Abstract
Tobacco addiction is a chronic disorder that is characterized by a negative affective state upon smoking cessation and relapse after periods of abstinence. Previous research has shown that an increased central release of corticotropin-releasing factor (CRF) at least partly mediates the deficit in brain reward function associated with nicotine withdrawal in rats. The aim of these studies was to investigate the role of CRF in the central nucleus of the amygdala (CeA), the lateral bed nucleus of the stria terminalis (BNST), and the nucleus accumbens shell (Nacc shell) in the deficit in brain reward function associated with precipitated nicotine withdrawal. The intracranial self-stimulation procedure was used to assess the negative affective aspects of nicotine withdrawal. Elevations in brain reward thresholds are indicative of a deficit in brain reward function. In all experiments, the nicotinic receptor antagonist mecamylamine (3 mg/kg) elevated the brain reward thresholds of the nicotine dependent rats (9 mg/kg/day of nicotine salt) and did not affect the brain reward thresholds of the saline-treated control rats. The administration of the nonspecific CRF1/2 receptor antagonist D-Phe CRF(12–41) into the CeA and the Nacc shell prevented the mecamylamine-induced elevations in brain reward thresholds in the nicotine dependent rats. Blockade of CRF1/2 receptors in the lateral BNST did not prevent the mecamylamine-induced elevations in brain reward thresholds in the nicotine dependent rats. These studies indicate that the negative emotional state associated with precipitated nicotine withdrawal is at least partly mediated by an increased release of CRF in the CeA and Nacc shell.
Keywords: Nicotine, corticotropin-releasing factor, dependence, reward deficit, rats
INTRODUCTION
Tobacco addiction is a chronic disorder that is characterized by loss of control over smoking, the appearance of withdrawal symptoms upon smoking cessation, and relapse after periods of abstinence (American Psychiatric Association 2000; McLellan et al, 2000; O’Brien 2003). Abrupt cessation of smoking typically mediates negative affective symptoms such as depressed mood, anxiety, irritability, and difficulty concentrating (American Psychiatric Association 2000). It has been hypothesized that the negative affective aspects of tobacco withdrawal provide a powerful motivation for the continuation of smoking (Koob et al, 1997; Markou et al, 1998). Experimental evidence suggests that nicotine is one of the main components of tobacco smoke that leads to and maintains the tobacco addiction (Bardo et al, 1999; Crooks and Dwoskin 1997; Stolerman and Jarvis 1995). The positive reinforcing effects of nicotine are at least partly mediated by the activation of neuronal nicotinic acetylcholine receptors (nAChRs). Blockade of nAChRs decreases the self-administration of nicotine in rats (Corrigall et al, 1994; Corrigall and Coen 1989; Donny et al, 1999; Watkins et al, 1999). In addition, mice that lack the β2-subunit of the nAChR self-administer less nicotine than wild-type controls (Picciotto et al, 1998). Nicotine withdrawal is associated with a deficit in brain reward function and somatic withdrawal signs in rats (Bruijnzeel and Markou 2004; Epping-Jordan et al, 1998; Harrison et al, 2001). Epping-Jordan and colleagues reported that systemic administration of the nAChR antagonist dihydro-β-erythroidine (DHβE) induces an elevation in brain reward thresholds (decrease in the reinforcing properties of intracranial self-stimulation) and an increase in somatic withdrawal signs in rats chronically treated with nicotine. Similarly, abrupt cessation of nicotine administration mediates an elevation in brain reward thresholds and an increase in somatic withdrawal signs (Epping-Jordan et al, 1998). The administration of nicotine after the discontinuation of chronic nicotine administration has been shown to mitigate somatic nicotine withdrawal signs (Malin et al, 1992). These findings indicate that nicotine stimulates the brain reward system and discontinuation of chronic nicotine administration leads to a negative emotional state in rats.
Accumulating evidence suggests that a hyperactivity of brain stress systems may lead to a deficit in brain reward function, which is one of the core symptoms of drug withdrawal and depression (Barr and Markou 2005; Bruijnzeel and Gold 2005). Clinical studies indicate that brain corticotropin-releasing factor (CRF) systems are hyperactive in patients with depressive disorders (Nemeroff et al, 1984; Zobel et al, 2000). Preclinical research indicates that intracerebroventricular (icv) administration of CRF induces an elevation in brain reward thresholds in rats, which is indicative of a deficit in brain reward function (Macey et al, 2000). The observation that increased CRF transmission plays a role in negative affective states has stimulated research into the role of CRF in drug addictions. These studies have provided evidence for a role of CRF in drug withdrawal-induced anxiety-like behavior. Blockade of CRF receptors has been shown to decrease anxiety-like behavior associated with withdrawal from alcohol, cocaine, and other drugs of abuse (Baldwin et al, 1991; Basso et al, 1999; Overstreet et al, 2004; Rassnick et al, 1993; Sarnyai et al, 1995). Moreover, in a recent study we demonstrated that the icv administration of the non-specific CRF1/2 receptor antagonist D-Phe CRF(12–41) prevents the elevations in brain reward thresholds associated with precipitated nicotine withdrawal in rats (Bruijnzeel et al, 2007).
Although extensive progress has been made into the understanding of the role of CRF in drug withdrawal, it is not known via which specific brain sites CRF mediates the deficit in brain reward function associated with nicotine withdrawal. Experimental evidence points towards a role for the central nucleus of the amygdala (CeA) and the lateral bed nucleus of the stria terminalis (BNST) in the negative emotional state associated with nicotine withdrawal. Spontaneous alcohol and cocaine withdrawal and precipitated cannabinoid and nicotine withdrawal have been shown to elevate extracellular CRF levels in the CeA (George et al, 2007; Merlo Pich et al, 1995; Richter and Weiss 1999; Rodriguez de Fonseca et al, 1997). In addition, alcohol withdrawal increases the release of CRF in the BNST, which can be reversed with subsequent alcohol intake (Olive et al, 2002). Another brain site that has been suggested to play an important role in drug addiction is the nucleus accumbens shell (Nacc shell). It has been suggested that the negative emotional state associated with drug withdrawal is at least partly mediated by a hypodopaminergic function in the Nacc shell (Barr et al, 2002; Rada et al, 2001). Although CRF and its receptors have been detected in the Nacc shell there is no experimental evidence suggesting a role for CRF in the Nacc shell during drug withdrawal (De Souza et al, 1985; Swanson et al, 1983). The administration of CRF into the lateral ventricles has been shown to induce behavioral activation in rats in a familiar environment, which is indicative of increased arousal and anxiety-like behavior, and a similar effect has been observed after the administration of CRF into the Nacc shell (Holahan et al, 1997). This suggests that the release of CRF into the Nacc shell could contribute to negative emotional states.
