Abstract
Pancreatic cancer has a poor prognosis. Improving survival will require diagnosis of early pancreatic cancer which can be defined based on resectability, size or curability. Pancreatic cancer progresses from non-invasive precursor lesions to invasive cancer over a variable time period. Retrospective review of CT scans done prior to diagnosis suggests that pancreatic cancer resectability may be significantly improved if detected as few as 6 months before clinical diagnosis. Since pancreatic cancer is relatively uncommon, to allow cost-effective screening the populations will have to be enriched for the disease using two “sieves”, first would be a population of subjects at higher than average risk of pancreatic cancer and the second sieve could be a characteristic phenotype among the members of the high-risk group, an abnormality seen on non-invasive imaging or a serologic marker of early pancreatic cancer. So far two high risk groups have been targets of screening for pancreatic cancer, viz., hereditary pancreatic cancer kindreds and new-onset diabetes. There is no serologic marker of early pancreatic cancer. Confirmation of diagnosis usually requires invasive procedures such endoscopic ultrasonography. Though much work still needs to be done, the developments in the field provide us with hope that screening for early pancreatic cancer could become a reality in the not-so-distant future.
Introduction
Pancreatic cancer (pancreatic cancer) is a devastating and poorly understood cancer. The life time risk of developing pancreatic cancer for men and women born today is 1.27%; in other words ~1 in 80 men and women will develop cancer in their lifetime. (http://seer.cancer.gov/statfacts/html/pancreas.html?statfacts_page=pancreas.html&x=9&y=20). In the United States in 2005 an estimated 32,180 cases of pancreatic cancer were diagnosed (1) and an estimated 31,800 deaths from pancreatic cancer occurred in the same time period (1), making it the fourth most common cause of cancer death in man, surpassing prostate cancer in the number of cancer-related deaths in 2005 (1). As can be seen from these grim statistics, the incidence and mortality of pancreatic cancer are nearly identical; less than 5% of individuals survive 5 years (1). Even more sobering is the fact that the survival in pancreatic cancer has not improved dramatically over het past 40 years (http://www.kreftregisteret.no).
Why is pancreatic cancer mortality so high?
One can identify three main reasons for the dismal prognosis of pancreatic cancer.
A) Disease is diagnosed at a late stage
The only hope of long-term survival in pancreatic cancer is if curative resection can be undertaken. However, since pancreatic cancer patients seldom exhibit disease-specific symptoms until late in the course of the disease, very few patients (<15–20%) have resectable disease by the time the diagnosis is made (1, 2). A proportion of patients thought to be resectable by imaging studies will be found to have metastatic or locally unresectable disease at surgery; the proportion varies from 15% to 50% depending on quality of pre-operative imaging (3–5). Finally, a subset of patients undergoing curative resection (up to 30%) will have positive resection margins (6), reflecting incomplete resection. Thus, eventually <10% of pancreatic cancer patients undergo a margin-negative (R0) resection.
B) Complete resection is not curative
If resectable disease were curable, the survival of pancreatic cancer could increase ~5-fold. Unfortunately surgery is only palliative in majority of patients undergoing resection; the median survival of patients undergoing curative resection (14–22 months) is only about 12–18 months longer than that of patients with unresectable disease (median survival 4–6 months) (2, 4). Thus ineffectiveness of aggressive and apparently complete resection of pancreatic cancer to effect a cure is another contributor to the poor survival in pancreatic cancer.
C) Adjuvant therapy is only palliative
If adjuvant therapy could eradicate the residual cancer after curative resection, more patients undergoing pancreatic resection could hope for long term survival. Such therapy would potentially also have an impact on locally unresectable disease. While some progress has been made in chemotherapy of pancreatic cancer and newer biologic agents promise better results, overall adjuvant therapy only prolongs life and rarely cures pancreatic cancer (7).
Thus, it is clear that unless newer chemotherapeutic agents can downstage locally unresectable cancers to allow resection, the greatest impact on resectability and survival would be through detection of early pancreatic cancer. Therefore, we believe that among the most compelling needs in pancreatic cancer research today is a) to identify high risk groups that would benefit from screening and early detection programs and b) to develop biomarkers of early pancreatic cancer.
Definition(s) of Early Pancreatic Cancer
“Early” pancreatic cancer may be defined based on resectability, size or curability. Here are three definitions in order of decreasing prevalence and increasing survival (Figure 1).
Figure 1.

“Early” Pancreatic Cancer- Definitions
A) Resectable pancreatic cancer
By this definition, 15–20% of pancreatic cancers would be classified as “early”. As already discussed complete resection of pancreatic cancer leads to improved median survival, but only 10–20% survive 5 years or longer (2, 6). Detecting resectable cancer is the first step in the fight against pancreatic cancer.
B) Small (≤20 mm) pancreatic cancer
According to the TNM classification, tumors ≤20 mm in size are classified as T1. “Early” pancreatic cancer defined as tumors ≤ 20 mm in size (8, 9) is also called “small” pancreatic cancer (8, 9). To put this in perspective, the median size of resected pancreatic cancers is 30 mm (6). Small cancers constitute only ~ 20% of resected pancreatic cancers (10). In an unpublished series of 300 patients with pancreatic ductal adenocarcinomas who underwent margin-negative (R0) resection between 1987 and 2000 at our center, 54 (18%) were small (i.e., ≤20mm). The 5 year survival after resection of small pancreatic cancers in case series and collective reviews has ranged from 30% to 60% (9–11), suggesting that detection of small pancreatic cancer will further enhance survival in pancreatic cancer.
C) Curable pancreatic cancer
The most stringent definition of “early” pancreatic cancer is that of curable pancreatic cancer. These cancers are generally well differentiated Stage I cancers or minute (≤10 mm) cancers. While 42–70% of tumors ≤20 mm are Stage I (9–11), over 85% of tumors ≤ 10 mm are Stage I (11). Five year survival in patients with Stage I disease and those with minute pancreatic cancer exceeds 75% (8, 11). However, some patients who don’t meet these criteria may survive >5 years and likely have biologically indolent tumors or tumors exquisitely sensitive to chemo-radiation (2, 6). The few patients with in situ cancer reported in literature have done very well following resection (11).
