Abstract
Objective
We sought to investigate the activity of bilateral parietal and premotor areas during a Go/No Go paradigm involving praxis movements of the dominant hand.
Methods
A sentence was presented which instructed subjects on what movement to make (S1; for example, “Show me how to use a hammer.”). After an 8-s delay, “Go” or “No Go” (S2) was presented. If Go, they were instructed to make the movement described in the S1 instruction sentence as quickly as possible, and continuously until the “Rest” cue was presented 3 s later. If No Go, subjects were to simply relax until the next instruction sentence. Event-related potentials (ERP) and event-related desynchronization (ERD) in the beta band (18–22 Hz) were evaluated for three time bins: after S1, after S2, and from −2.5 to −1.5 s before the S2 period.
Results
Bilateral premotor ERP was greater than bilateral parietal ERP after the S2 Go compared with the No Go. Additionally, left premotor ERP was greater than that from the right premotor area. There was predominant left parietal ERD immediately after S1 for both Go and No Go, which was sustained for the duration of the interval between S1 and S2. For both S2 stimuli, predominant left parietal ERD was again seen when compared to that from the left premotor or right parietal area. However, the left parietal ERD was greater for Go than No Go.
Conclusion
The results suggest a dominant role in the left parietal cortex for planning, executing, and suppressing praxis movements. The ERP and ERD show different patterns of activation and may reflect distinct neural movement-related activities.
Significance
The data can guide further studies to determine the neurophysiological changes occurring in apraxia patients and help explain the unique error profiles seen in patients with left parietal damage.
Keywords: apraxia, ERP, ERD, parietal, praxis representations, planning, executing, suppressing
Introduction
Non-human primate studies have shown that parietal and premotor areas play a large role in the preparation of correct performance of complex hand movements (Cavada and Goldman-Rakic, 1989; Gallese et al., 1994; Rizzolatti and Luppino, 2001; Rizzolatti et al., 1998; Wise et al., 1997). A type of complex hand movement performed by humans, called praxis movement, includes pantomime of tool use (transitive) and communicative (intransitive) gesture. Initially, the bulk of knowledge about praxis came from exploring the deficits of executing such movements in patients with ideomotor apraxia. These studies demonstrated that left hemisphere parietal and premotor area lesions generally produce apraxia (Geschwind, 1965; Hanna-Pladdy et al., 2001a; Heilman and Gonzalez Rothi, 2003). This idea was verified in physiological studies of normal praxis performance using electroencephalography (EEG) (Wheaton et al., 2005a; Wheaton et al., 2005b; Wheaton et al., 2005c) and functional magnetic resonance imaging (fMRI) (Bohlhalter et al., in press; Fridman et al., 2006).
Behaviorally, many types of errors are seen in subjects with apraxia, such as content (e.g., perservation, incorrect pantomime), temporal (e.g., sequence, incorrect repetitions) and spatial (e.g. amplitude, configuration) errors (Hanna-Pladdy et al., 2001a). From this work, patients with subcortical or left parietal lesions each demonstrated timing and spatial errors, while left parietal lesioned patients performed unrecognizeable movements that contained incorrect pantomime or failed to move at all. It becomes apparent that the scope of praxis motor control must include planning/preparation and executing, together with suppressing incorrect or invalid movement types. The left intraparietal sulcus has been implicated in having a major role in the full battery of praxis abilities (Buxbaum et al., 2005; Makuuchi et al., 2005).
The purpose of this study is to analyze the activity of bilateral parietal and premotor areas during three distinct phases of praxis motor control: preparation, execution, and suppression of a motor plan. To assess these phases, the Go/No Go paradigm is used, similar to a previously reported paradigm (Fridman et al., 2006). In the previous work, a similar stimulus presentation was used mainly to dichotomize between planning and execution phases. The Go/No Go paradigm provides us with the advantage of not only assessing the neural activation of planning and executing a praxis movement, but also in evaluating one mechanism of how a planned praxis movement is suppressed. This is useful, as it may provide some understanding of how we eliminate a praxis motor plan, which can have relevance to a particular set of errors seen in patients with apraxia. Certain errors are seen in patients with apraxia, such as perseveration and unrelated pantomimes, which reflect a loss of inhibitory control of a planned pantomime. These deficits are likely related to the inability to suppress a praxis motor plan. Subjects were visually cued to plan a specific praxis movement (S1). This is the planning/preparation phase. After a fixed delay, a second visual stimulus (S2) instructed the subject to make the movement (execution, “Go”) or not make the movement (suppression, “No Go”).
We evaluated the neural activity using EEG and focused on two analyses: event-related potentials (ERP) and beta band (18–22 Hz) event-related desynchronization (ERD) emulating the main findings of our previously reported self-paced praxis movement paradigm (Wheaton et al, 2005a,b). We hypothesized that presentation of the S1 cue will show increased and equivalent left parietal and premotor activity sustained during the planning/preparation phase. This would be reflected in pronounced and maintained slow negativity of ERP as seen in previous studies (Jankelowitz and Colebatch, 2002) and sustained ERD. After presentation of either S2 cue (Go or No Go), we expected an increase in left premotor areas primarily with activity surpassing the parietal cortex for both ERP and ERD. For each condition, we expected a left hemispheric dominance of ERP and ERD for planning/preparing, executing (after Go), and suppressing (after No Go).