Taken together, the above-discussed studies suggest that increased CRF transmission may play a role in the negative mood state associated with nicotine withdrawal. In addition, the CeA, BNST, and Nacc shell have been suggested to play an important role in drug withdrawal and/or relapse to drug seeking behavior (Koob 2008). The aim of the present experiments was to investigate the role of CRF in the CeA, lateral BNST, and Nacc Shell in the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. The effect of the administration of the non-specific CRF1/2 receptor antagonist D-Phe CRF(12–41) (CRF1 receptor, Ki = 56 nM; CRF2 receptor, Ki = 5.2 nM) into the CeA, BNST, or Nacc shell on the elevations in brain reward thresholds associated with precipitated nicotine withdrawal was investigated by using a discrete trial intracranial self-stimulation (ICSS) procedure (Gulyas et al, 1995; Perrin et al, 1999). This procedure was used to assess the negative affective aspects of nicotine withdrawal as it provides a quantitative measure of the emotional aspects of drug withdrawal (Bruijnzeel et al, 2006; Schulteis et al, 1995; Wise and Munn 1995). In all of the experiments, response latencies were assessed to determine if the administration of the CRF receptor antagonist into the specific brain sites would affect motor output (Markou and Koob 1992). Experiments that provide insight into the specific role of brain stress systems in the negative affective aspects of nicotine withdrawal may contribute to the development of non-addictive pharmacotherapies that reduce tobacco withdrawal symptomatology and improve relapse rates.
MATERIALS AND METHODS
Subjects
Male Wistar rats (Charles River, Raleigh, NC) weighing 250–300 g at the beginning of the experiments were used. Animals were group-housed (two per cage) in a temperature- and humidity-controlled vivarium and maintained on a 12 hr light-dark cycle (lights off at 6 PM). All testing occurred at the beginning of the light cycle. Food and water were available ad libitum in the home cages. All subjects were treated in accordance with the National Institutes of Health guidelines regarding the principles of animal care. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) and approved by the University of Florida Institutional Animal Care and Use Committee.
Drugs
Nicotine hydrogen tartrate salt, mecamylamine hydrochloride, and pentobarbital sodium salt were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA) and dissolved in sterile saline (0.9% sodium chloride). The CRF1/2 receptor antagonist [DPhe12, Nle21, 38CαMe Leu37]r/hCRF(12–41) (D-Phe CRF(12–41)) was synthesized by The Clayton Foundation Laboratories for Peptide Biology and kindly provided by Dr. Jean Rivier (Salk Institute for Biological Studies, La Jolla, CA). D-Phe CRF(12–41) was dissolved in distilled water and kept on ice until being used in the behavioral experiments. The CRF receptor antagonist was administered within one hour after being dissolved.
Surgical Procedures
Cannulae and electrode implantations
At the beginning of all the intracranial surgeries, the rats were anesthetized with an isoflurane/oxygen vapor mixture (1–3% isoflurane) and placed in a Kopf stereotaxic frame (David Kopf Instruments, Tujunga, CA) with the incisor bar set 3.3 mm below the interaural line (flat skull). The rats were prepared with 11 mm stainless steel 23 gauge cannulae above the CeA, lateral BNST, or Nacc Shell using flat skull coordinates according to Paxinos and Watson, 1998, and a previous study by Koob and colleagues (Funk et al, 2006). Bilateral cannulae were implanted 2.5 mm above the CeA (anterior-posterior [AP] −2.3, medial lateral [ML] ±4.0 mm, dorsal-ventral [DV] −4.7 from dura), 2.0 mm above the lateral BNST (AP −0.6, ML ±3.7 and 15° vertical tilt, DV −4.6 from dura), or 2.5 mm above the Nacc shell (AP +1.7 mm, ML ±1.0, DV −5.1 from dura). At the end of the surgery, 11 mm removable 30 gauge wire stylets were inserted in the cannulae to maintain patency. For the electrode implantations, the incisor bar was set 5 mm (CeA and BNST groups) above the interaural line or at the interaural line (Nacc shell group). The position of the incisor bar had to be adjusted for the electrode implantations in the Nacc shell group in order to be able to accommodate the cannulae and the electrode. The rats were prepared with stainless steel bipolar electrodes (model MS303/2 Plastics One, Roanoke, VA) 11 mm in length in the medial forebrain bundle at the level of the posterior lateral hypothalamus (AP −0.5 mm, ML ±1.7 mm, DV −8.3 mm from dura). The electrodes and cannulae were permanently secured to the skull using dental cement anchored with four skull screws.
Osmotic minipump implantations
The rats were prepared with osmotic minipumps (model 2ML4, 28 day pumps, Durect Corporation, Cupertino, CA) filled with either saline or nicotine hydrogen tartrate dissolved in saline. The pumps were implanted subcutaneously under isoflurane/oxygen (1–3% isoflurane) anesthesia. The nicotine concentration was adjusted to compensate for differences in body weight and to deliver a dose of 9 mg/kg/day of nicotine salt (3.16 mg/kg/day nicotine base).
Apparatus
The experimental apparatus consisted of twelve Plexiglas chambers (30.5 × 30 × 17 cm; Med Associates, Georgia, VT), each housed in a sound-attenuating melamine chamber (Med Associates, Georgia, VT). The operant conditioning chambers consisted of a metal grid floor and a metal wheel (5 cm wide) centered on a sidewall. A photobeam detector was attached next to the response wheel and recorded every 90 degrees of rotation. Brain stimulation was delivered by constant current stimulators (Model 1200C, Stimtek, Acton, MA). Subjects were connected to the stimulation circuit through bipolar leads (Plastics One, Roanoke, VA) attached to gold-contact swivel commutators (model SL2C Plastics One, Roanoke, VA). A computer controlled the stimulation parameters, data collection, and all test session functions.
