Abstract
The cannabinoid CB1 receptor (CB1) is one of the most abundant G protein-coupled receptors in the brain, but little is known about the mechanisms that modulate CB1 receptor signaling. Here we show that inhibition or null mutation of the epsilon isozyme of protein kinase C (PKCε) selectively enhances behavioral responses to the CB1 agonist WIN55,212-2 in mice, but not to the structurally unrelated CB1 agonist CP55,940. Binding affinity for [3H] WIN55,212-2 was increased in brain membranes from PKCε-/- mice compared with PKCε+/+ mice. There was no difference in binding of the inverse agonist [3H] SR141716A. In addition, repeated administration of WIN55,212-2 produced greater analgesic and thermal tolerance in PKCε-/- mice compared with PKCε+/+ mice. These results indicate that PKCε selectively regulates behavioral sensitivity, CB1 receptor binding and tolerance to WIN55,212-2.
Keywords: CANNABINOID; NEUROCHEMISTRY; SIGNAL TRANSDUCTION; NEUROPHARMACOLOGY; CB1 RECEPTOR; G PROTEIN; TOLERANCE; PROTEIN KINASE C EPSILON; WIN55,212-2
Introduction
The cannabinoid CB1 receptor (CB1) is a G protein-coupled receptor (GPCR) that is widely expressed in the central nervous system (Herkenham, 1991). In addition to mediating marijuana's psychoactive effects, CB1 receptors respond to the endogenous endocannabinoids arachidonylethanolamine (anandamide, AEA) and 2-arachidonylglycerol (2-AG) to regulate neuronal excitability and neurotransmitter release. CB1 receptors are coupled to Gi/o heterotrimeric G proteins and their activation leads to inhibition of adenylyl cyclase, activation of extracellular signal regulated kinases (ERK) 1 and 2, increased function of G protein-activated, inwardly rectifying K+ (GIRK) channels and inhibition of voltage-gated calcium channels (Howlett et al., 2004). The receptor is highly expressed in the cerebral cortex, hippocampus, striatum, cerebellum and reward centers of the limbic system (Herkenham, 1991). As a consequence, CB1 receptors play an important role in a variety of behavioral states including pain, learning and memory, drug reward, and feeding (Berghuis et al., 2007; Gardner & Vorel, 1998; Harkany et al., 2007; Kreitzer et al., 2002).
Although much is known about downstream signaling cascades regulated by CB1 receptor activation, there is very little information about signaling cascades that regulate CB1 receptor sensitivity to ligands. One study suggests that CB1 receptor function is regulated by protein kinase C (PKC) (Garcia et al., 1998), which is a family of phospholipid-dependent, serine-threonine kinases encoded by nine different genes (Olive & Messing, 2004). In anterior pituitary AtT20 cells treated with the CB1 agonist R-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de)-1,4-benzoxazin-6-yl]-1-napthalenylmethanone mesylate (WIN55,212-2) activation of PKC with phorbol 12-myristate, 13-acetate attenuates CB1-mediated suppression of P/Q type calcium currents and activation of GIRK currents (Garcia et al., 1998). In vitro, a mixture of PKC isozymes purified from rat brain phosphorylates CB1 at Ser 317 in the third intracellular loop. Mutation of this serine residue to alanine prevents phorbol ester mediated disruption of CB1 signaling, suggesting that PKC phosphorylates and negatively regulates CB1 signaling through phosphorylation of CB1 at Ser 317 (Garcia et al., 1998). It is not known if PKC similarly regulates the effects of WIN55,212-2 on neuronal CB1 receptors and, if so, which PKC isozymes are involved.