Progression of Pancreatic Cancer from Pre-invasive Precursor to Unresectable Cancer
The now famous Vogelgram of pancreatic cancer proposed by Hruban and colleagues (12) plots the histologic and molecular progression of preinvasive stages of pancreatic cancer. However, not much is known about progression of invasive pancreatic cancer. We have tried to recreate, based on published literature, the natural history of pancreatic cancer from its preinvasive stage to its diagnosis and correlate it with symptoms and changes on cross-sectional imaging (Figure 2).
Figure 2.

Model of Histologic and Radiologic Progression of Pancreatic Cancer
A) Pancreatic intraepithelial neoplasia (PanIN) I and II
Pancreatic cancer is thought to develop from pre-cancerous precursor lesions called pancreatic intraepithelial neoplasia or PanINs (12, 13). PanIN lesions do not cause symptoms and are not visible on cross-sectional imaging. In patients with familial pancreatic cancer markedly abnormal pancreatograms with dilated, cystic side branches have been described in patients with dysplasia without invasive cancer (14), findings that are not seen in sporadic pancreatic cancer.
B) Pancreatic intraductal carcinoma (PanIN3)
Also called intraductal carcinoma or carcinoma in situ, progresses to invasive cancer (12, 13). Only a few cases of resected intraductal carcinoma have been described in literature (11). These are generally asymptomatic and not visible on conventional imaging studies. However, pancreatic ductal dilation and cutoff may be a radiologic sign of intraductal carcinoma (11).
C) Minute pancreatic cancer
Further progression of the cancer leads to development of a small component of invasive disease (“minute” pancreatic cancer defined as cancer ≤10 mm in size (11)). In an autopsy study of 378 pancreata having small cystic lesions (15), invasion was found when epithelia spread about 5–8 mm, while it rarely infiltrated when the lesions were < 4 mm in size(15). Minute cancers remain asymptomatic. In a collective review of 36 minute (≤10 mm) pancreatic cancers, 33 had no symptoms and 3 presented with obstructive jaundice (11). Of the 35 patients in this study who underwent abdominal ultrasonography (US) and/or computed tomography (CT), 20 (57%) patients had duct dilation alone, and in only 9 patients (26%) was there visible tumor mass (11). The vast majority of these cancers (85–100%) is still confined to the pancreas (Stage 1) (8, 11) and is potentially curable.
D) Small pancreatic cancer
As discussed previously, small pancreatic cancers are defined as tumors <20 mm in size. Unlike large pancreatic cancer which is usually symptomatic, small cancers are generally asymptomatic or have non-specific symptoms. In a recent series of small pancreatic cancer 50% of patients were diagnosed incidentally on cross-sectional imaging (10). Extra-pancreatic spread is frequently present in small pancreatic cancer. In a collective review of 99 small pancreatic cancers, only 44% were confined to the pancreas (8); however, all were resectable (8). In a recent study of 16 small cancers only 37.5% were Stage I (10). Thus, metastatic spread of pancreatic cancer begins to occur when tumor grows >10 mm in size and the proportion of patients with metastases increases with increasing size of tumor.
E) Large pancreatic cancer
Majority of symptomatic pancreatic cancer patients have large pancreatic cancer defined as tumor ≥20 mm in size. Large tumors are almost always (>90%) visible on cross-sectional imaging and characteristically have not only local and regional metastases via angiolymphatic and perineural invasion but also involvement of adjacent veins and arteries. Among symptomatic patients a proportion has resectable disease. In a series of 616 patients with adenocarcinoma of the pancreas that underwent surgical resection, the median size of resected cancer was 30 mm (6).
Timeline of Progression from Pre -invasive Precursor to Clinical Diagnosis of Pancreatic Cancer
Pancreatic cancer is frequently unresectable at diagnosis and rapidly fatal once the diagnosis is made. However, the timeline of progression from resectable to unresectable disease is not known. The earliest changes of high grade dysplasia may appear many years before clinical diagnosis of cancer (16, 17). This is illustrated by the study in which atypical duct lesions preceded the diagnosis of infiltrating adenocarcinoma by 17 months, 9 years, and 10 years in 3 patients who had previously undergone pancreatic resection (2 for pancreatitis and one for adenocarcinoma 10 years previously) (16). In another study such changes (including carcinoma in situ) were seen 29 and 9 years before diagnosis of cancer (17). Once invasive cancer develops it appears to progress fairly rapidly, though the rate of progression varies considerably. We have seen patients with completely normal CT scans who within months were diagnosed with unresectable cancer (Figure 3). In others there appears to be ample opportunity to intervene as the first signs of cancer (dilated duct with cut off) appeared almost two years prior to detection (Figure 4).
Figure 3.

Can’t win them all! This 59 year old woman had a CT on 4/27/2005 (A) for post cholecystectomy pain which even on retrospective review shows a normal pancreas. Four months later, a repeat CT (B) done for continued narcotic requiring pain locally advanced pancreatic cancer confirmed by biopsy.
Figure 4.

This shows the pancreas cuts on yearly CTs done for surveillance on this patient with history of colon and endometrial cancer. The pancreas on the CT in 1994 (A) appeared entirely normal. In 1995 the pancreas showed a hint of a visible (but not abnormal) pancreatic duct with slight glandular atrophy (B). However, these findings were considered within normal limits. Comparison of the CT in 1996 (C) with earlier CTs suggested further dilatation of the pancreatic duct and led to the performance of EUS (inset) which revealed a 2 cm mass in the body of the pancreas. Patient was asymptomatic. Resection of the mass confirmed the diagnosis of T1N0M0 ductal adenocarcinoma. Patient is alive 9 years later despite developing ureteral cancer in the interim. She was subsequently shown to carry a mutation in the mLH1 gene confirming the suspicion of HNPCC.