Intracranial self-stimulation procedure
Rats were trained on a modified discrete-trial ICSS procedure (Kornetsky and Esposito 1979), as described previously (Markou and Koob 1992). The subjects were trained initially to turn the wheel on a fixed ratio 1 (FR1) schedule of reinforcement. Each quarter turn resulted in the delivery of a 0.5 second train of 0.1 millisecond cathodal square-wave pulses at a frequency of 100 Hz. After the successful acquisition of responding, defined as 100 reinforcements within 10 minutes, the rats were gradually trained on a discrete-trial current-threshold procedure. Each trial began with the delivery of a non-contingent electrical stimulus, followed by a 7.5 second response window within which the animal could respond to receive a second contingent stimulus identical to the initial non-contingent stimulus. A response during this 7.5 second response window was labeled as a positive response and the lack of a response was labeled as a negative response. During a 2-second period immediately after a positive response, additional responses had no scheduled consequences. The inter-trial interval (ITI), which followed either a positive response or the end of the response window, had an average duration of 10 seconds (7.5 – 12.5 seconds). Responses that occurred during the ITI resulted in a further 12.5 second delay of the onset of the next trial. During training on the discrete-trial procedure, the duration of the ITI and delay periods induced by time-out responses were gradually increased until animals performed consistently at standard test parameters. The rats were subsequently tested on the current-threshold procedure in which stimulation intensities varied according to the classical psychophysical method of limits. A test session consisted of four alternating series of descending and ascending current intensities starting with a descending series. Blocks of three trials were presented to the subject at a given stimulation intensity, and the intensity was altered systematically between blocks by 5 μA steps. The initial stimulus intensity was set 40 μA above the baseline current-threshold for each animal. Each test session typically lasted 30–40 minutes and provided two variables: brain reward thresholds and response latencies. The brain reward threshold for a descending series was defined as the midpoint between stimulation intensities that supported responding (i.e., positive responses on at least two of the three trials), and current intensities that failed to support responding (i.e., positive responses on fewer than two of the three trials for two consecutive blocks of trials). The threshold for an ascending series was defined as the midpoint between stimulation intensities that did not support responding and current intensities that supported responding for two consecutive blocks of trials. Four threshold estimates were recorded and the mean of these values was taken as the brain reward threshold for a specific subject. The response latency was defined as the time interval between the beginning of the non-contingent stimulus and a positive response. The response latency for each test session was defined as the mean response latency on all trials during which a positive response occurred.
Intracerebral microinjections
The bilateral injections were administered by using 30 gauge stainless steel injectors that extended 2.5 mm (length of injector tip, 13.5 mm; CeA and Nacc Shell) or 2.0 mm (length of injector tip, 13.0 mm; BNST) beyond the guide cannulae. The injection volume was 0.5 μl/side and this was infused over 66 seconds. The infusion speed was regulated by a Harvard Apparatus syringe pump (model 975) and the pump was equipped with 10 μl syringes (Hamilton, Rena, NE). The syringes were connected to the injectors with Tygon microbore PVC tubing (0.25 mm ID × 0.76 mm OD). The injectors were left in place for 30 seconds post-injection to allow diffusion from the injector tip. The dummy stylets, 11 mm, were re-inserted immediately after the injectors were removed.
Histology
At the end of the experiments, the rats were euthanized with an overdose of sodium pentobarbital (150 mg, intraperitoneally) and perfused via the ascending aorta with physiological saline (100 ml) followed by a 10% phosphate buffered formalin solution (150 ml). Brains were postfixed for 24 hours and cryoprotected in 10% sucrose in phosphate buffered saline for 48 hours. Sections were cut on a Leica CM3050 S cryostat (coronal sections of 40 μm at −15 °C). The sections were mounted on Fisher Superfrost Plus slides and stained with cresyl violet. The locations of the guide cannulae and injections sites were verified with light microscopy and with reference to a stereotaxic atlas of the rat brain (Paxinos and Watson 1998).
Experimental Design
Effect of D-Phe CRF(12–41) administered into the CeA, BNST or Nacc shell on precipitated nicotine withdrawal
Naïve rats were used for all the experiments. After recovery from the electrode implantations, the rats were trained on the ICSS procedure. When stable baseline brain reward thresholds were achieved (defined as less than 10% variation within a 5 day period), the rats were prepared with 28-day osmotic minipumps containing either saline or nicotine dissolved in saline (CeA: saline n=8, nicotine n=8; BNST: saline n=9, nicotine n=9; Nacc shell: saline n=9, nicotine n=9). Brain reward thresholds and response latencies were assessed daily throughout the experiment between 9:00 AM and 12:00 noon. The nAChR antagonist mecamylamine (3 mg/kg, sc) was used to precipitate nicotine withdrawal. Mecamylamine injections started at least 6 days after the implantation of the minipumps to allow time for the development of nicotine dependence. The CRF1/2 receptor antagonist D-Phe CRF(12–41) (5 – 500 ng per brain site) was administered 10 minutes prior to treatment with mecamylamine. The rats were placed in the ICSS test chambers 5 minutes after mecamylamine administration. It was ensured that the minimum time interval between the mecamylamine injections was at least 72 hours to reestablish/maintain nicotine dependence. The serum elimination half-life of mecamylamine is approximately 1 hour (Debruyne et al, 2003). At the end of the experiment the rats were perfused under pentobarbital anesthesia and the brains were removed for histological verification of the cannulae placements.
Statistical analyses
For all the experiments, brain reward thresholds and response latencies were expressed as percentages of the values obtained on the day prior to each test day. Percent changes in brain reward thresholds and response latencies were analyzed using a two-way repeated-measures analyses of variance (ANOVA) with the dose of D-Phe CRF(12–41) as the within-subjects factor and pump content (saline or nicotine) as the between-subjects factor. For all experiments, statistically significant interactions in the ANOVA were followed by the Newman-Keuls post-hoc test.