Methods
Subjects
Thirteen normal volunteers (6 males, 7 females) from 22 to 68 years of age (45.8 ± 19.4) were studied. Participants had no history of neurological disease or musculoskeletal problems impairing motor control of the arm. All subjects had normal or corrected vision.
Experimental Procedure
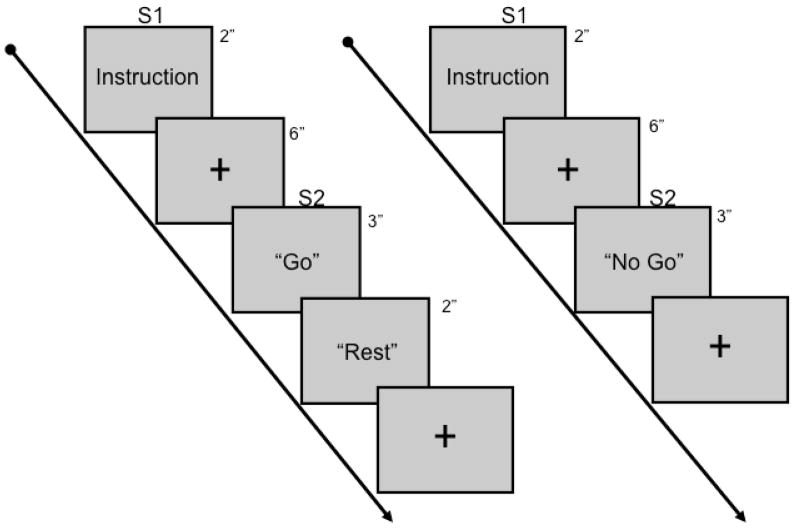
The paradigm used in this study is similar to that used in an fMRI study of parietal and premotor activation related to praxis movements (Fridman et al., 2006). There was one 5-min training session emulating the experimental sessions to ensure familiarization with the task, followed by four 18-min experimental sessions (Figure 1).
Figure 1.
Schematic of the paradigm used in this study.
Subjects sat comfortably in a reclining chair and were presented instructional word cues related to each task component. The cues were presented on a 16-inch LCD monitor using the Gentask application in Neurostim (Compumedics; Charlotte, NC). The monitor was positioned 24 in (61 cm) away from the face in the center of the visual field. The first cue (S1) was an instruction sentence (e.g., “Show me how to use a hammer.”), which was displayed on the screen for 2 s. The S1 instructions were developed from a list of 20 tool use movements and 20 communicative gestures. After presentation of this cue, a blue cross was positioned in the middle of the screen for 6 s. The subject was informed that the first cue served as the time to think about and plan the movement that was requested in the S1 cue. Following this, either “Go” or “No Go” (S2) was presented for 3 s. If “Go”, subjects were instructed to perform the movement with their right hand as quickly as possible until a “Rest” cue was presented afterwards. The movement was to be made repeatedly for the entire 3-s period. Subjects were instructed to attempt to perform the movements without raising the arm in an effort to minimize shoulder, trunk, and head movements. If “No Go”, the subjects were instructed to rest until presentation of the next S1. Presentation of “Go” and “No Go” were pseudorandomized and each accounted for 50% of all trials within each experimental condition. Tool use and gesture movements were pseudorandomized and a single movement was not repeated within an experimental session. There was a 5-min rest between each experimental session.
Data acquisition
High-density (64-channel) EEG was recorded using SynAmps (Compumedics; Charlotte, NC) with a linked ear reference. EEG was recorded at a 1 kHz sampling rate and a bandpass range of DC to 100 Hz. To ensure proper performance based on the S2, surface electromyography (EMG) was recorded from the abductor pollicis brevis (APB), flexor carpi ulnaris (FCU), biceps brachii (BB) and triceps brachii (TB) muscles in the right arm with a bandpass range of 5–200 Hz.
Data Analysis
ERP
Initial analysis was performed to assess the Go/No Go ERP. Raw data were bandpass filtered (0–50 Hz) and epoched. For each trial, onset of S2 served as time = 0, and epochs from 10.0 s before S2 onset to 3.0 s after were collected. Epochs with artifact were removed upon visual inspection. Transitive and intransitive data were pooled together to analyze overall praxis movement characteristics. Go trials were separated from No Go trials based on a unique S2 marker provided in the output of Stim. Individual averages of Go and No Go were analyzed for each subject. Baseline was subtracted from −10.0 to −9.5 s. For quantitative analysis of bilateral parietal and premotor, four regions of interest were evaluated: Left premotor (F1, F3, C1A, C3A); Right premotor (F2, F4, C2A, C4A); Left parietal (P1, P3, P1P, P3P); Right parietal (P2, P4, P2P, P4P). A grand total of approximately 300 trials were generated for both Go and No Go conditions.