To further understand the natural history of invasive cancer we did a study in which two radiologists blindly interpreted 62 CTs performed prior to clinical diagnosis (usually for reasons other than pancreas related symptoms) in 28 pancreatic cancer patients and 89 CTs in 89 controls, noting specific CT findings (18). The two radiologists agreed that CT findings definite or suspicious for pancreatic cancer were present in 50% of scans performed 2 to 6 months and 6 to 18 months prior to the diagnosis of pancreatic cancer (3/6 and 4/8 scans, respectively), but in only 7% (1/15) of scans performed greater than 18 months prior to diagnosis (18). Pancreatic ductal dilation and cutoff were early CT findings identified by both radiologists, and associated with near-perfect and substantial interobserver agreement (κ = 0.84 and 0.76, respectively). 95% confidence intervals of specificity for tumor absence ranged from 92% to 100% (18).
The key finding of this study (18) and that of studies of small pancreatic cancer (10) is that pancreatic ductal dilation and cutoff are clues to the presence of early pancreatic cancer. Though this study(18) did not specifically address the question of resectability of presymptomatic cancers, a look at the data suggests that the tumors appeared resectable in scans done >6 months prior diagnosis. Most scans done in the study were not dedicated pancreas protocol CTs, were done on older scanners and had large slice thickness (>5 mm) (18). It is unclear if high-resolution pancreas protocol CT done on modern multidetector scanners using sub-millimeter cuts can detect small or minute pancreatic cancers.
We have observed that many patients had “normal” CT scans even 6 months prior to clinical diagnosis. This suggests that the transition from resectable to unresectable disease occurs over a period of ~6 months prior to diagnosis. Thus detection of pancreatic cancer even 6 months prior to clinical diagnosis (when majority are asymptomatic) would lead significant increase in resectability rate.
Approaches to Screening for Early Pancreatic Cancer
Since sporadic pancreatic cancer patients seldom exhibit disease-specific symptoms until late in the course of the disease, detection of advanced stages of pre-invasive or early invasive pancreatic cancer will require screening asymptomatic patients for the disease. However, pancreatic cancer is relatively uncommon and it would not be cost-effective to screen for it in the general population. In a recent study it was observed that the pretest probability of dysplasia (or cancer) needs to be sufficiently high (=16%) in order for screening to be effective (19). Therefore, screening for asymptomatic pancreatic cancer will require at least two “sieves” to enrich the population to allow cost-effective screening (Figure 5). The first sieve would be a high-risk group, i.e., a population of subjects at higher than average risk of pancreatic cancer and the second sieve could be a characteristic phenotype among the members of the high-risk group, an abnormality seen on non-invasive imaging or a sensitive and specific serologic marker of early pancreatic cancer.
Figure 5.
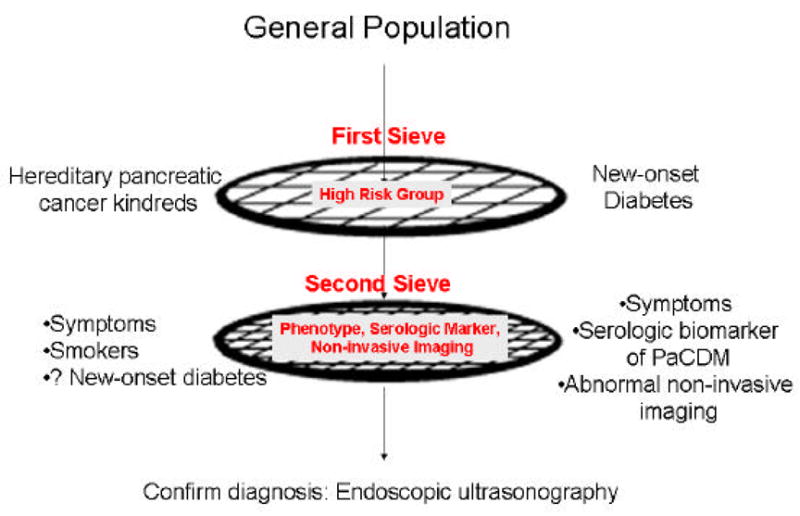
Step-wise Approach to Screening for Pancreatic Cancer
The First Sieve: Defining High Risk Groups for Pancreatic Cancer
The first step towards screening for asymptomatic, early pancreatic cancer is to define patient populations with a higher than average risk of pancreatic cancer. So far two high risk groups have been targets of screening for pancreatic cancer; one approach relies on detecting high grade preinvasive lesions in hereditary pancreatic cancer kindreds and other focusses on detecting early invasive cancer in subjects with new-onset diabetes.
1) Hereditary Pancreatic Cancer
About 10 percent of patients with pancreatic cancer report a family history of the disease. In a population-based case control study of 179 pancreatic cancers and 179 controls from Montreal, Canada, Ghadirian et al (20) found a 13-fold difference in the frequency of family history for pancreatic cancer between cases and controls (7.8% vs 0.6%, p<0.001). Pancreatic cancer may be inherited as part of a known cancer syndrome (viz., familial breast cancer, Peutz-Jeghers syndrome, hereditary non-polyposis colon cancer, or familial atypical multiple mole melanoma syndrome), may occur in association with hereditary pancreatitis or cystic fibrosis, or may occur in patients without any known associated cancers or diseases. Most inherited pancreatic cancers fall in the last category and do not occur in the context of a known syndrome; these are referred to as familial pancreatic cancers. However, most studies on familial pancreatic cancer have not excluded patients with known syndromes. The risk of pancreatic cancer in these syndromes varies.
a) Germline BRCA2 mutation
Germline mutations in the BRCA2 gene on chromosome 13q12-13 that truncate the BRCA2 protein are associated with a 60% to 85% lifetime risk of breast cancer, a 15% to 30% lifetime risk of ovarian cancer(21). Though the risk for the development of pancreatic cancer also appears to be increased in families with BRCA2 mutations, germline BRCA2 mutations remain a rare cause of pancreatic cancer. In a consecutive series of 245 patients who underwent pancreatoduodenectomy for adenocarcinoma of the pancreas(22), germline 6174delT mutation in BRCA2, a characteristic mutation identified in Ashkenazi Jewish patients that appears to increase the risk for pancreatic cancer (23), was seen only in 2 (0.8%) patients, neither of whom had a family history of pancreatic cancer. The frequency of BRCA2 mutations in pancreatic cancer-prone families is higher. In a study of 29 families with three or more relatives affected with pancreatic cancer, Murphy et al (24) reported BRCA2 germline mutations in 5 (17%). In a study of 26 European pancreatic cancer-prone families with at least two first-degree relatives with histologically diagnosed ductal adenocarcinoma from the German Cancer Foundation and European Registry of Hereditary Pancreatitis and Familial Pancreatic Cancer (EUROPAC), Hahn et al (25) found that five (19%) of the families harbored a germline mutation consisting of either a frameshift mutation (three families) or an unclassified variant of BRCA2.