RESULTS
Effect of D-Phe CRF(12–41) administered into the CeA on precipitated nicotine withdrawal
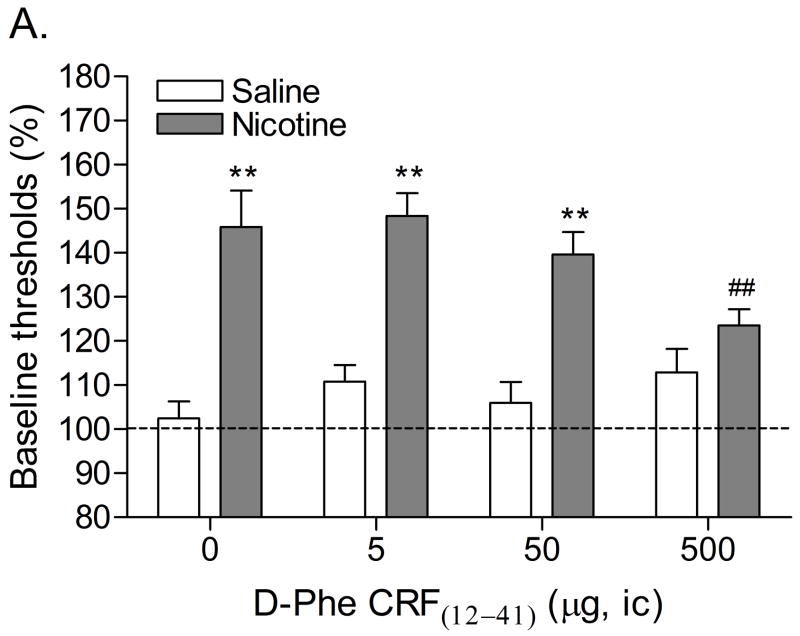
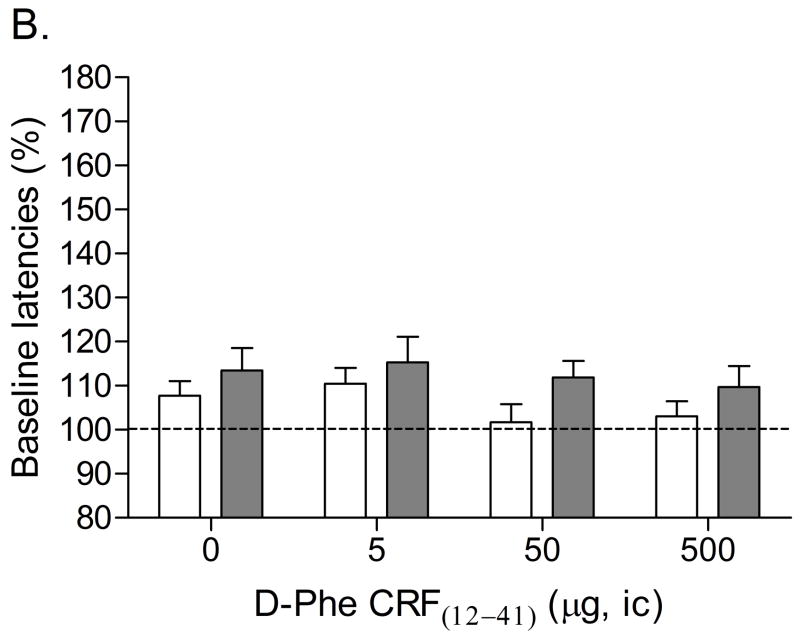
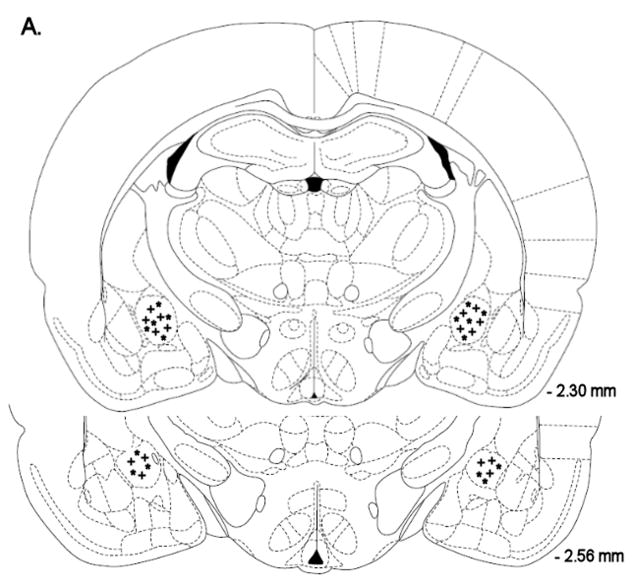
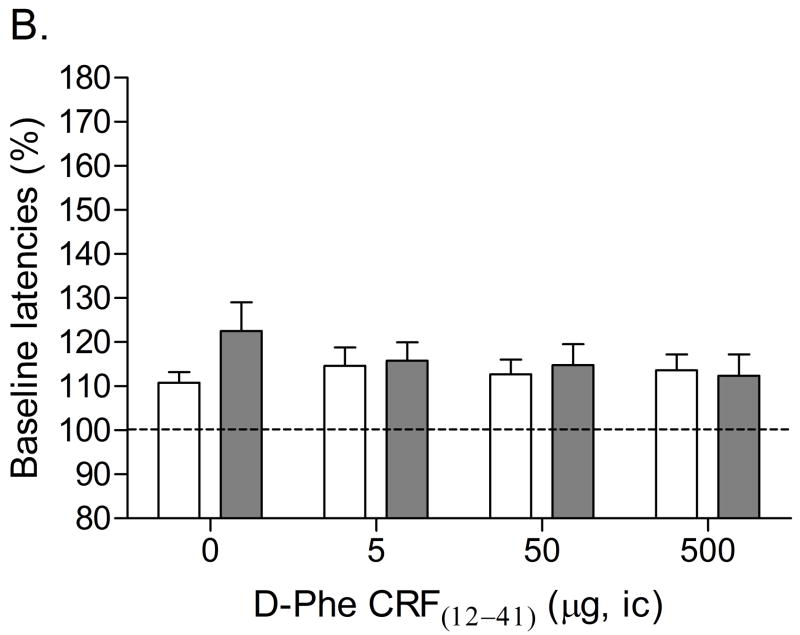
Mean (±S.E.M.) absolute brain reward thresholds before pump implantation for saline- and nicotine-treated rats were 87.14 ± 3.39 and 88.23 ± 6.66 μA [t(14)=0.15, n.s.], respectively. Mean (±S.E.M.) absolute response latencies for saline- and nicotine-treated rats were 2.90 ± 0.10 and 3.08 ± 0.15 seconds [t(14)=1.00, n.s.], respectively. Mecamylamine elevated the brain reward thresholds of the nicotine-treated rats, 146%, and did not affect the brain reward thresholds of the control rats (Figure 1A, Treatment: F1, 14=57.46, P<0.0001). Mecamylamine did not affect the response latencies of the nicotine-treated rats or the saline-treated rats (Figure 1B, Treatment: F1, 14=3.49, n.s.). The administration of D-Phe CRF(12–41) into the CeA prevented the mecamylamine-induced elevations in brain reward thresholds in the nicotine dependent rats and did not affect the brain reward thresholds of the rats that were chronically-treated with saline (Figure 1A; Dose × Treatment interaction: F3, 42=4.21, P<0.011). Newman-Keuls post-hoc comparisons indicated that 500 ng of D-Phe CRF(12–41) per brain site completely prevented the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. D-Phe CRF(12–41) did not affect the response latencies (Figure 1B; Dose: F3, 42=1.23, n.s.; Dose × Treatment interaction: F3, 42=0.17, n.s.). See figure 4A for a histological reconstruction of the injections sites.