Statistical analysis of data was performed on three distinct time bins and was determined upon visualization of the data due to the large size of the epoch. Time bin 1 was centered on the average peak response to S1, and was defined as −7.0 to −6.3 s. Time bin 2 was taken as a time bin between S1 and S2, and was defined as −2.5 to −1.5 s. Time bin 3 was between 0.3 – 0.6 s, centered on the peak activity occurring after S2. ANOVAs (2 conditions × 4 ROI) were run separately on each time bin followed by post-hoc analysis using the Greenhouse-Geisser correction method, with a statistical expectancy of p≤0.05 for significance and 0.1<p>0.05 as a standard for trends toward significance.
ERD
For our analysis, we focused on the beta band ERD, emulating the findings of our previous study using a self paced paradigm (Wheaton et al., 2005b). Raw data were bandpass filtered within the beta band (18–22 Hz), rectified, and epoched. Epochs were the same as the time-voltage analysis. Epochs with sustained artifact were removed upon visual inspection. For initial analysis, transitive and intransitive data were pooled together to analyze a characteristic praxis movement. Go trials were separated from No Go trials based on a unique S2 marker provided in the output of Stim. Individual averages of Go and No Go were analyzed for each subject. Baseline was subtracted from −10.0 to −9.5 s. ERD was quantified for each subject as %ERD relative to a reference interval from −9 to −8.25 s, based on previously defined methodology (Pfurtscheller and Andrew, 1999; Pfurtscheller and Aranibar, 1977). For quantitative analysis of bilateral parietal and premotor regions, analysis was performed on the same region of interest (ROI) as the time-voltage activity. A grand total of approximately 400 trials were generated for both Go and No Go conditions.
Statistical analysis of data was again performed on the three distinct time bins from the ERP analysis (Time bin 1, −7.0 to −6.3 s; Time bin 2, −2.5 to −1.5 s; Time bin 3, 0.3 – 0.6 s). ANOVAs (2 conditions × 4 ROI) were run separately on each time bin followed by post-hoc analysis using the Greenhouse-Geisser correction method, with a statistical expectancy of p≤0.05 for significance. Additional secondary analysis was performed to determine differences between transitive and intransitive movements within each condition for each ROI.
Results
Movement
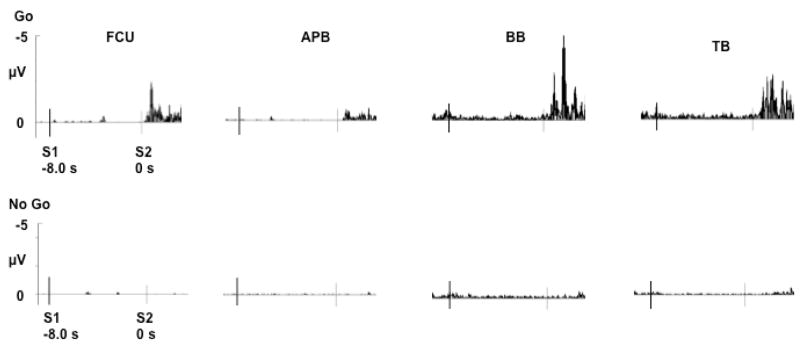
For each subject and condition, EMG over the superficial arm muscles revealed activity after presentation of the Go S2, but not the No Go S2 (Fig 2). For individual subjects and on average, the EMG onset began between 300 – 400 ms after presentation of the Go S2 stimulus, suggesting overall accordance with the directions of a rapid movement in response to the Go S2.
Figure 2.
Grand average surface EMG recorded for each condition over flexor carpi ulnaris (FCU), abductor pollicis brevis (APB), biceps brachii (BB) and triceps brachii (TB) muscles.
ERP
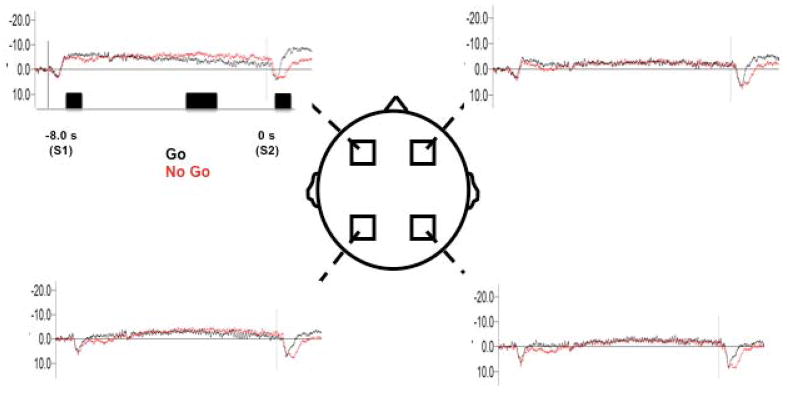
Overall, the ERP patterns demonstrate effects as a result of presentation of S1 and S2, which appears most pronounced over frontal regions (Figure 3). Descriptive data for the ERPs at each phase are provided in Table 1. Common across all subjects, presentation of S1 causes a sharp positive potential, which returns to a negative drift for the duration of the planning period. Following this, the Go S2 causes a positive peak with a large, sustained negative component following. The No Go S2 results in a positive peak, with a slower negative rise compared to the Go S2. Separate ANOVAs were run for each time bin across all conditions and regions. At time bin 1, there was no condition (F=1.34, p=0.31), region (F=2.11, p=0.15), or interaction effect (F=1.91, p=0.22). For time bin 2, there was no condition (F=1.1, p=0.71), region (F=2.02, p=0.24), or interaction effect (F=1.42, p=0.37). For time bin 3, there was an effect of condition (F=2.42, p=0.03), region (F=3.01, p=0.01) and interaction (F=2.66, p= 0.02). Further statistical analysis was made only on time bin 3. Figure 3 illustrates the ERP activations at each ROI.