b) Peutz-Jeghers Syndrome
Peutz-Jeghers syndrome is an autosomal dominant inherited disorder due to germline mutations of STK11/LKB1 characterized by mucocutaneous melanin pigmentation and hamartomatous gastrointestinal polyposis. An increased incidence and an earlier age of onset of pancreatic cancer have been reported in patients with Peutz-Jeghers syndrome. An individual patient meta-analysis of 210 subjects with the syndrome described in 6 publications reported a markedly increased risk (relative risk 132, 95% CI, range 44 to 261) for pancreatic cancer with a 36% cumulative risk from age 15 to 64 years old (25).
c) Hereditary Nonpolyposis Colorectal Cancer (HNPCC)
HNPCC is associated with an increased risk of developing a cancer of many organs including colon, endometrium, ovary, stomach, small bowel, hepatobiliary system, ureter, renal pelvis and breast. Pancreatic cancer has been reported in certain extended Lynch syndrome II families(26).
d) Familial Atypical Multiple Mole Melanoma Syndrome (FAMMM)
FAMMM is an autosomal dominant disorder characterized by multiple nevi (usually in the hundreds), multiple atypical nevi, and multiple melanomas. Cases of pancreatic carcinoma were reported in a subset of three FAMMM kindreds(27) harboring a 19-bp germline deletion in the CDKN2 gene that impaired the function of the p16 protein. Goldstein et al (28) reported that 10 of 19 melanoma-prone families had a CDKN2 mutation. Two individuals from the families with a p16 mutation developed pancreatic cancer prospective follow-up for 6 to 18 years. No cases of pancreatic cancer were seen in the families without the p16 mutation.
e) Hereditary Pancreatitis and Pancreatic Cancer
Chronic pancreatitis, like other chronic inflammatory states, predisposes patients to develop pancreatic ductal adenocarcinoma (29). Hereditary pancreatitis, inherited as an autosomal dominant trait, is a disease associated with mutations in the cationic trypsinogen gene that causes chronic pancreatitis. Lowenfels et al (30) reported that patients with hereditary pancreatitis have a markedly elevated risk many years after the onset of symptoms of pancreatitis (mean age, 13.9 ± 12.2 years) of developing pancreatic cancer (mean age, 56.9 ± 11.2 years), with a standardized incidence ratio of 54 (95% CI, range 23 to 105). Cigarette smoking increased the risk of pancreatic cancer in hereditary pancreatitis 154-fold compared with the general population (31). Smokers developed pancreatic cancer on average 20 years earlier than nonsmokers (31).
f) Familial Pancreatic Cancer
Familial pancreatic cancer kindreds have been defined as families having at least one pair of first-degree relatives with pancreatic cancer. The risk of pancreatic cancer in familial pancreatic cancer kindreds was best assessed in a study from Johns Hopkins University (32) in which the observed-to-expected rate of pancreatic cancer was evaluated in 5,179 individuals from 838 familial pancreatic cancer kindreds. In this group, the risk of pancreatic cancer increased as the number of first degree relatives with pancreatic cancer increased. Kindreds having three or more affected first degree relatives had a 32.0-fold increased risk of developing pancreatic cancer (95% CI, 10.4–74.7), those with two affected first degree relatives with pancreatic cancer had a 6.4-fold increased risk (95% CI, 1.8–16.4), and those with a single affected first degree relative had a 4.5-fold increased risk (95% CI, 0.54–16.3)(32). Risk was not increased among 369 spouses and other genetically unrelated relatives or in those with sporadic pancreatic cancer (32).
In summary, subjects with multiple (≥ 3) affected family members have a high lifetime risk of developing pancreatic cancer and therefore are an attractive target group for screening for pre-invasive and invasive pancreatic cancer. However, the limitation of this approach is that these groups with very high risk of pancreatic cancer collectively constitute <5% of all pancreatic cancer patients. Therefore, a different approach will be necessary to identify sporadic pancreatic cancer.
2) Diabetes And Pancreatic Cancer
The association between diabetes and pancreatic cancer has long been recognized. However, the assessment of diabetes as a clinically relevant screening target for pancreatic cancer is complicated by the fact that while long-standing diabetes is an etiologic factor for pancreatic cancer, new-onset diabetes is a manifestation of the cancer. Though most studies show an elevated risk of pancreatic cancer among persons with long-standing diabetes, the strength of this association is modest at best (33). In a meta-analysis of 20 epidemiologic studies, the pooled relative risk of pancreatic cancer for those whose diabetes was diagnosed at least 1 year prior to either diagnosis of pancreatic cancer or to pancreatic cancer death was 2.1 (95% CI, 1.6–2.8) (33). Many, but not all, cohort studies reveal that the risk of pancreatic cancer associated with diabetes decreases with increasing duration of follow-up (34–38). Additionally, while the number of persons with pancreatic cancer in the population is small, the number of older persons with longstanding diabetes is large. Thus long-standing diabetes as a marker for pancreatic cancer is likely to have limited clinical utility.