Figure 1.
Effect of D-Phe CRF(12–41) (saline, n = 8; nicotine, n = 8) administered into the CeA on the elevations in brain reward thresholds associated with mecamylamine-precipitated nicotine withdrawal (A). Effect of D-Phe CRF(12–41) on the response latencies of rats chronically treated with saline (n = 8) or nicotine (n = 8) and acutely treated with mecamylamine (B). Brain reward thresholds and response latencies are expressed as a percentage of the pre-test day values. D-Phe CRF(12–41) was administered bilaterally and the figure depicts the unilateral dose. Asterisks (** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Pound signs (## P<0.01) indicate lower brain reward thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle (0 μg of D-Phe CRF(12–41)). Abbreviation: ic, intracranial.
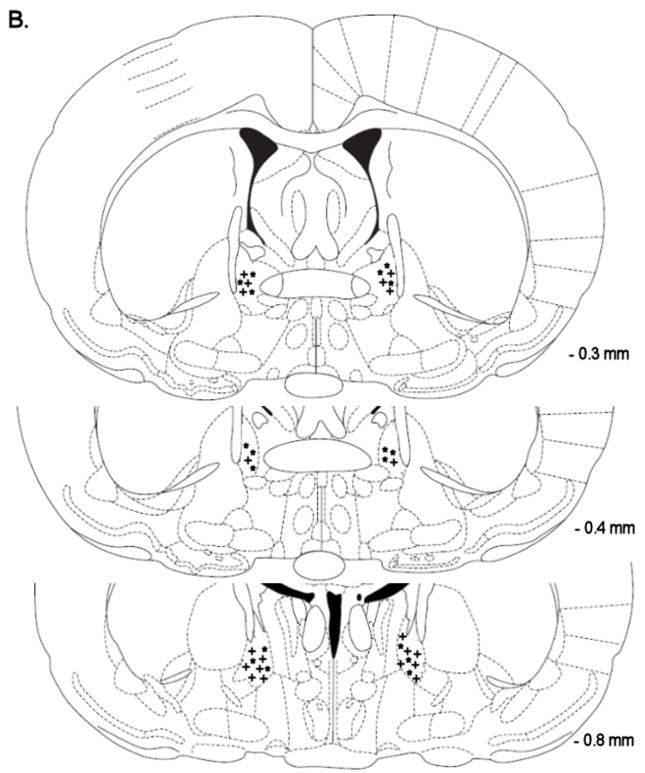
Figure 4.
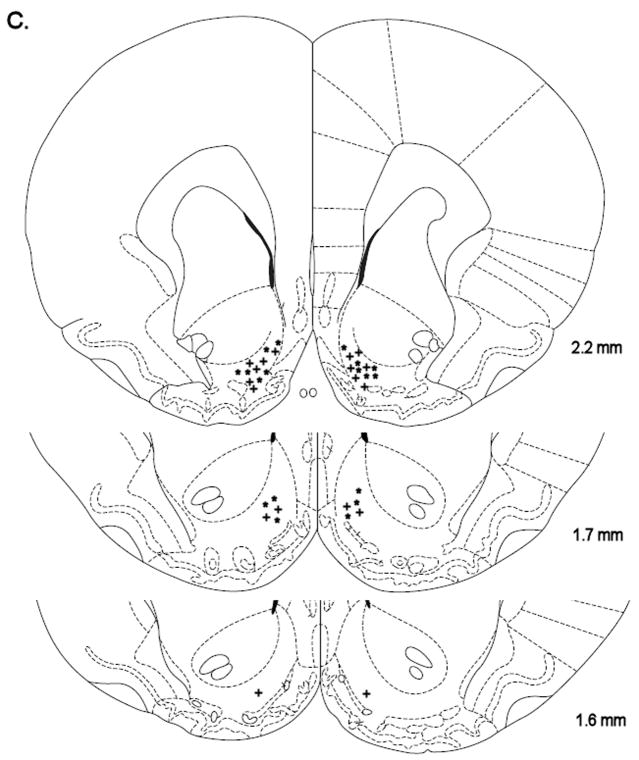
Histological reconstruction of bilateral injection sites in the CeA (A) of rats chronically treated with nicotine (+, n = 8) or saline (*, n = 8). Reconstruction of bilateral injection sites in the lateral BNST (B) of rats chronically treated with nicotine (+, n = 9) or saline (*, n = 9). Reconstruction of bilateral injections sites in the Nacc shell (C) of rats chronically treated with nicotine (+, n = 9) or saline (*, n = 9). The figures are copies from the Paxinos and Watson brain atlas (Paxinos and Watson 1998).
Effect of D-Phe CRF(12–41) administered into the BNST on precipitated nicotine withdrawal
Mean (±S.E.M.) absolute brain reward thresholds before minipump-implantation for the saline-treated rats and the nicotine-treated rats were 118.84 ± 8.78 and 118.93 ± 14.17 μA [t(16)=0.01, n.s.], respectively. Mean (±S.E.M.) absolute response latencies for the saline-treated rats and the nicotine-treated rats were 3.28 ± 0.16 and 2.99 ± 0.10 seconds [t(16)=1.61, n.s.], respectively. Similar to the first experiment, mecamylamine elevated the brain reward thresholds of the nicotine treated rats, 147%, and did not affect the brain reward thresholds of the saline-treated rats (Figure 2A, Treatment: F1, 16=17.33, P<0.0007). Mecamylamine did not affect the response latencies of the nicotine-treated rats or the saline-treated rats (Figure 2B, Treatment: F1, 16=3.21, n.s.). The administration of D-Phe CRF(12–41) into the BNST did not affect the brain reward thresholds (Dose: F3, 48=0.64, n.s.; Dose × Treatment: F3, 48=0.61, n.s.) or the response latencies (Dose: F3, 48=0.92, n.s.; Dose × Treatment: F3, 48=2.40, n.s.) of the saline-treated rats or the nicotine-treated rats. See figure 4B for a histological reconstruction of the injections sites.
Figure 2.
