Abstract
After periods of high-frequency firing, the normal rhythmically active serotonin (5HT)-containing neurosecretory neurons of the lobster ventral nerve cord display a period of suppressed spike generation and reduced synaptic input that we refer to as “autoinhibition.” The duration of this autoinhibition is directly related to the magnitude and duration of the current injection triggering the high-frequency firing. More interesting, however, is that the autoinhibition is inversely related to the initial firing frequency of these cells within their normal range of firing (0.5–3 Hz). This allows more active 5HT neurons to resume firing after shorter durations of inhibition than cells that initially fired at slower rates. Although superfused 5HT inhibits the spontaneous firing of these cells, the persistence of autoinhibition in saline with no added calcium, in cadmium-containing saline, and in lobsters depleted of serotonin suggests that intrinsic membrane properties account for the autoinhibition. A similar autoinhibition is seen in spontaneously active octopamine neurons but is absent from spontaneously active γ-aminobutyric acid cells. Thus, this might be a characteristic feature of amine-containing neurosecretory neurons. The 5HT cells of vertebrate brain nuclei share similarities in firing frequencies, spike shapes, and inhibition by 5HT with the lobster cells that were the focus of this study. However, the mechanism suggested to underlie autoinhibition in vertebrate neurons is that 5HT released from activated or neighboring cells acts back on inhibitory autoreceptors that are found on the dendrites and cell bodies of these neurons.
Biogenic amines appear to play key roles in the regulation of a wide array of physiological processes in both vertebrate and invertebrate nervous systems. They have been implicated in physiological processes and behaviors such as feeding (1), sleep (2, 3), repetitive motor acts such as locomotion (1), nociception (4), depression (5), and aggressive behavior, including the establishment of social hierarchies (6–9).
In vertebrates, spontaneously active serotonergic neurons of the midline raphe nuclei are implicated in these behaviors, and these neurons have been the subject of many studies (for reviews, see refs. 10–12). These cells fire at slow rates between 0.5 and 3 Hz, depending on the state of wakefulness of the animal (1, 13–15). They generate large, approximately 60-mV, action potentials with prominent afterhyperpolarizations, which are likely to be mediated by calcium-activated potassium currents (10, 16). Both a high density of inhibitory somatodendritic type 1A 5-hydroxytryptamine (serotonin; 5HT1A) autoreceptors and the afterhyperpolarization are suggested to be important mechanisms for regulating the pacemaker activity of these cells (10, 17). Periods of high-frequency firing, experimentally evoked by electrical stimulation, are followed by a “postactivation inhibition” during which no action potentials are generated. The duration of the period of suppressed spike generation is linearly related to the stimulus intensity (13, 18). The high density of inhibitory 5HT receptors localized in the somatodendritic regions of 5HT neurons (17), together with the observation that application of 5HT inhibits their spontaneous activity, led to the suggestion that the postactivation inhibition was mediated by 5HT released by the same cells (for review, see ref. 10). Changes in intrinsic ionic currents also were considered as possible explanations for the autoinhibition, but this suggestion has not received much attention.
Other vertebrate central nervous system amine-containing neurons, including the dopaminergic cells in the substantia nigra (19) and the retina (20), also show spontaneous activity. These cells are similar to the raphe 5HT neurons in that they fire large action potentials at slow rates, which are followed by a pronounced afterhyperpolarization. In addition, a postactivation inhibition linked to the intensity of stimulation has been described for the dopaminergic neurons of the substantia nigra (19, 21). The firing of these cells is also inhibited by application of exogenous dopamine, and the cells are capable of releasing dopamine from their dendrites (for review, see ref. 22). Although the lobster amine neurons that we describe here also show a postactivation inhibition, in this communication we demonstrate that this is an internal property of these neurons and not related to the effect of released serotonin.
In lobsters, the amines serotonin and octopamine play important roles in aggressive behavior (7, 23), and they modulate postures connected with dominance status (24). The neurosecretory neuron systems for both amines have been mapped by immunostaining (25, 26), and the somata of these cells are easily accessible for electrophysiological recording. The lobster serotonergic neurosecretory system consists of only four cells: one pair in the fifth thoracic ganglion and a second in the first abdominal (A1) ganglion (25). Like the vertebrate raphe cells, lobster serotonin-containing neurons usually are spontaneously active in the range of 0.5–3 Hz and produce large (50–60 mV) overshooting action potentials with prominent afterhyperpolarizations (27). These cells release 5HT via central and peripheral sets of endings (28). They act as gain setters on central circuits involved in the control of posture (27, 29), and they exert important modulatory effects on peripheral targets such as muscles (23), sensory organs (30), and the heart (31). The spontaneous rhythms of these cells are shaped by synaptic input (32–34; unpublished data). Bath application of 5HT to these cells inhibits their firing (but see below and ref. 32). The octopaminergic neurosecretory system consists of 14 pairs of cells situated in the anterior and posterior cell clusters of thoracic ganglia and in the posterior neuromeres of the subesophageal ganglion (26). They too have both central and peripheral release sites, but thus far none of their synaptic inputs have been identified. Unlike the serotonergic neurosecretory cells, octopaminergic cells usually do not show spontaneous activity, although on occasion they show regular firing of large (40–55 mV) overshooting action potentials followed by prominent afterhyperpolarizations (see below).