Pancreatic Cancer Induces Diabetes
There is a large body of evidence that supports the notion that pancreatic cancer causes glucose intolerance (39, 40).
a) Most pancreatic cancer patients have glucose intolerance
Pancreatic cancer has one of highest rates of prevalence diabetes in any disease. When formally tested using oral glucose tolerance tests and WHO criteria for diabetes, nearly two-thirds of pancreatic cancer patients have diabetes (41–43). In our studies, using the 1997 ADA criteria (44) diabetes was present in nearly half the patients with pancreatic cancer at diagnosis (45).
b) There is a clos e temporal relationship between diagnosis of pancreatic cancer and diagnosis of diabetes
The diabetes of pancreatic cancer is generally of short duration and not long-standing. We found that in 60 pancreatic cancer patients with diabetes, in 88% the diagnosis of diabetes was made within 24 months of the diagnosis of pancreatic cancer (45). These data are very similar to that of Gullo et al. (40).
c) Resection of tumor improves glucose tolerance
Glucose tolerance of 7 pancreatic cancer patients undergoing subtotal pancreatectomy, of whom 6 were diabetic before surgery, was studied before and 3 months after surgery (46). After surgery, diabetes status improved in all. There was no evidence of diabetes in 2 patients not requiring insulin before surgery and insulin was no longer necessary after surgery in two other patients with insulin-dependent diabetes. In the remaining two diabetic patients insulin requirements markedly decreased after surgery (46). We observed a similar improvement in glucose tolerance in 9 pancreatic cancer patients with recently diagnosed diabetes who underwent pancreatico-duodenectomy (unpublished). While post-operative weight loss might account for some improvement in fasting blood glucose, we believe this consistent, and sometimes dramatic, improvement in glucose tolerance in spite of partial pancreatic resection strongly suggests the presence of a diabetogenic factor released by pancreatic cancer.
d) Cell culture supernatants from pancreatic cancer cell lines induce glucose intolerance in SCID mice
Daily intraperitoneal injection of 300 ul of supernatants from pancreatic cancer cell line MIA PaCa2 into SCID mice led to significant increase in blood glucose levels. Intravenous glucose tolerance tests, successfully performed in 9 animals (4 controls and 5 treated), demonstrated a significantly reduced glucose tolerance in animals treated with cell culture supernatant compared to control mice. The authors concluded that pancreatic cancer cell line MIA PaCa2 produces one or more soluble factors able to cause hyperglycemia in vivo (47).
e) Pancreatic cancer cells selectively stimulate islet cells to secrete amylin but not insulin
Amylin is thought to mediate insulin resistance due to its effect on muscles. The observation that pancreatic cancer cells selectively stimulate amylin release (48, 49) led to the hypothesis that pancreatic cancers produce an amylin-releasing factor. Such a factor remains to be identified. However, hyper-amylinemia itself is not a satisfactory marker for pancreatic cancer (45).
In summary, the very high prevalence of new-onset diabetes which improves after tumor resection coupled with experimental evidence noted suggests that pancreatic cancer is causally related to the diabetes in pancreatic cancer.
Pancreatic Cancer-induced Diabetes may be useful as a marker of undiagnosed pancreatic cancer
The following pieces of evidence support this hypothesis:
a) Glucose intolerance is an early manifestation of pancreatic cancer and occurs at a resectable stage of disease
Development of glucose intolerance is not dependent on size of tumor or stage of disease. Tsuchiya et al. (8) reported that 48 of 79 (60.8%) patients with small pancreatic cancers (≤20 mm in size) had abnormal glucose tolerance. We (45) and others (41, 42) have reported that 55%-65% of patients with resectable pancreatic cancer have glucose intolerance and diabetes. Permert et al. (42) reported that 64% of patients with resectable tumors had diabetes upon formal glucose tolerance testing. In our series, 12 of 22 (54%) resectable cancers had diabetes (45). Animal studies also show that development of glucose intolerance coincides with appearance of visible pancreatic tumors in hamsters (50). The fact that glucose intolerance occurs early in pancreatic cancer makes it an attractive biomarker for pancreatic cancer.
b) New-onset Diabetes defines a High-risk Group for Pancreatic Cancer
Based on such observations, new-onset diabetes has been suggested as a possible target for screening for pancreatic cancer (39, 40, 42, 51, 52). To test the hypothesis we assembled a population-based cohort of 2,122 Rochester, Minnesota, residents age ≥ 50 years, and identified those who developed pancreatic cancer within 3 years of meeting criteria for diabetes. In this study subjects with new-onset diabetes had an 8 times increased risk compared to the general population of having pancreatic cancer diagnosed within 3 years of meeting criteria for diabetes. In this study 1 in 125 subjects with new-onset diabetes had pancreatic cancer. Recent studies (53, 54) show an even higher prevalence of pancreatic cancer in subjects with new-onset diabetes (5.2 to 13.6%) as they targeted selected high-risk subjects with recently diagnosed diabetes. These studies and the earlier epidemiologic studies suggest that subjects with new-onset diabetes are a high-risk group for having pancreatic cancer.
Based on such observations, new-onset diabetes has been suggested as a possible target for screening for pancreatic cancer (39, 40, 42, 51, 52). The subject of screening new-onset diabetes for pancreatic cancer still has a number of unanswered questions.
Would screening subjects with new-onset diabetes detect resectable disease?
The fact that diabetes occurs early in the course of pancreatic cancer in conjunction with the observation that subjects with new-onset have an 8-fold increased risk of having pancreatic cancer would suggest that screening new-onset diabetics could lead to diagnosis of resectable pancreatic cancer. Studies on screening new-onset diabetes have, however, found that majority of patients have unresectable disease. Further analyses of these studies provide an explanation for this paradox. In studies on prevalence of cancer in new-onset diabetes, patients with physician diagnosed diabetes were investigated for cancer mostly after development of cancer symptoms. The poor rate of resectability in all these studies shows that the strategy to use symptoms such as jaundice and anorexia as clues to suspect pancreatic cancer in new-onset diabetes is unlikely to detect resectable cancer as these symptoms are generally associated with unresectable pancreatic cancer (53, 54) On the other hand, in studies showing a very high prevalence of diabetes in early pancreatic cancer, subjects with resectable and small pancreatic cancers were screened for (asymptomatic) diabetes. Thus, from a screening perspective, the lesson to be learned from these studies is that one will to have screen asymptomatic subjects for new-onset diabetes and screening such subjects for cancer may detect resectable disease.
What proportion of pancreatic cancer subjects could be detected by screening new-onset diabetes?
About ~50% of subjects with pancreatic cancer will meet ADA diagnostic criteria for diabetes (45). However only half of these patients would potentially benefit from screening as in the other 50%, the diabetes will be diagnosed too close (< 6 months) to the diagnosis of cancer to be beneficial from a screening perspective.