Seven PKC isozymes (α, β, γ, δ, ε, η, and θ) can be activated by tumor promoting phorbol esters, which are widely used probes of PKC function. We chose to study the epsilon isozyme of PKC because mice that lack this enzyme show enhanced behavioral responses to ethanol (Hodge et al., 1999) and morphine (Newton et al., 2007). The endogenous cannabinoid system regulates ethanol intake in rodents (Hansson et al., 2007; Hungund et al., 2002) and cannabinoid and opioid receptor systems have been shown to interact (Caille et al., 2007; Caille & Parsons, 2006; Vigano et al., 2005). In addition, both CB1 and PKCε are abundantly expressed throughout the nervous system (Choi et al., 2002; Garcia-Navarro et al., 1994), especially in presynaptic terminals (Saito et al., 1993). We found that inhibition or deletion of PKCε selectively increased behavioral sensitivity to the CB1 agonist WIN55,212-2 but not 2-[(1S,2R,5S)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol (CP55,940). We also found that brain membranes from PKCε-/- mice showed increased binding affinity for WIN55,212 but not for 5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (SR141716A). Behavioral tolerance to WIN55,212-2 was also increased in PKCε-/- mice compared with PKCε+/+ mice. Together, our findings suggest that PKCε negatively regulates the actions of WIN55,212-2 at the CB1 receptor.
Materials and Methods
Animals
PKCε-/- mice (Khasar et al., 1999) were maintained on inbred 129S4/SvJae and C57BL6/J backgrounds and crossed to generate PKCε+/- C57BL/6J X129S4/SvJae F1 hybrid mice. These mice were intercrossed to generate F2 hybrid PKCε+/+ and PKCε-/- littermates for experiments. We used 8 to 16 week-old male mice for all studies. Mice were housed under a 12:12 h light-dark cycle in groups of 5 per cage with food and water ad libitum. All procedures were conducted in accordance with NIH and Gallo Center Institutional Animal Care and Use Committee guidelines. Cannabinoid-induced analgesia and hypothermia were measured concurrently on the same animals; other studies were performed using drug-naïve mice. EC50 values were calculated from sigmoid dose response curves using Prism 4.0 (GraphPad Software, San Diego, CA).
Reagents
WIN55,212-2 and CP55,940 were purchased from Sigma Aldrich (St. Louis, MO) and were dissolved in 0.9% saline containing 0.1% Tween 80. The peptides Tat-εV1-2 (YGRKKRRQRRRC-CEAVSLKPT) and Tat-scrambled-εV1-2 (YGRKKRRQRRRC-CLSETKPAV) were synthesized by Anaspec (San Jose, CA). N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) was purchased from Tocris Bioscience (Ellsville, MO). SR141716A was purchased from Sequoia Research Products (Pangbourne, UK) and was dissolved in 1:1:18 ethanol:Tween 80:normal saline. [3H] WIN55,212-2 was purchased from Perkin Elmer (Waltham, MA). [3H] SR141716A was purchased from American Radiolabeled Chemicals Inc. (St. Louis, MO).
Analgesia
We measured analgesia by the tail-flick test (Reggio et al., 1991), using a beam of light focused on the underside of the tail as the nociceptive stimulus (Columbia Instruments, Columbus, OH). Mean baseline tail-flick response time was calculated from three consecutive stimulations along the length of the tail. We used a maximal stimulation time of 10 s. One PKCε-/- mouse did not flick its tail within this time period and was eliminated from this study. For each mouse, we measured baseline tail flick latency and then tail flick latency following treatment with WIN,55,212-2 or CP55,940. Peak analgesia occurred 1 h after s.c. administration of drug; therefore, we tested drug responses 1 h after administration. To confirm that WIN55,212-2 responses were mediated by CB1 receptors, we pretreated some mice with SR141716A (3 mg/kg) 15 min prior to administering WIN55,212-2. Analgesia was calculated as the percent of maximal possible effect: (%MPE = [(test latency-baseline latency)/(10-baseline latency] × 100).
Hypothermia
We measured hypothermia immediately following analgesia by inserting a thermocouple thermometer (Type J, Barnant Co., Barrington, IL) 2 cm into the mouse's rectum. Hypothermia was calculated as the change in core body temperature from baseline (Adams & Martin, 1996).