In addition to the two kinds of amine neurons, GABA-containing inhibitory cells (I1 cells) of the abdominal ganglia also are spontaneously active. The action potentials recorded in the somata of these neurons are only 5–15 mV in amplitude, but they, too, fire at steady frequencies between 1.5 and 10 Hz. The I1 cells are electrically coupled to their contralateral partners and send axons through the third ganglionic roots to innervate the superficial flexor muscles (35).
The lobster 5HT-containing neurons thus share many similarities with the vertebrate brain 5HT cells, including spontaneous generation of large action potentials at slow firing rates of 0.5–3 Hz, prominent afterhyperpolarizations after each spike, and a suppression of rhythmical firing after 5HT exposure. Recently, Hörner et al. (33) reported an autoinhibition period after high-frequency firing of the 5HT cells that resembled the postactivation inhibition described for the vertebrate cells. In this publication, we present a more thorough investigation of the properties of this autoinhibition. Unlike studies of vertebrate amine neurons, the lobster isolated central nervous system (ventral nerve cord) preparation allows us to record from the same identified 5HT cell from different animals. Thus, it is possible in a detailed series of experiments to use different stimulation protocols, media of altered saline composition, treatments with many pharmacological reagents, animals of different social or molt cycle status, etc. The results of these studies suggest (i) that the autoinhibition of firing after high-frequency discharge of the cells is an intrinsic property of the cells based on internal membrane properties and is not a result of a 5HT-mediated autoinhibition as has been suggested for the vertebrate cells, and (ii), surprisingly, that the duration of the autoinhibition is inversely related to the initial firing rate of the cell over the normal range of firing of the cells. Thus, how cells were being used before high-frequency stimulation influences their subsequent usage after the interruption by the period of enhanced activity.
MATERIALS AND METHODS
Animals.
Experiments were performed on isolated nerve cords of lobsters (Homarus americanus). Adult lobsters (ca. 500 g) were purchased locally, and juvenile lobsters (2.5–4.0 g) were supplied by a rearing facility at the New England Aquarium. The animals were kept at 14–16°C in recirculating artificial seawater tanks on a 12-h day/12-h night schedule and were fed shrimp or squid three times a week.
5HT Depletion.
Juvenile lobsters were depleted of serotonin by repeated injections of 5,7-dihydroxytryptamine (5,7-DHT, Sigma). A total of eight injections of 20 μl of a solution containing 8 mg of 5,7-DHT, 4 mg of ascorbate, and 2 mg of Mops buffer per 100 μl of saline, were given to the animals on a twice a week schedule(for full description of the original method used for depletion in mature lobsters, see ref. 37). Benton et al. (36) demonstrated that a similar protocol with 4 injections of 5,7-DHT abolished 5HT immunostaining in neuropil regions of embryonic lobsters, and they further confirmed by HPLC the absence of 5HT (reduction greater than 90%). Injections were given into the ventral hemolymph sinus. Confirmation that 5HT was depleted from each animal was made by 5HT immunostaining (for method, see ref. 37) after the electrophysiological recordings (data not shown). 5HT cells were easily identified in most of these preparations because of a brownish staining in the cytoplasm, resulting from the depletion procedure.
Dissections.
The animals were anesthetized by reducing their body temperature on ice. After the appendages were removed, the bodies were placed in cold saline of the following composition: 462 mM NaCl, 16 mM KCl, 26 mM CaCl2, 8 mM Mg Cl2, and 11 mM glucose, buffered with 10 mM Hepes at pH 7.4 (29). Ventral nerve cords including the six abdominal, five thoracic, and subesophageal ganglion were removed by dissection and pinned ventral side up in a Sylgard-coated (Dow-Corning) Petri dish. The ganglia chosen for recordings were desheathed, and the covering layer of glial cells was puffed away with a stream of saline from a Pasteur pipette. Preparations were continuously superfused with oxygenated saline at 12–15°C. Chemical transmission was interrupted by perfusing the preparation with saline with no added calcium (replaced by MgCl2) or by perfusion with cadmium-containing saline (0.13, 1.3, or 2.6 mM CdCl2), compensated for osmolarity by lower amounts of CaCl2. Concentrations of 100 μM CdCl2 have been shown to block the actions of 5HT on lobster stomatogastric ganglion cells (38), and 2 mM CdCl2 interrupted chemical transmission in crayfish central nervous system preparations (39).
Electrophysiology.