Figure 3.
Grand average ERP seen for Go and No Go conditions over each ROI. Black rectangles indicate time bins for statistical analysis
Table 1.
Summary of ERP responses in latency and amplitude to the S1 and S2 for both Go and No Go conditions. Measures are given for the last peak amplitude and latency (row 1) before the large positive response (row 2), and the maximal response after the positivity (row 3).
| Go | Left Parietal | Left Premotor | Right Parietal | Right Premotor |
|---|---|---|---|---|
| S1 (lat (s), amp (μV)) | −7.68, −2.42 | −7.98, −1.19 | −7.84, 1.67 | −7.98, −1.52 |
| −7.66, 5.43 | −7.72, 3.09 | −7.66, 5.14 | −7.72, 3.51 | |
| −7.04, −2.56 | −7.0, −6.83 | −7.05, −2.76 | −7.43, −4.97 | |
| S2 | 0.06, −2.72 | 0.07, −3.54 | 0.07, −3.08 | 0.06, −3.55 |
| 0.37, 7.1 | 0.36, 6.39 | 0.37, 7.99 | 0.36, 6.63 | |
| 1.52, −3.74 | 1.03, −8.55 | 1.61, −2.05 | 1.31, −5.25 | |
| No Go | Left Parietal | Left Premotor | Right Parietal | Right Premotor |
| S1 | −7.86, −1.50 | −7.89, 0.05 | −7.81, −0.28 | −7.86, 0.35 |
| −7.64, 6.62 | −7.67, 3.37 | −7.64, 7.96 | −7.66, 4.29 | |
| −7.20, 0.42 | −7.4, −5.43 | −7.4, 0.58 | −7.40, −4.47 | |
| S2 | 0.26, 0.2 | 0.16, −4.4 | 0.2, −1.73 | 0.17, −1.78 |
| 0.67, 7.99 | 0.41, 6.9 | 0.4, 8.64 | 0.41, 7.54 | |
| 0.98, 0.83 | 0.95, −3.13 | 1.03, 0.78 | 0.95, −1.82 |
Time bin 3
We found significantly greater activation in the Go condition versus the No Go condition over the left premotor area (p=0.03), but not over any other areas. There were no hemispheric differences between ROI for either Go or No Go. For the Go condition, activation over the left premotor areas was significantly greater than the left parietal areas (p=0.03). Right premotor areas were significantly greater than right parietal areas (p=0.04). For the No Go condition, activation over the left premotor areas was significantly greater than the left parietal areas (p=0.05). Right premotor activation was significantly greater than the right parietal areas (p=0.03).
ERD
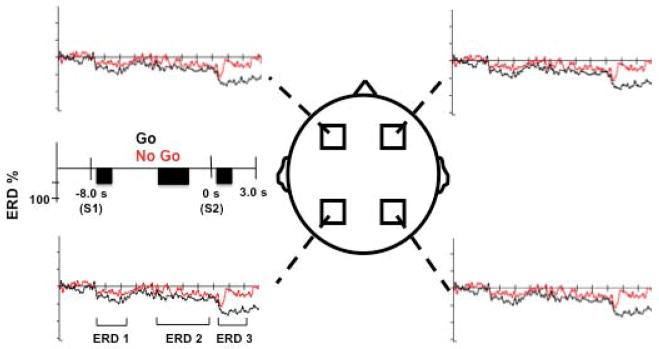
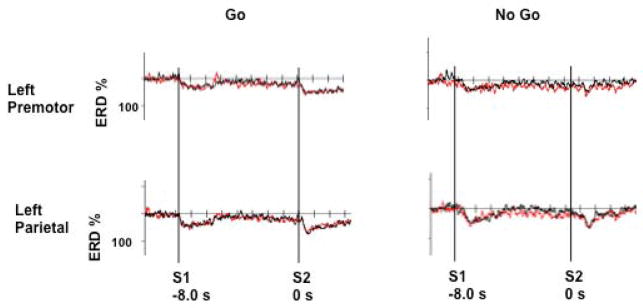
Overall, three distinct ERD patterns are seen in the data (Figure 4). The first occurs after presentation of S1, with a sharp ERD, followed by ERD decline back to near baseline in both conditions. The second appears as a slowly increasing ERD for the duration of the planning/preparation phase in both conditions, consistent across all subjects. The third occurs after presentation of S2, remains for the duration of the epoch in the Go condition, and is a transient ERD response in No Go.