Locomotor activity
Locomotor activity was monitored using 46 × 46 cm open field chambers (Med Associates, St. Albans, VT) in dark, sound attenuating boxes. Mice received an injection of vehicle or WIN55,212-2 and were immediately placed in the test chamber. Their distance traveled was recorded for 1 h.
Analgesia and hypothermia following repeated WIN55,212-2 treatment
On day 1, mice were tested for acute sensitivity to WIN55,212-2-induced analgesia and hypothermia, as described above. On days 2-7, they received twice daily s.c. injections (at 09:00 and 17:00) of equipotent doses (ED75) of WIN55,212-2 (0.9 mg/kg for PKCε-/- and 2.5 mg/kg for PKCε+/+ mice), as determined from the acute dose response curve for each genotype. This dose was chosen based on prior studies of cannabinoid tolerance (Cook et al., 1998; Sim-Selley & Martin, 2002). On day 8, mice were retested for WIN55,212-2-induced analgesia and hypothermia using the same doses (0.5 - 4.0 mg/kg) used on day 1. The magnitude of tolerance to analgesia was calculated by subtracting the mean % MPE on day 8 from the mean % MPE on day 1. The magnitude of tolerance to hypothermia was calculated by subtracting the core body temperature on day 8 from the core body temperature on day 1.
Analgesia following intracerebroventricular microinjection of Tat-εV1-2
PKCε+/+ mice were anesthetized with ketamine 100 mg/kg i.p. and xylazine 10 mg/kg i.p. A mouse stereotaxic tracking device (RBM-IT Anilam, Painesville, OH) and stereotaxic table (Kopf, Tujunga, CA) were used to implant 26-gauge guide cannulae into the right and left ventricles (coordinates relative to bregma: anteroposterior, 0; mediolateral, ± 1.1; dorsoventral, 3.0 (Franklin & Paxinos, 1992)). Cannulae were stabilized with acrylic dental cement (Lang Dental Manufacturing, Wheeling, IL), and dummy cannulae and dust caps were put in place (Plastics One, Wallingford, CT). Ten days later, Tat-εV1-2 or Tat-scrambled-εV1-2 (20 μM each) were infused bilaterally using 3 mm, 26-gauge cannulae (Plastics One, Wallingford, CT) attached to 1μl syringes (Hamilton, Reno, NV) and a PHD 2000 pump (Harvard Apparatus, Holliston, MA). The injection volume was 1 μl with a flow rate of 1 μl/10 min. Thirty min after infusion, mice were injected with 1 mg/kg WIN55,212-2 or vehicle s.c. and tested 1 h later for tail-flick analgesia and hypothermia. Cannulae placement was verified by histological analysis of brain sections.
Radioligand binding to whole brain membranes
[3H] WIN55,212-2 saturation binding was performed as described (Nakazi et al., 2000). The maximal binding capacity (Bmax) and the dissociation constant (Kd) were calculated by nonlinear curve fitting using Prism 4.0 (GraphPad Software, San Diego, CA). [3H] SR141716A saturation binding was measured as described (Breivogel et al., 2001) except that the amount of protein per tube was 100 μg and 2.5 μM of the CB1 inverse agonist/antagonist AM 251 (Tocris Cookson, Bristol, UK) was used to measure nonspecific binding. Competitive [3H] WIN55,212-2 binding was measured in a manner similar to saturation binding except that a fixed concentration of the tracer ligand was used and the competing ligand was SR141716A. The effect of the non-hydrolyzable guanine nucleotide guanylylimidodiphosphate (GPP(NH)p), 100μM) on agonist binding to the CB1 receptor was conducted in a manner similar to that described by (Lambert & Childers, 1984) except that [3H] WIN55,212-2 was used as agonist and SR141716A was the competing ligand. Nonlinear curve fitting and area under the curves were analyzed using Prism 4.0 (GraphPad Software, San Diego, CA).