Intracellular recordings from neuronal somata were performed with glass microelectrodes (12–25 MΩ resistance) filled with 2 M potassium acetate or 3 M KCl. Electrical signals were amplified with an Axoprobe 1A amplifier and stored on video tape and on a personal computer after being digitized with a TL-1 DMA interface and processed with the pclamp 5.5 software package (all Axon Instruments, Foster City, CA). The cells were physiologically and morphologically identified with criteria defined in earlier publications [A1- and fifth thoracic ganglion-5HT cells (27), octopamine-containing cells (26), spontaneously active GABA cells (35)]. Nerve roots and connectives were stimulated by placing their cut ends into closely fitting suction electrodes. Electrical stimuli were generated and delivered by stimulus isolation units (model 850A, WPI Instruments, Waltham, MA) in conjunction with pulse modules (model 831, WPI) and an interval generator (model 830, WPI).
RESULTS AND DISCUSSION
Effects of Serotonin on Spontaneously Active Lobster 5HT Cells.
A characteristic feature of vertebrate serotonergic cells is the inhibition of their spontaneous activity by applied 5HT or by 5HT1A receptor agonists (10). This characteristic, together with the observed high density of somatic and dendritic autoreceptors (17), led to the hypothesis that the postactivation inhibition of firing of these cells is caused by 5HT released by the cells themselves.
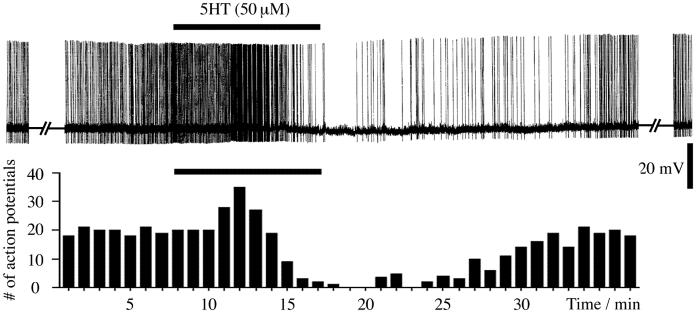
Perfusion of a lobster ventral nerve cord with serotonin (50 μM) reversibly inhibits the spontaneous generation of action potentials in A1–5HT neurons (Fig. 1), as previously demonstrated by Ma and Weiger (32). However, a closer look at the original data and an evaluation of our more recent results revealed an initial increase in the rate of firing of the cells that is later compensated for by an inhibition of their activity and ultimately by a sustained suppression of firing. Along with the suppression of firing, we observed a hyperpolarization of the membrane potential of the cells (range, 1–3 mV) that only slowly recovered to the initial level of membrane potential during the washout of 5HT (unpublished observations). When the nerve cord was returned to normal saline (10 min of 5HT exposure in the experiment shown in Fig. 1), it took 6–8 min, for the cells to begin to fire spontaneously again and some 20–30 min to return to their initial spontaneous rate. After prolonged perfusion with 5HT and/or application of higher concentrations of the amine, it required considerably longer periods of washout to return to the initial firing rate (ref. 32; unpublished data).
Figure 1.
Serotonin causes a biphasic action on the spontaneous activity of A1–5HT cells. Application of 50 μM serotonin leads to an initial increase, then a decrease in firing, and, ultimately, a complete suppression of spontaneous spike activity. The inhibitory effect is reversed after superfusion with normal saline, and the cell slowly resumes its initial rate of firing. (Upper) Spontaneous action potentials of an A1–5HT cell, before, during, and after superfusion with 5HT. (Lower) Numbers of action potentials per minute over time. Bars, 10 min of 5HT superfusion.
The biphasic response to 5HT suggests that at least two different types of 5HT receptors exist on these neurosecretory neurons: an excitatory type responding with a relatively short latency and an inhibitory type with later onset but stronger net effect. It remains possible, however, that synaptic activation or inhibition of other neurons contributes to the observed effects. For the excitatory component, this is highly unlikely, because experiments in saline with no-added Ca2+ or with Cd2+ present, in which all spontaneous synaptic activity disappears, still show an enhanced activity of the A1–5HT cells in the presence of 5HT.
Autoinhibition in the A1–5HT Cells.