Figure 4.
Grand average ERD responses seen for each ROI. Brackets demonstrate the three ERD phases described in the Results section. Black rectangles indicate time bins for statistical analysis.
Separate ANOVAs were run for each time bin across all conditions and regions. At time bin 1, there was no effect of condition (F=1.97, p=0.2), but there was a region (F=4.78, p=0.0008) and interaction effect (F=4.39, p= 0.0009). At time bin 2, there was no effect of condition (F=0.98, p=0.48), region (F=1.32, p=0.3) or interaction (F=1.08, p=0.38). At time bin 3 there was an effect of condition (F=5.53, p=0.0003), region (F=3.98, p=0.001) and interaction (F=4.64, p=0.0005). Further statistical analysis was made only on time bins 1 and 3.
Time bin 1
We found significantly increased ERD over the left parietal versus left premotor areas for both Go (p=0.04) and No Go conditions (p=0.01), but not over the right parietal versus right premotor areas for either Go (p=0.21) or No Go (p=0.57). When comparing the Go versus No Go conditions, no significant differences were observed.
Time bin 3
We found significantly increased ERD over the left parietal versus left premotor areas for both Go (p=0.009) and No Go (p=0.04) and for right parietal versus right premotor areas for Go (p=0.04) but not for No Go (p=0.2). When comparing Go versus No Go, there was a significant difference for Go versus No Go left parietal (p=0.01), but not for any other regions (left premotor, p=0.68; right premotor, p=0.50; right parietal, p=0.12).
After presentation of S2, we noticed clear distinctions between the ERD characteristics. For the Go condition, ERD was sustained for the duration of the epoch, while the No Go condition revealed ERD, which quickly returned to baseline within 800 ms of the S2 cue at each area.
Differences between movement types
Secondary analysis failed to reveal any differences in ERD at time bins 1, 2, or 3 between transitive and intransitive movement in either Go or No Go conditions (Fig 5).
Figure 5.
Illustration of the left parietal and premotor areas for transitive only (black) and intransitive only (red) conditions. Data show no differences between the two movement types.
Discussion
Parietofrontal networks for praxis have been shown during pre-movement and movement onset for self-paced praxis movements (Wheaton et al., 2005a). However, a limitation of self-paced studies is that there is no distinct separation of preparation and execution. Additionally, self-paced studies are unable to assess other aspects of motor control, such as suppressing a planned movement. To better evaluate distinct phases of activation, an S1–S2 paradigm was implemented. In the current study, preparation occurred after the instructional S1 cue. Evaluation of the ERP components demonstrated a classical frontal predominant negativity after presentation of Go, which was greater than the No Go (Smith et al., 2008). After S2, frontal ERP activation bilaterally was greater than parietal areas bilaterally (Figure 3). Focusing on the beta band, we saw increased ERD over parietal and premotor areas bilaterally during preparation of praxis hand movements. However, this increased ERD was greatest over the left parietal area with no significant difference between Go and No Go conditions. After presentation of S2 (Go or No Go), there was a significant difference in ERD, with Go conditions being greater than No Go conditions. Unlike the ERP, ERD within both conditions was greatest at the left parietal area. This finding elaborates the role of left parietal cortex to involve not only preparing and executing, but also suppressing the motor plan.
The presence of activity over left parietal and premotor areas further underscored the importance of these regions in performance of praxis. It has been considered that coherent networks exist that can integrate activity related to various aspects of planning a complex motor task to actual performance of the task (Burnod et al., 1999; Wise et al., 1997). For praxis movements, this could involve knowledge of tools (or gestures), the normal motor parameters in situations performing the associated task, and then developing a motor pattern based on this information. Preparation ERD is particularly important. In this study, there was no difference in the ERD related to preparation movements between Go or No Go trials. This is expected because subjects were told to always plan the movements that they were instructed, regardless of the type of S2 that would be presented. This allowed us to know that the ERD activity was related to preparation, and did not overlap with any motor activity.