Effect of D-Phe CRF(12–41) (saline, n = 9; nicotine, n = 9) administered into the BNST on the elevations in brain reward thresholds associated with mecamylamine-precipitated nicotine withdrawal (A). Effect of D-Phe CRF(12–41) on the response latencies of rats chronically treated with saline (n = 8) or nicotine (n = 8) and acutely treated with mecamylamine (B). Brain reward thresholds and response latencies are expressed as a percentage of the pre-test day values. D-Phe CRF(12–41) was administered bilaterally and the figure depicts the unilateral dose. Abbreviation: ic, intracranial.
Effect of D-Phe CRF(12–41) administered into the Nacc Shell on precipitated nicotine withdrawal
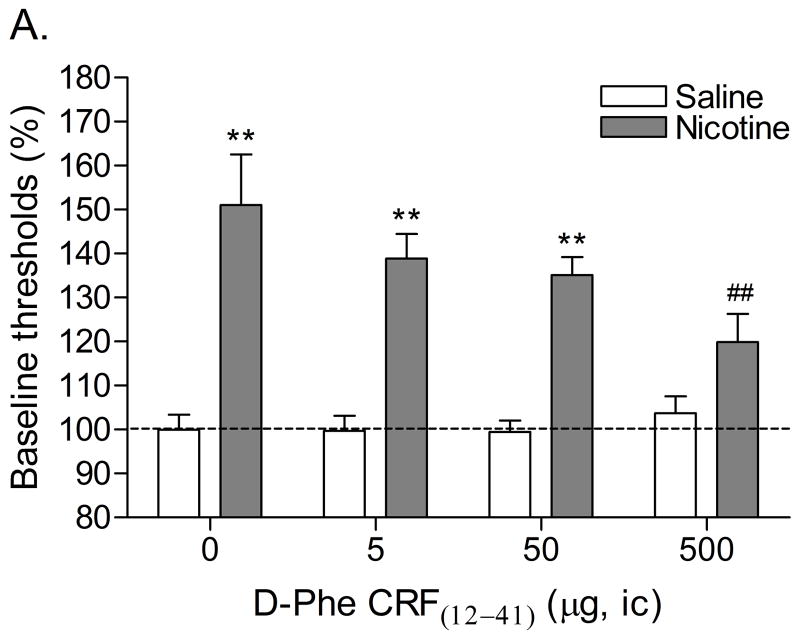
Mean (±S.E.M.) absolute brain reward thresholds before minipump-implantation for the saline-treated rats and the nicotine-treated rats were 139.21 ± 14.54 and 136.99 ± 15.18 μA [t(16)=0.11, n.s.], respectively. Mean (±S.E.M.) absolute response latencies for the saline-treated rats and the nicotine-treated rats were 3.61 ± 0.17 and 3.93 ± 0.17 seconds [t(16)=1.33, n.s.], respectively. Mecamylamine elevated the brain reward thresholds of the nicotine treated rats, 151%, and did not affect the brain reward thresholds of the saline-treated rats (Figure 3A, Treatment: F1, 16=59.44, P<0.0001). Mecamylamine did not affect the response latencies of the nicotine-treated rats or the saline-treated rats (Figure 3B, Treatment: F1, 16=0.77, n.s.). The administration of D-Phe CRF(12–41) into the Nacc shell prevented the mecamylamine-induced elevations in brain reward thresholds in the nicotine dependent rats and did not affect the brain reward thresholds of the rats that were chronically-treated with saline (Figure 3A; Dose × Treatment interaction: F3, 48=3.48, P<0.023). Post-hoc analysis indicated that the highest dose of D-Phe CRF(12–41), 500 ng per site, completely prevented the mecamylamine-induced elevations in brain reward thresholds in the nicotine-treated rats. The administration of D-Phe CRF(12–41) into the Nacc Shell did not affect the response latencies of the saline-treated rats or the nicotine-treated rats (Dose: F3, 48=0.35, n.s.; Dose × Treatment: F3, 48=1.07, n.s.). See figure 4C for a histological reconstruction of the injections sites.
Figure 3.
Effect of D-Phe CRF(12–41) (saline, n = 9; nicotine, n = 9) administered into the Nacc shell on the elevations in brain reward thresholds associated with mecamylamine-precipitated nicotine withdrawal (A). Effect of D-Phe CRF(12–41) on the response latencies of rats chronically treated with saline (n = 9) or nicotine (n = 9) and acutely treated with mecamylamine (B). Brain reward thresholds and response latencies are expressed as a percentage of the pre-test day values. D-Phe CRF(12–41) was administered bilaterally and the figure depicts the unilateral dose. Asterisks (** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Pound signs (## P<0.01) indicate lower brain reward thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle (0 μg of D-Phe CRF(12–41)). Abbreviation: ic, intracranial.
DISCUSSION
These results demonstrate that the nAChR antagonist mecamylamine elevates the brain reward thresholds of rats that are chronically treated with nicotine and does not affect the brain reward thresholds of saline-treated control rats, which is in line with previous studies (Bruijnzeel and Markou 2004; Epping-Jordan et al, 1998; Watkins et al, 2000). The administration of D-Phe CRF(12–41) (500 ng/site) into the CeA and the Nacc shell prevented the mecamylamine-induced elevations in brain reward thresholds. In contrast, the administration of D-Phe CRF(12–41) into the lateral BNST did not prevent the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. Our findings extend and corroborate previous findings by demonstrating that antagonism of CRF1/2 receptors in the CeA and Nacc shell, but not in the lateral BNST, prevents the negative affective state of precipitated nicotine withdrawal in rats (Bruijnzeel et al, 2007; Epping-Jordan et al, 1998).
These studies focused on investigating the role of CRF in the CeA, lateral BNST, and Nacc shell in nicotine withdrawal as all of these structures are considered part of the extended amygdala, are highly interconnected, and have overlapping afferent and efferent connections (Alheid and Heimer 1988; Heimer et al, 1991). In addition, it has been suggested that the extended amygdala plays a critical role in the negative affective state associated with drug withdrawal (Koob and Le Moal 2005). Extensive evidence points toward a role for CRF in the CeA in drug addictions. Withdrawal from alcohol (Merlo Pich et al, 1995), cannabis (Rodriguez de Fonseca et al, 1997), cocaine (Richter and Weiss 1999), and nicotine (George et al, 2007) has been shown to induce an increased release of CRF in the CeA. It has also been shown that alcohol withdrawal-induced anxiety-like behavior in the elevated plus maze test can be reversed by the administration of the nonspecific CRF1/2 receptor antagonist α-helical CRF(9–41) in the lateral ventricles (Baldwin et al, 1991) or the CeA (Rassnick et al, 1993). Koob and colleagues demonstrated that alcohol intake in rats is increased after chronic exposure to alcohol vapor (Roberts et al, 2000) and the increased alcohol intake in the alcohol dependent animals can be prevented by the administration of D-Phe CRF(12–41) into the CeA prior to the alcohol self-administration sessions (Funk et al, 2006). The results of our study demonstrated that antagonism of CRF receptors in the CeA prevented the elevations in brain reward thresholds associated with nicotine withdrawal. This suggests that the release of CRF into the CeA may at least partly mediate the deficit in brain reward function associated with nicotine withdrawal. Drug intake during the withdrawal phase could possibly diminish negative affective states by decreasing the release of CRF in the CeA.