Are we ready to screen new-onset diabetes for pancreatic cancer?
The short answer is “not yet”. Type 2 diabetes is very common and pancreatic cancer-induced diabetes relatively rare. Currently type 2 diabetes and pancreatic cancer-induced diabetes are indistinguishable. The prevalence of pancreatic cancer in new-onset diabetes is too low (<1%) to justify screening new-onset diabetes for pancreatic cancer. Therefore, from a public health perspective screening new-onset diabetes for pancreatic cancer would not be cost-effective unless a serologic or other marker is identified that can identify a subset of new-onset diabetes who have a very high (>10%) likelihood of having underlying pancreatic cancer.
B) The Second Sieve: Further Enriching the High Risk Population for Pancreatic Cancer
Pancreatic cancer is a relatively rare disease with the incidence in the general population of 9/100,000 population. Even in subjects >65 years of age the incidence is 62.8/100,000 (http://seer.cancer.gov/csr/1975_2002/results_merged/sect_22_pancreas.pdf). Other than rare “loaded” families with a very high incidence of pancreatic cancer, most other high risk groups do not have a high enough incidence to justify direct surveillance with invasive testing. Thus in a population with a 6–10 fold higher risk of pancreatic cancer (e.g., most familial cancer kindreds, new-onset diabetes) the incidence of pancreatic cancer will be approximately 360 to 630/100,000 or 0.4 to 0.6% which is much lower than the 16% suggested as the target for screening (19). To enrich the population further, one could use demographic and clinical characteristics, non-invasive imaging techniques, or biochemical markers.
Demographic and Clinical features
In the study from University of Washington (14) family members with symptoms of abdominal pain, nausea, weight loss, and diarrhea were more likely to have pancreatic dysplastic changes and were considered good candidates for screening. A similar approach has been used in patients with new-onset diabetes (53, 54). While this approach may work for familial pancreatic cancer, development of severe symptoms (severe abdominal or back pain, jaundice) is often an indicator of advanced disease in sporadic cancer.
Non-invasive imaging
The recent development of multi-detector CT scanners allows for marked increases in both scanning speed and spatial resolution (i.e., thinner slice thickness). Greater lesion contrast is achieved because there is less volume averaging and more uniform enhancement during each vascular phase (due to the decreased scanning time and the acquisition of multiple phases of vascular enhancement) (55, 56). Fast scanning, high-resolution computerized tomography scanners with < 1 mm slice thickness are now available and have been used to screen for lung cancer in smokers (57, 58). Though promising, the role of CT scanning in screening for small pancreatic cancer remains to be established.
Serologic Biomarker of Early Pancreatic Cancer
An important tool for diagnosis of early pancreatic cancer would be a serologic biomarker of early disease which could be the second “sieve” (Figure 5). Currently CA 19–9 is the best pancreatic cancer tumor marker. However, it is a stage-sensitive tumor marker and its sensitivity for resectable cancer is poor (~25%) and its sensitivity is likely to be even lower for Stage I disease.
Though a humoral mediator has not yet been identified, there are several lines of evidence to suggest that pancreatic cancer-induced diabetes is caused not so much by local effects of tumor infiltration, as by remote effects impairing glucose metabolism. Many groups, including ours, are actively investigating the possibility of identifying a unique serologic marker of diabetes associated with pancreatic cancer.
Invasive imaging
In the absence of a non-invasive imaging technique or serologic marker, imaging using invasive endoscopic procedures such as endoscopic ultrasound and endoscopic retrograde cholangio-pancreatography has been used to screen for pancreatic cancer. A successful screening strategy to detect pancreatic cancer using endoscopic ultrasound (EUS) and endoscopic retrograde cholangio-pancreatography (ERCP), applied to one form of familial pancreatic cancer has already been reported (14). Recently the Johns Hopkins group reported that they were able to detect pre-cancerous lesions in their experience with a EUS-based screening of asymptomatic individuals at risk for hereditary pancreatic cancer (59).
However, it is unclear if lessons learnt from study of hereditary cancer are necessarily applicable to sporadic cancer. For example, diffuse abnormalities reported on endoscopic ultrasound and endoscopic retrograde cholangiopancreatography, viz., “dilated, irregular main duct with large side-branch sacculations and irregularities”(14), are not features of sporadic cancer.
Confirming the Diagnosis of Pancreatic Cancer
If non-invasive imaging is equivocal or negative, endoscopic ultrasonography (EUS) will be necessary to conclusively diagnose pancreatic cancer. EUS utilizes high frequency ultrasound probes placed in the stomach and duodenum juxtaposed to the pancreas, and can identify small pancreatic and periampullary tumors. EUS has exquisite sensitivity to detect tumors as small as 10 mm in size. It also has the capability of providing histologic confirmation of the diagnosis using fine needle aspiration (60). Thus the technology now exists to detect and confirm the diagnosis of even minute pancreatic cancers.
EUS has been successfully used to detect high-risk lesions in families with a very high incidence of pancreatic cancer (14). However, in subjects in whom the pre-test probability of having pancreatic cancer is low as in familial cancer kindreds with two family members with pancreatic cancer or new-onset diabetes, using EUS directly to screen for pancreatic cancer may be problematic due high likelihood of false positive findings. EUS can detect cystic spaces as small as 2 to 3 mm in size (Michael Levy, personal communication). Thus EUS is capable of at least visualizing preinvasive stages of pancreatic cancer, both PanINs and intraductal papillary mucinous neoplasms. However, PanIN lesions are very common in individuals without pancreatic disease. Autopsy studies in older individuals have shown that 28% of pancreata had grossly recognizable PanIN lesions, a significant proportion (98%) of which was PanIN 1 and 2 (15). EUS may also detect benign lesions and non-neoplastic masses (61).
The commonest abnormalities seen in high risk kindreds are changes consistent with chronic pancreatitis (61). A recent study showed that the frequency of EUS abnormalities in patients without clinical evidence of chronic pancreatitis increases with age, particularly after 60 years of age (62). In another study (63) alcohol consumption and cigarette smoking affected the endosonographic appearance of the pancreas in a dose-dependent fashion; these variables also were independent predictors of significant pancreatic abnormalities in healthy individuals.