However, after S2 presentation, there was a difference between trials, showing peak ERD activity in parietal areas for both Go and No Go. After presentation of “No Go”, it was possible that there was an inhibitory process involving similar areas that are also present in preparatory processes, primarily including the left parietal cortex. It was previously demonstrated that the main effect of a No Go stimulus is over bilateral medial frontal cortex in adults, while children have an additional bilateral temporal and parietal source of activity, indicating early executive control processes are more distributed in children than adults (Jonkman et al., 2007). This work is similar to findings in fMRI that show bilateral posterior activation in children but not adults to the No Go stimuli (Bunge et al., 2002a). Sources of inhibitory activity have been attributed to premotor areas (Sasaki et al., 1993; Sasaki et al., 1989). In the present study, we suggest that both parietal and premotor activation are essential, as we saw the continued ERD at both parietal and premotor areas throughout the task (Figure 4). This suggests that these neural areas work together because of the unique nature of the motor task. Planning and executing higher-order movements require extensive parietofrontal networks (Wheaton et al., 2005a), a property that, we suggest, may be maintained during task suppression as well. Activity related to motor inhibition has been demonstrated at parietal and premotor areas, which could relate to the increased activity after presentation of No Go (Shibata et al., 1998). Conjunction analysis in fMRI found inhibitory processes related to “Stop” and “No Go” tasks in mesial, medial, and inferior frontal and parietal areas (Rubia et al., 2001). Additionally, during a “No Go” decision in fMRI, activation included bilateral premotor areas, left dorsal premotor areas, and left inferior parietal sulcus (Watanabe et al., 2002).
We must consider the possibility that the activity said to be in the left parietal area is not specific to that area, but comes in part by volume conduction from adjacent areas. Volume conduction is a potential problem because of the use of linked ear referencing, and other left hemisphere activity will be occurring due to exclusive use of the right upper limb in this study. Clinical evidence certainly points to involvement of the left parietal area. Clinical-pathological correlation shows apraxia of either hand derives from mainly left parietal and/or frontal lesions (Geschwind, 1965; Haaland et al, 2000; Schnider et al, 1997). The importance of the left hemisphere in praxis has been demonstrated in an fMRI study illustrating that praxis planning is left-lateralized regardless of whether the movement is made with the left or right hand (Bohlhalter et al, in press). As well, corticocortical networks in healthy persons performing praxis movements with the non-dominant left hand demonstrated left parietofrontal networks (Wheaton et al, 2008). However, use of more sophisticated EEG analysis methods to better define precise cortical activations related to such tasks is a focus of ongoing studies.
Overall, there were differences noted in the regional activation profiles of the ERP (largely left frontal) and the beta ERD (left parietofrontal) to the S2, which suggest distinct cortical mechanisms underlying the properties of these signals. This is similar to previous studies comparing the mapping of alpha band ERD and ERP in a cued Go/No Go wrist flexion motor task (Filipovic et al., 2001). Based on our hypotheses of parietofrontal involvement particular for this study and motor task, the largely frontal ERP was unexpected. However, during the interval of S1 and S2 for both ERD and ERP we saw increased activity suggesting that both were similarly involved with planning (Fig 4, 5). Regional differences in execution/suppression in response to the S2 need to be better evaluated to understand the distinctions.
In this study design, it is conceivable that parietal and premotor areas may not be as necessary at the onset of a movement, since there is an extensive planning period in the task. However, parietal and premotor areas, and possibly networks between the structures, can be involved in online control of movement (Cunnington et al., 2002; Hanakawa et al., 2003; Johnson et al., 2002; Wise et al., 1997). This effect may be paradigm-dependent, since it is not generally seen in response selection studies, where mainly frontal areas initiate the appropriate motor response (Bunge et al., 2002b). In similar Go/No-Go studies, only sensorimotor, mesial frontal, thalamic and cerebellar activity is seen during execution (Watanabe et al., 2002). In our paradigm, normal subjects have an extensive planning period and can formulate a clear motor program, and still involve predominant left parietal activity related to executing the praxis movement. This concept is similar to studies using fMRI for praxis movements (Fridman et al., 2006). Our study shows no difference between transitive and intransitive movements. It must be pointed out that this analysis is focused on voltage activity and beta band ERD, emulating previous work (Wheaton et al., 2005b). It is possible that other EEG analysis methods may indicate differences in movement type. This specific question was outside the scope of this study. Further work exploring other EEG analyses is needed to evaluate if other activations detect differences between the two movement types.
Much of the human evidence of parietofrontal networks and the deficits that can arise from lesions in these areas comes from studies of patient groups with ideomotor apraxia, where stroke patients have spatial and/or temporal errors of tool use pantomime and/or gesture hand movements. Lesions generally arise in left parietal and/or premotor areas (Haaland et al., 2000). Failure to execute these movements relate to the lesions that arise in left parietal and or premotor areas and disable normal planning or execution mechanisms. Spatial, content, and occurrence errors are particularly characteristic in patients with apraxia (Rothi et al., 1988). Using similar methods, qualitative error analysis has demonstrated that timing errors are present in patients with apraxia (Hanna-Pladdy et al., 2001b). An intriguing concept is that temporal errors also exist (including increased, decreased, or irregular rates of production) and perseverative errors are also seen. The loss of inhibitory control of an ongoing motor command (i.e., increased or irregular rates of production), or extinguish a previously used one (i.e., perseveration) may require intact left parietal or premotor mediated inhibition mechanisms. Based on the finding of loss of inhibitory control seen in the work of Hanna-Pladdy et al (2001), we suggest that this process may be similar to the No Go activation seen in this study where the peak activation was seen over the left parietal areas. Perhaps the left parietal areas mediate control related to inhibitory processes of complex motor tasks. However, we do not propose that this No Go mechanism is related to the full battery of spatial and temporal errors. Future studies will better elaborate these mechanisms.