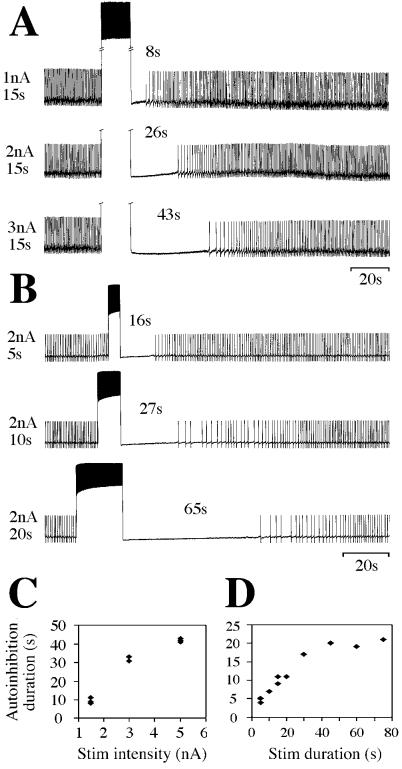
Current injections into spontaneously active 5HT neurons cause increases in their rates of firing of up to 40 Hz depending on the amount of current injected (Fig. 2). This activation is followed by a pronounced afterhyperpolarization (up to −10 mV) and the autoinhibition period during which no spontaneous action potentials are generated. Throughout the silent period, the membrane potentials of cells slowly increase until the cells begin to resume their previous rates of firing. The duration of the autoinhibition can be up to several minutes. In addition, during the autoinhibition period, synaptic input to 5HT cells is markedly altered. Previously reported long-lasting excitatory postsynaptic potentials from medial and lateral giant fibers (ref. 33; unpublished data) are dramatically shortened (from many hundreds of milliseconds to <100 ms), whereas long-lasting inhibitory postsynaptic potentials from unidentified sources activated by nerve connective stimulation almost completely disappear. At the same time, shorter inhibitory postsynaptic potentials probably originating from GABAergic cells in the A3 ganglion and shorter excitatory postsynaptic potentials of unknown origin are not affected at all (ref. 34; unpublished data). Some of the changes seen in synaptic input could result from actions of released 5HT on nearby nerve terminals. On the other hand, these effects could result from stimulation-induced changes in the intrinsic properties of A1–5HT cells. Further experiments will be needed to sort between these possibilities. In confirmation of previous results of Ma and coworkers (27), stimulation of one A1–5HT cell had no effect on the spontaneous rhythm of the contralateral 5HT cell, indicating that pairs of A1–5HT cells are not synaptically coupled.
Figure 2.
The duration of the autoinhibition of A1–5HT cells is directly correlated with the amount of current injected during stimulation (A and C) and the duration of the period of stimulation (B and D). A and B are chart recordings showing three trials in which either the stimulus intensity or the stimulus duration were varied. The data presented in C and D are from two other preparations, in which the recorded cells maintained constant spontaneous firing rates throughout the experiments.
The duration of the autoinhibition period is positively correlated with both the stimulus intensity (the amount of current injected, Fig. 2A) and the stimulus duration (Fig. 2B). If, instead of a constant current to activate the cells, short current pulses of 2 ms duration are used to trigger action potentials, the same effect is observed. If these shorter pulses are just below the threshold to activate additional spikes, however, no autoinhibition is seen. This excludes that it is the constant depolarization of the membrane that triggers the autoinhibition, but instead it appears to be related to the action potential mechanism. Because both the magnitude of the injected current and the stimulus duration are directly correlated with the total number of spikes per stimulation and because each spike should trigger a release of 5HT in the ganglionic neuropil, the autoinhibition, at first glance, seems as if it could be based on 5HT activation of inhibitory autoreceptors, as has been proposed for vertebrate 5HT containing raphe cells (40, 41).
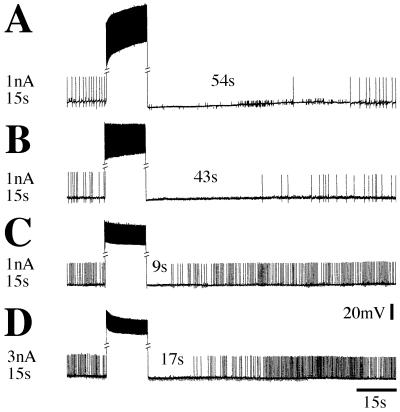
Therefore, in an effort to prevent the release of 5HT, we depleted juvenile lobsters of 5HT by repeated injections of 5,7-DHT. In vertebrate brain 5HT cells, 5,7-DHT exposure apparently causes cell degeneration (10, 42). Lobster cells, in contrast, not only survive this treatment (25, 36) but also maintain spontaneous spike activity, despite the fact that little or no 5HT remains in the synaptic neuropil. Moreover, the characteristics of the autoinhibition seen in A1 neurons depleted of 5HT were no different from those of untreated juveniles (Fig. 3 A and B).
Figure 3.
Autoinhibition is maintained in 5HT-depleted animals and in saline with lowered levels of calcium. As with adult animals, after high-frequency activation, A1–5HT cells of juvenile lobsters display periods of autoinhibition (A). The characteristics of the autoinhibition, an increase in duration with higher intensities of stimulation and an inverse relationship to the initial firing frequency, seemed unchanged in juvenile lobsters that were depleted of serotonin by repeated injections of 5,7-DHT (B). Superfusion of the preparation (same as in B: 5HT-depleted animal) with saline with no added calcium leads to the disappearance of afterhyperpolarizations and an increase in the spontaneous firing rates of 5HT cells (to 2.4 Hz in this example). As anticipated, the duration of the period of autoinhibition is short at this high initial rate of firing (C), but by increasing the intensity of stimulation, autoinhibition clearly can be seen (D).