The exquisite sensitivity of EUS to detect subtle changes in architecture comes with the problem of uncertainty of the clinical relevance of the findings. In high risk individuals the uncertainty can lead to unnecessary morbid pancreatic resection. Therefore, careful control studies including individuals matched for age, gender, smoking and alcohol consumption need to be performed to determine the significance of EUS findings noted in high risk groups.
In summary, considerable progress has been made in understanding the natural course of progression of pancreatic cancer, identifying individuals at higher than average risk of pancreatic cancer and our ability to confirm the presence of asymptomatic pancreatic cancer or high-risk lesions. The identification of a serologic marker of early pancreatic cancer would have significant impact on screening for early pancreatic cancer. While number questions remain to be answered, the developments in the field provide us with hope that screening for early pancreatic cancer will be a reality soon.
Acknowledgments
Grant Support: Dr Chari’s research was funded by grants from NIH (R01 CA 100685) and the Lustgarten Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal AMT, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer Statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–9. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maire F, Sauvanet A, Trivin F, et al. Staging of pancreatic head adenocarcinoma with spiral CT and endoscopic ultrasonography: an indirect evaluation of the usefulness of laparoscopy. Pancreatology. 2004;4:436–40. doi: 10.1159/000079617. [DOI] [PubMed] [Google Scholar]
- 4.Karmazanovsky G, Fedorov V, Kubyshkin V, et al. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30:488–500. doi: 10.1007/s00261-004-0279-z. [DOI] [PubMed] [Google Scholar]
- 5.Roche CJ, Hughes ML, Garvey CJ, et al. CT and pathologic assessment of prospective nodal staging in patients with ductal adenocarcinoma of the head of the pancreas. AJR Am J Roentgenol. 2003;180:475–80. doi: 10.2214/ajr.180.2.1800475. [DOI] [PubMed] [Google Scholar]
- 6.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya R, Noda T, Harada N, Miyamoto T, Tomioka T, et al. Collective review of small carcinomas of the pancreas. Annals of Surgery. 1986;203:77–81. doi: 10.1097/00000658-198601000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa H, Okada S, Saisho H, et al. Clinicopathologic features of small pancreatic adenocarcinoma. A collective study. Cancer. 1996;78:986–90. doi: 10.1002/(SICI)1097-0142(19960901)78:5<986::AID-CNCR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu Y, Yasui K, Matsueda K, et al. Small carcinoma of the pancreas is curable: new computed tomography finding, pathological study and postoperative results from a single institute. J Gastroenterol Hepatol. 2005;20:1591–4. doi: 10.1111/j.1440-1746.2005.03895.x. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa O, Ohigashi H, Imaoka S, et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter-- collective review of Japanese case reports. Hepatogastroenterology. 1999;46:8–15. [PubMed] [Google Scholar]
- 12.Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–72. [PubMed] [Google Scholar]
- 13.Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–95. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 14.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kimura W. How many millimeters do atypical epithelia of the pancreas spread intraductally before beginning to infiltrate? Hepatogastroenterology. 2003;50:2218–24. [PubMed] [Google Scholar]
- 16.Brat DJ, Lillemoe KD, Yeo CJ, et al. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol. 1998;22:163–9. doi: 10.1097/00000478-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Brockie E, Anand A, Albores-Saavedra J. Progression of atypical ductal hyperplasia/carcinoma in situ of the pancreas to invasive adenocarcinoma. Ann Diagn Pathol. 1998;2:286–92. doi: 10.1016/s1092-9134(98)80020-8. [DOI] [PubMed] [Google Scholar]
- 18.Gangi S, Fletcher JG, Nathan MA, et al. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol. 2004;182:897–903. doi: 10.2214/ajr.182.4.1820897. [DOI] [PubMed] [Google Scholar]
- 19.Rulyak SJ, Kimmey MB, Veenstra DL, et al. Cost-effectiveness of pancreatic cancer screening in familial pancreatic cancer kindreds. Gastrointest Endosc. 2003;57:23–9. doi: 10.1067/mge.2003.28. [DOI] [PubMed] [Google Scholar]
- 20.Ghadirian P, Boyle P, Simard A, et al. Reported family aggregation of pancreatic cancer within a population-based case-control study in the Francophone community in Montreal, Canada. Int J Pancreatol. 1991;10:183–96. doi: 10.1007/BF02924156. [DOI] [PubMed] [Google Scholar]
- 21.Arnold MA, Goggins M. BRCA2 and predisposition to pancreatic and other cancers. Expert Rev Mol Med. 2001;2001:1–10. doi: 10.1017/S146239940100309X. [DOI] [PubMed] [Google Scholar]
- 22.Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360–4. [PubMed] [Google Scholar]
- 23.Ozcelik H, Schmocker B, Di Nicola N, et al. Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nat Genet. 1997;16:17–8. doi: 10.1038/ng0597-17. [DOI] [PubMed] [Google Scholar]
- 24.Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–93. [PubMed] [Google Scholar]
- 25.Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214–21. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 26.Watson P, Lynch HT. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993;71:677–85. doi: 10.1002/1097-0142(19930201)71:3<677::aid-cncr2820710305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Bergman W, Watson P, de Jong J, et al. Systemic cancer and the FAMMM syndrome. Br J Cancer. 1990;61:932–6. doi: 10.1038/bjc.1990.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein AM, Fraser MC, Struewing JP, et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995;333:970–4. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- 29.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 30.Lowenfels AB, Maisonneuve P, Whitcomb DC. Risk factors for cancer in hereditary pancreatitis. International Hereditary Pancreatitis Study Group. Med Clin North Am. 2000;84:565–73. doi: 10.1016/s0025-7125(05)70240-6. [DOI] [PubMed] [Google Scholar]