The intra-CeA dose of D-Phe CRF(12–41) that prevented the elevations in brain reward thresholds was about 20 times lower than the icv dose required to prevent the elevations in brain reward thresholds associated with nicotine withdrawal (1 μg intra-CeA [total bilateral dose] vs. 20 μg icv)(Bruijnzeel et al, 2007). This suggests that the current total bilateral dose, 1 μg of D-Phe CRF(12–41), would not have prevented the deficit in brain reward function associated with nicotine withdrawal when administered into the lateral ventricles. This rules out the possibility that in the present studies D-Phe CRF(12–41) diffused into the lateral ventricles and then mediated its effects by acting upon other brain sites. It is also unlikely that D-Phe CRF(12–41) prevented the elevations in brain reward thresholds by activating brain reward systems. The icv administration of D-Phe CRF(12–41) does not alter brain reward thresholds in drug free rats (Macey et al, 2000). In addition, in the present study we showed that intra-CeA, BNST, or Nacc shell administration of D-Phe CRF(12–41) does not affect the brain reward thresholds of the chronic saline/acute mecamylamine control group.
Some studies suggest that the BNST may be involved in specific aspects of drug addictions. Discontinuation of chronic alcohol administration increases extracellular CRF levels in the BNST, which subsides with subsequent alcohol intake (Olive et al, 2002). In addition, naloxone induces a dose-dependent increase in c-fos mRNA in the BNST of morphine dependent animals (Frenois et al, 2002). Evidence for a role of the BNST in negative affective states is provided by Aston-Jones and colleagues (Delfs et al, 2000). They reported that the blockade of β-noradrenergic receptors or activation of α2-adrenergic receptors in the BNST prevents opioid withdrawal-induced conditioned place aversion. At this point in time, we are not aware of any studies which reported that blockade of CRF receptors in the BNST prevents the negative affective state associated with drug withdrawal. The results of our study suggest that the activation of CRF receptors in the BNST does not play a role in the negative emotional state associated with nicotine withdrawal. This is in agreement with a previous study showing that blockade of CRF receptors in the BNST does not reduce alcohol intake in alcohol dependent rats (Funk et al, 2006). It is unlikely that the doses of D-Phe CRF(12–41) were too low in the present study as the same doses prevented the negative emotional state associated with nicotine withdrawal when administered into the CeA or Nacc shell. In addition, a previous study reported that the intra-BNST administration of doses of D-Phe CRF(12–41), that are within the current dose-response range (10 or 50 ng/side administered bilaterally), prevent stress-induced reinstatement of cocaine seeking behavior (Erb and Stewart 1999).
Emerging evidence suggests that CRF in the CeA and BNST may have a distinct role in various aspects of drug addictions and anxiety and fear responses (Erb and Stewart 1999; Walker et al, 2003). The present studies suggest that CRF in the CeA, but not in the BNST, plays a role in the negative affective state associated with nicotine withdrawal. CRF in the CeA, but not in the BNST, has also been suggested to play a role in the increased alcohol intake in alcohol dependent animals (Funk et al, 2006). Furthermore, the administration of the CRF1/2 receptor antagonist alpha-helical CRF(9–41) into the CeA, but not into the BNST, reduced the severity of naloxone-precipitated somatic morphine withdrawal signs (McNally and Akil 2002). The aforementioned studies suggest that CRF signaling in the CeA mediates acute drug withdrawal signs such as elevations in brain reward thresholds, somatic signs, and increased drug intake. In contrast, CRF transmission in the BNST may at least partly mediate protracted drug withdrawal signs such as stress-induced reinstatement of drug seeking behavior. For example, Erb and Stewart demonstrated that the BNST, but not the CeA, plays a role in stress-induced reinstatement of cocaine seeking behavior (Erb and Stewart 1999).
Neurochemical changes in the Nacc shell have been implicated in the negative affective state associated with drugs withdrawal. For example, nicotine withdrawal has been associated with a decrease in dopamine levels and an increase in acetylcholine levels in the Nacc shell in rats (Rada et al, 2001). The Nacc shell contains CRF-immunoreactive cells and moderate levels of CRF1 and CRF2 receptors have been detected in this brain site (De Souza et al, 1985; Merchenthaler et al, 1982; Rominger et al, 1998; Swanson et al, 1983). However, very little research has been conducted to investigate the role of CRF in the Nacc shell in drug addiction. The results of the present study indicate that the administration of the CRF1/2 receptor antagonist D-Phe CRF(12–41) into the Nacc shell prevents the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. Thus, this suggests that the negative affective state associated with nicotine withdrawal is at least partly mediated by the release of CRF in the Nacc shell. Research by Koob and colleagues suggests that CRF in the Nacc shell does not play a role in alcohol intake in alcohol dependent rats (Funk et al, 2006). However, emerging evidence suggests that CRF in the Nacc shell has behavioral effects and could play a role in psychiatric disorders. The icv administration of CRF has been shown to increase locomotor activity in rats in a familiar environment and to increase anxiety-like behavior in a novel environment (Sutton et al, 1982; Takahashi et al, 1989). Kelley and colleagues demonstrated that the administration of CRF into the Nacc shell also increases locomotor activity in rats in a familiar environment (Holahan et al, 1997), which suggests that CRF might mediate some of its behavioral effects by stimulating CRF receptors in the Nacc shell. In a recent study it was demonstrated that the administration of CRF into the Nacc shell increases cue-induced motivation to obtain sucrose pellets (Pecina et al, 2006). It was suggested that the CRF release in the Nacc shell may also increase the motivation to obtain other positive reinforcers such as drugs of abuse and therefore play a role in the reinstatement of drug seeking behavior.