In a series of preparations, we exchanged the normal saline superfusion for solutions with no added calcium or with cadmium (0.13–2.6 mM). As these solutions penetrated the preparations, the afterhyperpolarizations and the synaptic input to the 5HT cells gradually disappeared, the cells depolarized by 4–6 mV, and their firing frequencies increased to as high as 12 Hz. Our attempts to reduce the firing rate of 5HT cells to values observed in normal saline by tonic injection of hyperpolarizing current failed, because the cells adapted rapidly to their original high rates. Autoinhibition, however, persisted even after superfusion with low-calcium saline for 2 h, although it could be elicited only with higher intensities of stimulation and its duration was markedly shorter than in normal saline (for explanation, see next paragraph). Autoinhibition under superfusion of saline with no added calcium even appeared in A1 cells of 5HT-depleted juvenile lobsters (Fig. 3 C and D). Because synaptic input to 5HT cells vanished in the absence of calcium or with added cadmium in depleted and normal animals, suggesting that transmitter release was largely prevented under these conditions, we speculated that autoinhibition might be caused by stimulation-evoked changes in the intrinsic membrane properties of these cells rather than activation of inhibitory autoreceptors by released serotonin.
The Duration of Autoinhibition Is Inversely Related to the Spontaneous Firing Rate of A1–5HT Cells.
The duration of the period of autoinhibition after identical stimuli to cells in different preparations was variable. Therefore, we searched for parameters that might be correlated with these differences. We could not correlate the variability with differences in the resting potential of cells, with small differences in the increase in firing frequencies resulting from current injections, or with the magnitude of the afterhyperpolarization at the end of the period of stimulation.
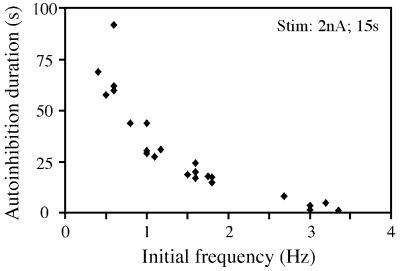
As mentioned above, we observed that 5HT cells firing at increased frequencies in saline with no added calcium showed a reduced period of autoinhibition. Therefore, we decided to measure the duration of the periods of autoinhibition shown by A1–5HT cells under identical activation paradigms, among a group of cells firing at different initial rates in normal saline. Most 5HT cells show initial frequencies of firing between 0.5 and 1.5 Hz, whereas a smaller number show initial activities of up to 3.5 Hz (see also ref. 27). The autoinhibition period after standardized stimuli turned out to be considerably shorter in cells that had higher initial firing rates (Fig. 4). In the example shown in Fig. 4 (2 nA of stimulation for 15 s), cells firing initially at less than 1 Hz showed periods of autoinhibition of up to 1.5 min, whereas cells firing initially at 3 Hz showed virtually no autoinhibition.
Figure 4.
The duration of the autoinhibition period is inversely related to the initial firing frequency of cells. Data from 12 different A1–5HT cells were chosen to cover the range of initial frequencies between 0.5 and 3.4 Hz that were observed in our experiments. During the recording period with each cell (up to 8 h), the initial firing rates often changed. In those cases, autoinhibition was measured at several different spontaneous frequencies, accounting for the additional data points in this figure. All cells were stimulated by current injections of 2 nA for 15 s.
Because the range of 0.5–3 Hz is the normal range of spontaneous firing of these cells, this inverse relationship allows for a faster activation of the cells that were being used at the higher rate after a period of interruption. With vertebrate 5HT cells, higher rates of firing (in the 3-Hz range) correlate closely with the state of awakeness of animals (3, 43). The range in vertebrate cells is from silent (with animals in rapid eye movement sleep) to 3 Hz in awake, behaving animals. One presumes that a higher tonic release of 5HT may be important in aspects of lobster behavior as well, but this direct linkage has not yet been made. The ability to more rapidly restore the activity of 5HT cells showing higher initial rates of activity relates both to the generation of action potentials and to restoring these cells to the influence of long-lasting excitatory and inhibitory synaptic inputs that govern the use of these neurons (see above; ref. 33; unpublished data). A similar period of suppressed activity and reduced excitability after high-frequency activation, which is related to the initial firing rate of a neuron, has been seen in tonically stimulated crayfish stretch receptor neurons (44, 45).
To observe autoinhibition in 5HT cells with high initial spontaneous firing rates, the stimulus intensity has to be increased. This was seen in the experiments with no added calcium and with cadmium-containing saline in which 5HT cells fired at rates of as high as 12 Hz. Under the calcium blocked condition, cells that fired at 3–4 Hz showed similar durations of autoinhibition as cells firing at 3–4 Hz in normal saline. Thus, whereas there may be an influence of released 5HT on the function of A1–5HT neurons, e.g., on synaptic inputs to the cells, there are not likely to be any important effects on the spike initiation zone of these cells. Instead, the persistence of autoinhibition without added calcium and in 5HT-depleted cells suggests that an intrinsic mechanism, e.g., a conductance increase triggered by high-frequency activity, could serve as the basis for this interesting phenomenon. Although a conductance increase was considered as a possible source of the postactivation inhibition in some studies (10), this possibility has not been investigated in any detail in vertebrate spontaneously active 5HT neurons.
