), only the stem-cell daughter inherits the apical microdomain, whereas the post-mitotic cell that separates from the ventricular zone does not.
The finding of three distinct domains containing Ncad/βcat, Afadin/ZO1 and PAR3/aPKC at the apical surface of the neural progenitor cells was achieved because of the way in which the imaging was performed. The authors imaged the apical surface of the cell en face from the ventricular surface, with the successive sections proceeding inward from that plane of observation. Another study, which analysed cross-sections through the neuroepithelium, had previously concluded that a ring of adherens junction proteins—the so-called ‘cadherin hole'—might be distributed asymmetrically during asymmetric division (Kosodo et al, 2004). The plane of sectioning used in this study probably made it difficult for the authors to distinguish the microdomain structure of Ncad/βcat, Afadin/ZO1 and PAR3/aPKC, which was revealed by the en face observations of Marthiens and ffrench-Constant. The unique orientation of their optical sections clearly revealed distinct patterns of distribution for these three types of membrane protein at the apical surface and an unequal inheritance of the PAR3/aPKC complex during asymmetrical cell division.
As with any new finding, this study raises many questions that remain to be addressed. How are these protein microdomains established for the junctional and polarity proteins? Are they established during cellular membrane assembly or are they spatially arranged once the proteins reach the membrane? The adherens junctional domains could be established first, thereby limiting the polarity proteins to the most apical position in the cell. Conversely, the apical protein domain could be established first and exclude the junctional proteins. Another important question relates to the signal that determines whether a cell remains in the proliferative stem-cell state or becomes post-mitotic. The polarity proteins could be responsible for this decision, or a signalling mechanism could be activated by the presence of the polarity proteins and/or inactivated by the absence of these proteins. For example, aPKC is known to target the Lgl (Lethal giant larvae) protein to the basolateral membrane (Knoblich, 2008). Another possibility is that the signal is something that binds to or is restricted to the apical domains by the polarity proteins; possible candidates include prominin 1—also known as Prom1 and CD133—and Numb. Prom1 is present in the midbody and the primary cilium of dividing neuroepithelial cells, and has been proposed to regulate the switch between cellular proliferation and differentiation (Dubreuil et al, 2007), whereas Numb blocks Notch signalling during asymmetric cell division and promotes neuronal cell fate in one of the two daughter cells (Knoblich, 2008). In addition, Numb and Numbl are known to have essential roles in cadherin cell adhesion and in the maintenance of the apico-basal polarity of radial glial cells (Rasin et al, 2007).
By observing neurogenesis from the ventricular surface, Marthiens and ffrench-Constant have described a new three-dimensional ring structure composed of three functional protein microdomains that is present at the apical crescent membrane of neural stem cells during mitosis. This new way of looking at neurogenesis will probably lead to the identification of novel cell-fate determinants and mechanisms of cell-fate specification in both embryonic and adult neural stem cells.

References
- Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Brauninger M (2007) Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol 176: 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Marquardt T, Thaler JP, Carter N, Andrews SE, Pfaff SL, Hunter T (2008) Instructive role of aPKCzeta subcellular localization in the assembly of adherens junctional in neural progenitors. Proc Natl Acad Sci USA 105: 335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6: 777–788 [DOI] [PubMed] [Google Scholar]
- Knoblich JA (2008) Mechanisms of asymmetric stem cell division. Cell 132: 583–597 [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F (2008) Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol 10: 93–101 [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB (2004) Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J 23: 2314–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthiens V, ffrench-Constant C (2009) Adherens junction domains are split by asymmetric division of embryonic neural stem cells. EMBO Rep 10: 515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR (2004) Cortical neurons arise in symmetric and asymmetric division zones and migrates through specific phase. Nat Neurosci 7: 136–144 [DOI] [PubMed] [Google Scholar]
- Rasin MR et al. (2007) Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci 10: 819–827 [DOI] [PubMed] [Google Scholar]
- Yingling J, Youn YH, Darling D, Toyo-oka K, Pramparo P, Hirotsune S, Wynshaw-Boris A (2008) Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell 132: 474–486 [DOI] [PMC free article] [PubMed] [Google Scholar]