D-Phe CRF(12–41) is a nonspecific CRF receptor antagonist and therefore the present studies did no distinguish between CRF1 and CRF2 receptor subtypes. Extensive evidence suggests that the CRF1 receptor plays a pivotal role in drug withdrawal and relapse. Systemic administration of the small-molecule non-peptide CRF1 receptor antagonist MPZP has been shown to decrease nicotine withdrawal-induced anxiety-like behavior and to prevent increased nicotine intake after a period of abstinence (George et al, 2007). Furthermore, the non-peptide CRF1 receptor antagonist MTIP blocks alcohol withdrawal induced anxiety-like behavior, excessive alcohol self-administration in alcohol dependent rats, and stress-induced reinstatement of alcohol seeking behavior (Gehlert et al, 2007). Conflicting findings have been reported with regard to the role of the CRF2 receptor in drug withdrawal. Stimulation of CRF2 receptors decreases alcohol intake in alcohol dependent animals and decreases alcohol withdrawal-induced anxiety-like behavior (Funk and Koob 2007; Valdez et al, 2004). In contrast, CRF2 receptor knockout mice display decreased somatic morphine withdrawal signs, which suggests that activation of CRF2 receptors contributes to drug withdrawal (Papaleo et al, 2008). The above discussed studies would suggest that the activation of the CRF1 receptor may play an important role in the negative affective state of nicotine withdrawal. At this point in time, additional studies are needed before firm conclusions can be drawn about the role of the CRF2 receptor in drug withdrawal.
In the present studies, the role of CRF in nicotine withdrawal was investigated in rats passively exposed to nicotine. It should be noted that passive exposure to nicotine and nicotine self-administration may have different effects on brain chemistry and brain reward function (Epping-Jordan et al, 1998; Jacobs et al, 2003; Kenny and Markou 2006). Therefore, additional studies are warranted to investigate the role of CRF in changes in brain reward function after discontinuing chronic nicotine self-administration. Previous research has shown that extended access to nicotine self-administration, 23 hours per day, leads to nicotine dependence as indicated by precipitated somatic withdrawal signs (O’Dell et al, 2007). Somatic withdrawal signs were not recorded in the present study. However, future studies may investigate the effect of the administration of CRF receptor antagonists in specific brain sites on affective and somatic withdrawal signs as this may help to delineate the neuronal substrates underlying the negative affective and somatic withdrawal signs.
Taken together, the present findings indicate that blockade of CRF1/2 receptors in the CeA and Nac Shell, but not in the lateral BNST, prevents the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. These studies point toward an important role for CRF in the CeA and Nacc shell in the negative affective state associated with smoking cessation. Further studies are warranted to investigate the role of specific CRF receptor subtypes in the extended amygdala in the negative affective state associated with smoking cessation.
Acknowledgments
This work was funded by National Institute on Drug Abuse grants (DA023575 and DA020504) to Adrie Bruijnzeel. The authors would like to thank Dr. Jean Rivier (The Clayton Foundation Laboratories for Peptide Biology, The Salk Institute for Biological Studies, San Diego, CA) for generously providing D-Phe CRF(12–41).
Footnotes
Disclosure/Conflict of Interest
The author(s) declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A. Psychostimulant withdrawal as an inducing condition in animal models of depression. Neurosci Biobehav Rev. 2005;29:675–706. doi: 10.1016/j.neubiorev.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23:475–482. doi: 10.1016/s0165-6147(02)02086-2. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Lewis B, Bajpai LK, Morey TE, Dennis DM, Gold M. Severe deficit in brain reward function associated with fentanyl withdrawal in rats. Biol Psychiatry. 2006;59:477–480. doi: 10.1016/j.biopsych.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in rats. Neuropharmacology. 2004;47:572–579. doi: 10.1016/j.neuropharm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, Barre L. Short-term pharmacokinetics and brain distribution of mecamylamine as a preliminary to carbon-11 labeling for nicotinic receptor investigation. J Pharm Sci. 2003;92:1051–1057. doi: 10.1002/jps.10302. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, et al. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Cador M, Caille S, Stinus L, Le MC. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur J Neurosci. 2002;16:1377–1389. doi: 10.1046/j.1460-9568.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2, 6-dimethyl-imidazo[1, 2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas J, Rivier C, Perrin M, Koerber SC, Sutton S, Corrigan A, et al. Potent, structurally constrained agonists and competitive antagonists of corticotropin-releasing factor. Proc Natl Acad Sci U S A. 1995;92:10575–10579. doi: 10.1073/pnas.92.23.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Heimer L, de Olmos J, Alheid GF, Zaborszky L. “Perestroika” in the basal forebrain: opening the border between neurology and psychiatry. Prog Brain Res. 1991;87:109–165. doi: 10.1016/s0079-6123(08)63050-2. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Kalin NH, Kelley AE. Microinfusion of corticotropin-releasing factor into the nucleus accumbens shell results in increased behavioral arousal and oral motor activity. Psychopharmacology (Berl) 1997;130:189–196. doi: 10.1007/s002130050228. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, De Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866:82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, et al. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McNally GP, Akil H. Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neuroscience. 2002;112:605–617. doi: 10.1016/s0306-4522(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Vigh S, Petrusz P, Schally AV. Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. Am J Anat. 1982;165:385–396. doi: 10.1002/aja.1001650404. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Research advances in the understanding and treatment of addiction. Am J Addict. 2003;12(Suppl 2):S36–S47. [PubMed] [Google Scholar]
- O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, et al. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Ghozland S, Ingallinesi M, Roberts AJ, Koob GF, Contarino A. Disruption of the CRF(2) Receptor Pathway Decreases the Somatic Expression of Opiate Withdrawal. Neuropsychopharmacology. 2008;33:2878–2887. doi: 10.1038/npp.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Pecina S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MH, Sutton SW, Cervini LA, Rivier JE, Vale WW. Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of corticotropin-releasing factor receptors. J Pharmacol Exp Ther. 1999;288:729–734. [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Merlo Pich E, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl) 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Rominger DH, Rominger CM, Fitzgerald LW, Grzanna R, Largent BL, Zaczek R. Characterization of [125I]sauvagine binding to CRH2 receptors: membrane homogenate and autoradiographic studies. J Pharmacol Exp Ther. 1998;286:459–468. [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav Neurosci. 1989;103:648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Wise RA, Munn E. Withdrawal from chronic amphetamine elevates baseline intracranial self-stimulation thresholds. Psychopharmacology (Berl) 1995;117:130–136. doi: 10.1007/BF02245178. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]