There are some limitations to this concept. Our suppression of praxis (No Go S2 cue) reflects the need to suppress a motor plan since the correct outcome in this condition is no movement. However, this is not completely analogous to the impairments seen in ideomotor apraxia of the inability to suppress incorrect movements, which is demonstrated in errors such as perseveration, unrecognizable movements, or unrelated pantomimes. Such error patterns are difficult to reproduce adequately in healthy subjects without other confounds. We suggest that there may be similar mechanisms as several error profiles in ideomotor apraxia consist of the inability to suppress an improper motor plan. In the No Go, we have the advantage of suppressing a planned pantomime. In patients with apraxia it is not always clear if these above-mentioned errors are not, in part, due to improper motor plans or failed execution. In either case, the behavioral result involves inappropriate mechanisms to suppress a flawed movement that results from damaged neural representations of the motor task.
In addition, the study has other limitations. There is potential that effects of aging played a role in the results seen here. Previous studies have demonstrated a broader recruitment of parietal and frontal areas as a function of aging, reflected in oscillatory activity in a visuomotor task (Derambure, et al. 1993; Labyt, et al. 2003, Labyt, et al 2004). In this study we are focusing on the presence of both parietal and frontal activity, which is known to be present in praxis motor control tasks. The effect of neural recruitment magnitude across aging is potentially limited by the ROI analysis used here, though this effect should be better evaluated in future studies. As well, the extended time period between S1 and S2 might contain elements that could contribute to neural activation unrelated to the task. For this reason, we sought to evaluate a later time bin for the interval between S1 and S2 to ensure that the EEG signals indicated persistent planning throughout the time period before S2. The ERP demonstrates a continued negativity at the frontal regions (Figure 3), a feature of continued planning of the task condition between S1 and S2 in similar invasive (Ikeda et al., 1999) and surface (Jankelowitz and Colebatch, 2002) recordings. We see ERD maintained between S1 and S2 (Figure 4), also suggestive of ongoing neural processes related to task planning.
Normal subject preparation, execution, and suppression mainly involve activity of left parietal cortex. This evidence can guide further studies to determine the neurophysiological changes occurring in apraxia patients and help explain the unique error profiles seen in patients with left parietal damage.
Acknowledgments
This research was supported by the Intramural Research Program of the NINDS, NIH. Special thanks to Devee Schoenberg for professional editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bohlhalter S, Hattori N, Wheaton L, Fridman E, Shamim E, Garraux G, et al. Gesture-subtype dependent left lateralization of praxis planning: an event related functional MRI study. Cerebral Cortex. doi: 10.1093/cercor/bhn168. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002a;33:301–11. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002b;17:1562–71. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Burnod Y, Baraduc P, Battaglia-Mayer A, Guigon E, Koechlin E, Ferraina S, et al. Parieto-frontal coding of reaching: an integrated framework. Exp Brain Res. 1999;129:325–46. doi: 10.1007/s002210050902. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Johnson-Frey SH, Bartlett-Williams M. Deficient internal models for planning hand-object interactions in apraxia. Neuropsychologia. 2005;43:917–29. doi: 10.1016/j.neuropsychologia.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 2002;15:373–85. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- Derambure P, Defebvre L, Dujardin K, Bourriez JL, Jacquesson JM, Destee A, et al. Effect of aging on the spatio-temporal pattern of event-related desynchronization during a voluntary movement. Electroencephalogr Clin Neurophysiol. 1993;89:197–203. doi: 10.1016/0168-5597(93)90133-a. [DOI] [PubMed] [Google Scholar]
- Filipovic SR, Jahanshahi M, Rothwell JC. Uncoupling of contingent negative variation and alpha band event-related desynchronization in a go/no-go task. Clin Neurophysiol. 2001;112:1307–15. doi: 10.1016/s1388-2457(01)00558-2. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Immisch I, Hanakawa T, Bohlhalter S, Waldvogel D, Kansaku K, et al. The role of the dorsal stream for gesture production. Neuroimage. 2006;29:417–28. doi: 10.1016/j.neuroimage.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Gallese V, Murata A, Kaseda M, Niki N, Sakata H. Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport. 1994;5:1525–9. doi: 10.1097/00001756-199407000-00029. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II Brain. 1965;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123 (Pt 11):2306–13. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Immisch I, Toma K, Dimyan MA, Van Gelderen P, Hallett M. Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol. 2003;89:989–1002. doi: 10.1152/jn.00132.2002. [DOI] [PubMed] [Google Scholar]