Anti-CB1-pSer316 immunoreactivity
Mice were sacrificed by CO2 inhalation and decapitation. Amygdala, hippocampus, cortex, striatum and cerebellum were immediately dissected on ice. Tissues were homogenized at 4°C in modified RIPA buffer containing 50 mM Tris-HCl (pH 7.4) 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% sodium deoxycholate, 1 mM PMSF, Complete™ protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), and 1:100 dilution of serine/threonine phosphatase inhibitor cocktail 1 (Sigma-Aldrich, St. Louis, MO). The homogenates were mixed at 4°C for 30 min and centrifuged at 6,000 g for 5 min at 4°C. Supernatants were collected and adjusted to a concentration of 2 mg/ml. The concentrated sample buffer solution contained 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 5% β-mercaptoethanol, 10% glycerol and 0.002% bromophenol blue. Samples were boiled at 90°C for 7 min and then passed through a 27-gauge needle. Equal amounts of protein (40 μg/lane) were loaded onto duplicate 4-12% Tris-bis glycine SDS-polyacrylamide gels. The separated proteins were transferred to nitrocellulose membranes (Hybond™-C Extra, Amersham Biosciences, Buckinghamshire, England), then blocked for 1 h at 25°C with either 5% BSA in 0.01 M TBS containing 0.1% Tween 20 for the anti-phospho-CB1 antibody or with 5% nonfat dry milk in 1X PBS containing 0.1% Tween 20 for the anti-CB1 antibody. Each pair of membranes was then incubated with anti-phospho-CB1 (Ser 316) antibody (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) or with anti-CB1 antibody (1:500 dilution; Santa Cruz Biotechnology) overnight at 4°C. Specificity of the anti-phospho-CB1 (Ser 316) antibody for the phosphorylated form of CB1 in mouse brain has been demonstrated by the manufacturer (Santa Cruz Biotechnology catalog #sc-17555). Membranes incubated with anti-CB1 were also incubated with anti-actin (clone AC40 at 1:2000 dilution; Sigma-Aldrich) or anti- glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:2500 dilution; Abcam, Cambridge, MA) to control for protein loading. Membranes were washed 1X TBS or PBS containing 0.1% Tween 20, 3 times for 5 min, then incubated with peroxidase-conjugated goat anti-rabbit IgG (1:1000 dilution; Roche Applied Science). Immunoreactivity was visualized by enhanced chemiluminescence (Pierce, Rockford, IL).
Immunoreactive bands were detected using X-ray film and the optical density of each band was quantified using a flat bed scanner and the program Image J (http://rsb.info.nih.gov/ij). Values for CB1 immunoreactivity were normalized to GAPDH immunoreactivity in the same sample. Values for phospho-CB1 were measured relative to total CB1 immunoreactivity in the same sample. The results for each brain area were analyzed using unpaired, two-tailed t-tests.