Other mechanisms could contribute to or underlie the autoinhibition as well. For example, in explaining a postexcitatory depression of spike activity accompanied by hyperpolarization in sensory neurons in various species of animals (45–47), the participation of an electrogenic sodium pump has been invoked. The poststimulus reduction in activity in these cases has been attributed mainly to activation of an electrogenic Na pump through accumulation of Na+ during the high-frequency stimulation. This mechanism is believed to underlie adaptation in sensory neurons. A posttetanic hyperpolarization involving the Na pump also has been seen in lizard motor neuron terminal regions (46). However, we see no simple explanation for how the involvement of such a pump could account for an inverse relationship between the initial firing rate of a cell and the duration of autoinhibition after high-frequency usage. In the experimental systems mentioned above, an involvement of calcium-activated K+ currents during the early part of the inhibitory period also has been demonstrated (47, 48). Such a current seems not to play a major role in autoinhibition in lobster 5HT neurons, because the autoinhibition persists under conditions preventing calcium entry into the cells (20).
Voltage clamp and further pharmacological studies will be required to elaborate the mechanism underlying autoinhibition in the lobster aminergic neurons studied here. Preliminary pharmacological characterization (unpublished data) of the action potentials of these neurons demonstrate that tetrodotoxin (1 mM) completely blocks both the action potential and any underlying generator potential. The afterhyperpolarization of the action potential is reduced in magnitude by charybdotoxin (10 nM), teraethylammonium (0.5 mM), and 4-aminopyridine (100 nM), completely blocked in the presence of Cd2+, and unaffected by apamin (1 mM). Partial or complete block of the afterhyperpolarization leads to an increase in the firing rates of cells. These results suggest that calcium-activated BK channels are important contributors to the afterhyperpolarization.
Autoinhibition Is a Characteristic of Aminergic Cells.
Autoinhibition was observed in every spontaneously active A1- and fifth thoracic ganglion-5HT cell tested. As long as action potentials retained their typical shape with a characteristic afterhyperpolarization, 5HT cells from different preparations with similar initial firing rates displayed very similar durations of autoinhibition after standardized stimuli.
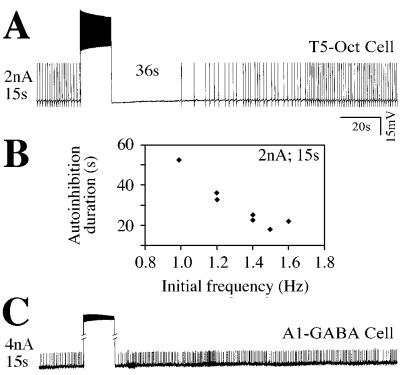
Although octopamine-containing neurosecretory cells usually are not spontaneously active, we did record overshooting action potentials of about 40–55 mV amplitude with a prominent afterhyperpolarization from two octopaminergic cells in the fifth thoracic ganglion and from one cell in the third thoracic and one in the fourth thoracic ganglia. The shape of spikes recorded from the somata of these cells and their spontaneous frequencies of firing (0.5–2 Hz) were similar to those of the 5HT cells. Of most relevance here, however, is that these cells also displayed autoinhibition (Fig. 4A) whose duration was inversely related to the initial firing frequency of the cell (Fig. 4B) and was directly related to the stimulus intensity and duration. Moreover, unidentified synaptic inputs to these cells triggered by stimulation of the abdominal connectives were altered during the autoinhibition period. As with the A1–5HT cells, long-lasting excitatory postsynaptic potentials of 400–500 ms duration were shortened to about 100 ms by high-frequency firing of the cells, and these potentials returned to control durations within 25–30 s after the end of the stimulation.
We also examined 12 spontaneously active GABA cells (I1 cells) from abdominal ganglia 1–3 for autoinhibition after high-frequency firing. These cells were spontaneously active at 1.5–10 Hz and showed action potentials of 5–15 mV amplitude in their somata (action potentials did not invade these regions of the cell). Stimulation protocols were used with GABA cells that generated discharge frequencies similar to those seen in 5HT neurons. None of the GABA cells showed autoinhibition, even with stimulation intensities that triggered long interruptions (>2 min) in the firing rhythms of 5HT cells. For example, a serotonergic neuron with the same initial frequency as the GABA cell shown in Fig. 5C (approximately 1.5 Hz) would display autoinhibition for at least 60 s after the level of stimulation used in this experiment.
Figure 5.