- Hanna-Pladdy B, Daniels SK, Fieselman MA, Thompson K, Vasterling JJ, Heilman KM, et al. Praxis lateralization: errors in right and left hemisphere stroke. Cortex. 2001a;37:219–30. doi: 10.1016/s0010-9452(08)70569-0. [DOI] [PubMed] [Google Scholar]
- Hanna-Pladdy B, Heilman KM, Foundas AL. Cortical and subcortical contributions to ideomotor apraxia: analysis of task demands and error types. Brain. 2001b;124:2513–27. doi: 10.1093/brain/124.12.2513. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Gonzalez Rothi LJ. Apraxia. In: Heilman KM, Valenstein E, editors. Clinical Neurophysiology. New York: Oxford University Press; 2003. pp. 215–235. [Google Scholar]
- Ikeda A, Yazawa S, Kunieda T, Ohara S, Terada K, Mikuni N, et al. Cognitive motor control in human pre-supplementary motor area studied by subdural recording of discrimination/selection-related potentials. Brain. 1999;122 (Pt 5):915–31. doi: 10.1093/brain/122.5.915. [DOI] [PubMed] [Google Scholar]
- Jankelowitz SK, Colebatch JG. Movement-related potentials associated with self-paced, cued and imagined arm movements. Exp Brain Res. 2002;147:98–107. doi: 10.1007/s00221-002-1220-8. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Rotte M, Grafton ST, Hinrichs H, Gazzaniga MS, Heinze HJ. Selective activation of a parietofrontal circuit during implicitly imagined prehension. Neuroimage. 2002;17:1693–704. doi: 10.1006/nimg.2002.1265. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Sniedt FL, Kemner C. Source localization of the Nogo-N2: a developmental study. Clin Neurophysiol. 2007;118:1069–77. doi: 10.1016/j.clinph.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Labyt E, Szurhaj W, Bourriez JL, Cassim F, Defebvre L, Destee A, et al. Changes in oscillatory cortical activity related to a visuomotor task in young and elderly healthy subjects. Clin Neurophysiol. 2003;114:1153–66. doi: 10.1016/s1388-2457(03)00058-0. [DOI] [PubMed] [Google Scholar]
- Labyt E, Szurhaj W, Bourriez JL, Cassim F, Defebvre L, Destee A, et al. Influence of aging on cortical activity associated with a visuo-motor task. Neurobiol Aging. 2004;25:817–27. doi: 10.1016/j.neurobiolaging.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Makuuchi M, Kaminaga T, Sugishita M. Brain activation during ideomotor praxis: imitation and movements executed by verbal command. J Neurol Neurosurg Psychiatry. 2005;76:25–33. doi: 10.1136/jnnp.2003.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Andrew C. Event-Related changes of band power and coherence: methodology and interpretation. J Clin Neurophysiol. 1999;16:512–9. doi: 10.1097/00004691-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol. 1977;42:817–26. doi: 10.1016/0013-4694(77)90235-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–96. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Rothi LJG, Mack L, Verfaellie M, Brown P, Heilman K. Ideomotor apraxia, error pattern analysis/Aphasiology. 1988;2:381–388. [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Gemba H, Nambu A, Matsuzaki R. No-go activity in the frontal association cortex of human subjects. Neurosci Res. 1993;18:249–52. doi: 10.1016/0168-0102(93)90062-u. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Gemba H, Tsujimoto T. Suppression of visually initiated hand movement by stimulation of the prefrontal cortex in the monkey. Brain Res. 1989;495:100–7. doi: 10.1016/0006-8993(89)91222-5. [DOI] [PubMed] [Google Scholar]
- Shibata T, Shimoyama I, Ito T, Abla D, Iwasa H, Koseki K, et al. The synchronization between brain areas under motor inhibition process in humans estimated by event-related EEG coherence. Neurosci Res. 1998;31:265–71. doi: 10.1016/s0168-0102(98)00046-7. [DOI] [PubMed] [Google Scholar]
- Schnider A, Hanlon RE, Alexander DN, Benson DF. Ideomotor apraxia: behavioral dimensions and neuroanatomical basis. Brain Lang. 1997;58:125–36. doi: 10.1006/brln.1997.1770. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clin Neurophysiol. 2008;119:704–14. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, et al. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage. 2002;17:1207–16. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Nolte G, Bohlhalter S, Fridman E, Hallett M. Synchronization of parietal and premotor areas during preparation and execution of praxis hand movements. Clin Neurophysiol. 2005a;116:1382–90. doi: 10.1016/j.clinph.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Shibasaki H, Hallett M. Temporal activation pattern of parietal and premotor areas related to praxis movements. Clin Neurophysiol. 2005b;116:1201–1212. doi: 10.1016/j.clinph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Yakota S, Hallett M. Posterior parietal negativity preceding self-paced praxis movements. Exp Brain Res. 2005c;163:535–9. doi: 10.1007/s00221-005-2314-x. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Bohlhalter S, Nolte G, Shibasaki H, Hattori N, Fridman E, et al. Cortico-cortical networks in patients with ideomotor apraxia as revealed by EEG coherence analysis. Neurosci Lett. 2008;433:87–92. doi: 10.1016/j.neulet.2007.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]