- 31.Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. Jama. 2001;286:169–70. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 32.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 33.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta- analysis. JAMA. 1995;273:1605–9. [PubMed] [Google Scholar]
- 34.Ragozzino M, Melton LJd, Chu CP, et al. Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. Journal of Chronic Diseases. 1982;35:13–9. doi: 10.1016/0021-9681(82)90025-x. [DOI] [PubMed] [Google Scholar]
- 35.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. Journal of the National Cancer Institute. 1997;89:1360–5. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 36.Calle EE, Murphy TK, Rodriguez C, et al. Diabetes mellitus and pancreatic cancer mortality in a prospective cohort of United States adults.[see comment] Cancer Causes & Control. 1998;9:403–10. doi: 10.1023/a:1008819701485. [DOI] [PubMed] [Google Scholar]
- 37.Adami HO, McLaughlin J, Ekbom A, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes & Control. 1991;2:307–14. doi: 10.1007/BF00051670. [DOI] [PubMed] [Google Scholar]
- 38.Chow WH, Gridley G, Nyren O, et al. Risk of pancreatic cancer following diabetes mellitus: a nationwide cohort study in Sweden. J Natl Cancer Inst. 1995;87:930–1. doi: 10.1093/jnci/87.12.930. [DOI] [PubMed] [Google Scholar]
- 39.Noy A, Bilezikian JP. Clinical review 63: Diabetes and pancreatic cancer: clues to the early diagnosis of pancreatic malignancy. J Clin Endocrinol Metab. 1994;79:1223–31. doi: 10.1210/jcem.79.5.7962312. [DOI] [PubMed] [Google Scholar]
- 40.Gullo L, Pezzilli R, Morselli-Labate AM. Diabetes and the risk of pancreatic cancer. Italian Pancreatic Cancer Study Group. N Engl J Med. 1994;331:81–4. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 41.Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D, et al. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–93. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Permert J, Ihse I, Jorfeldt L, et al. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg. 1993;159:101–7. [PubMed] [Google Scholar]
- 43.Permert J, Larsson J, Ihse I, et al. Diagnosis of pancreatic cancer. Alteration of glucose metabolism. Int J Pancreatol. 1991;9:113–7. doi: 10.1007/BF02925586. [DOI] [PubMed] [Google Scholar]
- 44.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 45.Chari ST, Klee GG, Miller LJ, Raimondo M, DiMagno EP. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–5. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 46.Permert J, Ihse I, Jorfeldt L, et al. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–50. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 47.Basso D, Brigato L, Veronesi A, et al. The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res. 1995;15:2585–8. [PubMed] [Google Scholar]
- 48.Wang F, Larsson J, Abdiu A, et al. Dissociated secretion of islet amyloid polypeptide and insulin in serum- free culture media conditioned by human pancreatic adenocarcinoma cell lines. Int J Pancreatol. 1997;21:157–64. doi: 10.1007/BF02822387. [DOI] [PubMed] [Google Scholar]
- 49.Ding X, Flatt PR, Permert J, et al. Pancreatic cancer cells selectively stimulate islet beta cells to secrete amylin. Gastroenterology. 1998;114:130–8. doi: 10.1016/s0016-5085(98)70641-9. [DOI] [PubMed] [Google Scholar]
- 50.Ahren B, Andren-Sandberg A. Glucose tolerance and insulin secretion in experimental pancreatic cancer in the Syrian hamster. Res Exp Med. 1993;193:21–6. doi: 10.1007/BF02576207. [DOI] [PubMed] [Google Scholar]
- 51.Moossa AR, Levin B. Collaborative studies in the diagnosis of pancreatic cancer. Seminars in Oncology. 1979;6:298–308. [PubMed] [Google Scholar]
- 52.Jain M, Howe GR, St Louis P, et al. Coffee and alcohol as determinants of risk of pancreas cancer: a case-control study from Toronto. International Journal of Cancer. 1991;47:384–9. doi: 10.1002/ijc.2910470313. [DOI] [PubMed] [Google Scholar]
- 53.Damiano J, Bordier L, Le Berre JP, et al. Should pancreas imaging be recommanded in patients over 50 years when diabetes is discovered because of acute symptoms? Diabetes & Metabolism. 2004;30:203–7. doi: 10.1016/s1262-3636(07)70111-8. [DOI] [PubMed] [Google Scholar]
- 54.Ogawa Y, Tanaka M, Inoue K, et al. A prospective pancreatographic study of the prevalence of pancreatic carcinoma in patients with diabetes mellitus. Cancer. 2002;94:2344–9. doi: 10.1002/cncr.10493. [DOI] [PubMed] [Google Scholar]
- 55.McNulty NJ, Francis IR, Platt JF, et al. Multi--detector row helical CT of the pancreas: effect of contrast- enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97–102. doi: 10.1148/radiology.220.1.r01jl1897. [DOI] [PubMed] [Google Scholar]
- 56.Johnson CD. Pancreatic carcinoma: developing a protocol for multi-detector row CT. Radiology. 2001;220:3–4. doi: 10.1148/radiology.220.1.r01jl483. [DOI] [PubMed] [Google Scholar]
- 57.Tiitola M, Kivisaari L, Huuskonen MS, et al. Computed tomography screening for lung cancer in asbestos-exposed workers. Lung Cancer. 2002;35:17–22. doi: 10.1016/s0169-5002(01)00294-x. [DOI] [PubMed] [Google Scholar]
- 58.Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222:773–81. doi: 10.1148/radiol.2223010490. [DOI] [PubMed] [Google Scholar]
- 59.Canto M, Wroblewski L, Goggins M, Petersen G, Brune K, Charles Yea, Giardello F, Hruban R. Screening for pancreatic neoplasia in hgh-risk individuals: The Johns Hopkins Experience. Gastroenterology. 2002;122(supplement 1):A-17. [Google Scholar]
- 60.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–95. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 61.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 62.Rajan E, Clain JE, Levy MJ, et al. Age-related changes in the pancreas identified by EUS: a prospective evaluation. Gastrointest Endosc. 2005;61:401–6. doi: 10.1016/s0016-5107(04)02758-0. [DOI] [PubMed] [Google Scholar]
- 63.Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol. 2004;2:405–9. doi: 10.1016/s1542-3565(04)00126-0. [DOI] [PubMed] [Google Scholar]