Autoinhibition occurs in spontaneously active amine cells but not in GABA-containing cells. Spontaneously active, octopamine-containing neurosecretory cells show autoinhibition (A) with characteristics similar to those described for 5HT cells. To illustrate, the inverse relationship between the duration of the autoinhibition period and the initial firing frequency of cells is shown in the graph (B). In contrast, spontaneously active GABA cells from abdominal ganglia immediately resume their initial firing rate at the end of the stimulation period (C).
Thus, although our sample of spontaneously active cell types is small, these results suggest that autoinhibition might be a special feature of aminergic neurosecretory neurons in lobsters. In vertebrate systems, the 5HT cells of midline raphe nuclei and dopaminergic cells of the substantia nigra also display a stimulus-dependent postactivation inhibition (19). Whether the interesting features of the lobster system of an inverse relationship between the duration of the autoinhibition and the initial firing rate of the cell will be seen in other systems with spontaneously active cells remains to be established.
In summary, we report here an autoinhibition of the firing of lobster aminergic neurons that has certain unique characteristics. The autoinhibition does not appear to be caused by the release of the amine neurotransmitter. Instead, it seems to be an intrinsic property of these neurons. The duration of the autoinhibition is directly related to the magnitude and duration of the period of stimulation but is inversely related to the initial firing rate of the cell. Such a mechanism allows for faster reuse of the neuron after a period of interruption of its normal, relatively constant tonic firing pattern.
Acknowledgments
We thank Drs. Barbara Beltz and Jean Benton for depleting animals of serotonin for these studies. We also thank Mr. Jason Goldstein and Dr. Marianne Farrington for supplying the juvenile animals from a rearing facility at the New England Aquarium and Elizabeth Wilkinson, Amy Hower, Dr. Geoffrey Ganter, and Dr. Margaret Bradley for assistance with animal keeping in our laboratories at Harvard Medical School. This research was supported by the Alexander von Humboldt Stiftung (to R.H. and M.H.), the National Science Foundation (Grants IBN-9728551 and IBN-9601288, to E.A.K. and D.H.E.), and a National Research Service Award postdoctoral fellowship from National Institutes of Health-National Institute of Mental Health (to S.C.).
ABBREVIATIONS
- 5HT
5-hydroxytryptamine (serotonin)
- 5
7-DHT, 5,7-dihydroxytryptamine
- A1
first abdominal ganglion
- GABA
γ-aminobutyric acid
References
- 1.Veasey S C, Fornal C A, Metzler C W, Jacobs B L. Neuroscience. 1997;79:161–169. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- 2.McGinty D J, Harper R M. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs B L, Fornal C A. Pharmacol Rev. 1991;43:563–578. [PubMed] [Google Scholar]
- 4.Mason P, Gao K. Pain Forum. 1998;7:143–150. [Google Scholar]
- 5.Stockmeier C A, Shapiro L A, Dilley G E, Kolli T N, Friedman L, Rajkowska G. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raleigh M J, Mcguire M T, Brammer G L, Pollack D B, Yuwiler A. Brain Res. 1991;559:181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- 7.Huber R, Smith K, Delago A, Isaksson K, Kravitz E A. Proc Natl Acad Sci USA. 1997;94:5939–5942. doi: 10.1073/pnas.94.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards D H, Kravitz E A. Curr Opin Neurobiol. 1997;7:812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- 9.Ferris C F, Melloni R H, Jr, Koppel G, Perry K W, Fuller R W, Delville Y. J Neurosci. 1997;17:4331–4040. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aghajanian G K, Sprouse J S, Rasmussen K. In: Psychopharmacology: The Third Generation of Progress. Meltzer H Y, editor. New York: Raven; 1987. pp. 141–149. [Google Scholar]
- 11.Mason P, Leung C G. Prog Brain Res. 1996;107:269–282. doi: 10.1016/s0079-6123(08)61870-1. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs B L, Fornal C A. Curr Opin Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- 13.Wang R Y, Aghajanian G K. Brain Res. 1977;132:186–193. doi: 10.1016/0006-8993(77)90719-3. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham K A, Lakoski J M. Eur J Pharmacol. 1988;148:457–462. doi: 10.1016/0014-2999(88)90128-8. [DOI] [PubMed] [Google Scholar]
- 15.Mason P. J Neurophysiol. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- 16.Aghajanian G K. Nature (London) 1985;315:501–503. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- 17.Pazos A, Palacios J M. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- 18.Aghajanian G K, VanderMaelen C P. Brain Res. 1982;238:462–469. doi: 10.1016/0006-8993(82)90124-x. [DOI] [PubMed] [Google Scholar]
- 19.Grace A A, Bunney B S. J Neurosci. 1984;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feigenspan A, Gustincich S, Bean B P, Raviola E. J Neurosci. 1998;17:6776–6789. doi: 10.1523/JNEUROSCI.18-17-06776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kita T, Kita H, Kitai S T. Brain Res. 1986;372:21–30. doi: 10.1016/0006-8993(86)91454-x. [DOI] [PubMed] [Google Scholar]
- 22.Cheramy A, Leviel V, Glowinski J. Nature (London) 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- 23.Kravitz E A. Science. 1988;241:1775–1781. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- 24.Livingstone M S, Harris-Warrick R M, Kravitz E A. Science. 1980;208:76–79. doi: 10.1126/science.208.4439.76. [DOI] [PubMed] [Google Scholar]
- 25.Beltz B S, Kravitz E A. J Neurosci. 1983;3:585–602. doi: 10.1523/JNEUROSCI.03-03-00585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider H, Trimmer B A, Rapus J, Eckert M, Valentine D F, Kravitz E A. J Comp Neurol. 1993;329:129–142. doi: 10.1002/cne.903290109. [DOI] [PubMed] [Google Scholar]
- 27.Ma P M, Beltz B S, Kravitz E A. J Neurophysiol. 1992;68:36–54. doi: 10.1152/jn.1992.68.1.36. [DOI] [PubMed] [Google Scholar]
- 28.Beltz B S, Kravitz E A. J Neurosci. 1987;7:533–546. doi: 10.1523/JNEUROSCI.07-02-00533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris-Warrick R M, Kravitz E A. J Neurosci. 1984;4:1976–1993. doi: 10.1523/JNEUROSCI.04-08-01976.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasztor V M, Bush B M H. Nature (London) 1987;326:793–795. doi: 10.1038/326793a0. [DOI] [PubMed] [Google Scholar]
- 31.Cooke I M, Hartline D K. J Exp Biol. 1975;63:33–52. doi: 10.1242/jeb.63.1.33. [DOI] [PubMed] [Google Scholar]
- 32.Ma P M, Weiger W S. J Neurophysiol. 1993;69:2015–2029. doi: 10.1152/jn.1993.69.6.2015. [DOI] [PubMed] [Google Scholar]
- 33.Hörner M, Weiger W A, Edwards D H, Kravitz E A. J Exp Biol. 1997;200:2017–2033. doi: 10.1242/jeb.200.14.2017. [DOI] [PubMed] [Google Scholar]
- 34.Weiger W A, Ma P M. J Neurophysiol. 1993;69:2003–2014. doi: 10.1152/jn.1993.69.6.2003. [DOI] [PubMed] [Google Scholar]
- 35.Otsuka M, Kravitz E A, Potter D D. J Neurophysiol. 1967;30:725–752. doi: 10.1152/jn.1967.30.4.725. [DOI] [PubMed] [Google Scholar]
- 36.Benton J, Huber R, Ruchhoeft M, Helluy S, Beltz B. J Neurobiol. 1997;33:357–373. doi: 10.1002/(sici)1097-4695(199710)33:4<357::aid-neu2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Livingstone M S, Schaeffer S F, Kravitz E A. J Neurobiol. 1981;12:27–54. doi: 10.1002/neu.480120104. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Harris-Warrick R M. J Neurophysiol. 1995;74:1929–1937. doi: 10.1152/jn.1995.74.5.1929. [DOI] [PubMed] [Google Scholar]
- 39.Heitler W J, Pitman R M, Cobb J L, Leitch B. J Neurocytol. 1991;20:109–123. doi: 10.1007/BF01279615. [DOI] [PubMed] [Google Scholar]
- 40.Sprouse J S, Aghajanian G K. Eur J Pharmacol. 1986;128:295–298. doi: 10.1016/0014-2999(86)90782-x. [DOI] [PubMed] [Google Scholar]
- 41.Hjorth S. J Neurochem. 1993;60:776–779. doi: 10.1111/j.1471-4159.1993.tb03217.x. [DOI] [PubMed] [Google Scholar]
- 42.Verge D, Daval D, Patey A, Gozlan H, El Mestikwy S, Hamon M. Eur J Pharmacol. 1985;113:463–464. doi: 10.1016/0014-2999(85)90099-8. [DOI] [PubMed] [Google Scholar]
- 43.Trulson M E, Jacobs B L. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 44.Eyzaguirre C, Kuffler S W. J Gen Physiol. 1955;39:121–153. doi: 10.1085/jgp.39.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sokolove P G, Cooke I M. J Gen Physiol. 1971;57:125–163. doi: 10.1085/jgp.57.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morita K, David G, Barrett J N, Barrett E F. J Neurophysiol. 1993;70:1874–1884. doi: 10.1152/jn.1993.70.5.1874. [DOI] [PubMed] [Google Scholar]
- 47.Parker D, Hill R, Grillner S. J Neurophysiol. 1996;76:540–553. doi: 10.1152/jn.1996.76.1.540. [DOI] [PubMed] [Google Scholar]
- 48.Jansen J K S, Nicholls J G. J Physiol. 1973;229:635–656. doi: 10.1113/jphysiol.1973.sp010158. [DOI] [PMC free article] [PubMed] [Google Scholar]