RESULTS
PKCε-/- mice show increased behavioral sensitivity to WIN55,212-2
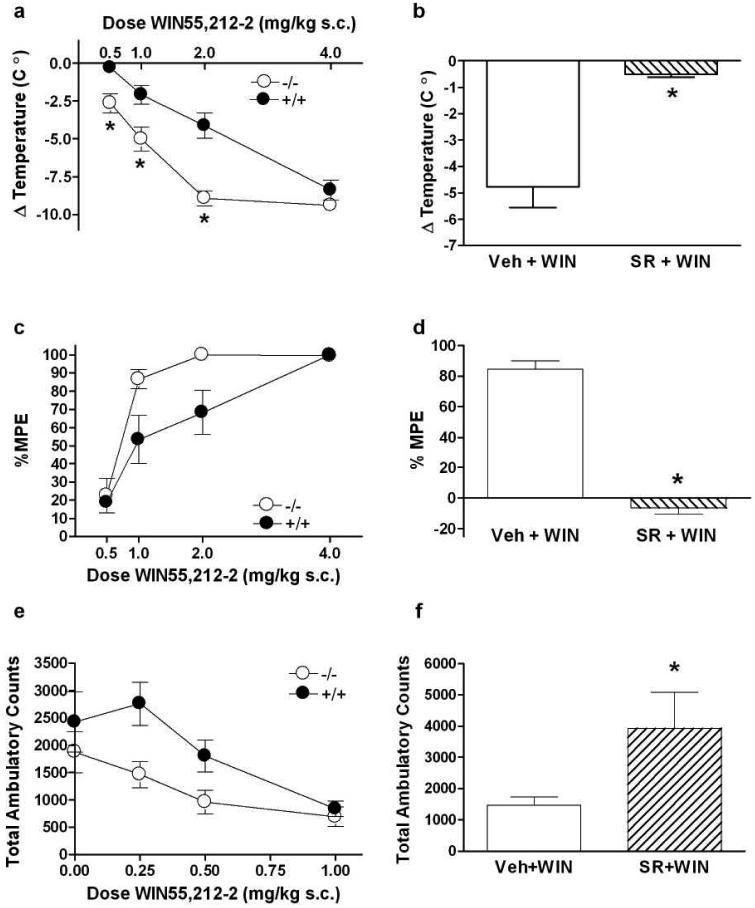
CB1 agonists produce hypothermia, analgesia, and hypolocomotion in rodents (Adams & Martin, 1996). We measured these responses to determine if the effects of WIN55,212-2 differ between PKCε+/+ and PKCε-/- mice. In agreement with previous studies, we found that there were no baseline differences in analgesia (Newton et al., 2007), locomotor behavior (Hodge et al., 1999) and hypothermia (Olive et al., 2000). We found that PKCε-/- mice show greater hypothermia than PKCε+/+ littermates (Figure 1a). A two-factor ANOVA showed an effect of genotype (F(1, 63) = 41.19, p<0.0001) and dose (F(3, 63) = 59.63, p<0.0001) with an interaction between these factors (F(3, 63) = 3.331 p<0.025). Nonlinear regression analysis showed that the log EC50 values for WIN55,212-2 -induced hypothermia were nearly statistically different (p<0.06) between PKCε-/- mice (-0.78 ± 6.5 mg/kg) and PKCε+/+ mice (0.28 ± 0.85 mg/kg). The maximal responses were significantly different, (p<0.005), indicating greater efficacy of WIN55,212-2 for producing hypothermia in PKCε-/- mice. There were no baseline differences between the genotypes (data not shown) (Figure 1a). To determine if the increased effect of WIN55,212-2 is CB1 receptor-mediated, we administered the CB1 receptor inverse agonist/antagonist SR141716A to PKCε-/- mice and found that it completely blocked the hypothermia produced by an ED84 dose of WIN55,212-2 (Figure 1b).
Figure 1.
PKCε-/- mice show enhanced responses to the CB1 agonist WIN55,212-2. (a) WIN55,212-2 produced greater hypothermia in PKCε-/- mice when compared with PKCε+/+ mice. *p<0.05 by Bonferroni post-hoc tests. (b) The effect of an ED84 dose of WIN55,212-2 (WIN; 1 mg/kg) was blocked by 3 mg/kg SR141716A (SR) but not by vehicle (Veh) in PKCε-/- mice. *p<0.0002 by two-tailed, unpaired t-test. (c) PKCε-/- mice showed greater tail-flick analgesia than PKCε+/+ mice. (d) The ED84 analgesic dose of WIN55,212-2 (1.7 mg/kg) was blocked by 3 mg/kg SR141716A (SR) and not by vehicle (Veh) in PKCε-/- mice *p<0.0002, two-tailed, unpaired t-test. (e) PKCε-/- mice showed greater WIN55,212-2-induced locomotor suppression than PKCε+/+ mice. (f) Locomotor suppression produced by 1 mg/kg WIN55,212-2 was reversed by 3 mg/kg SR141716A (SR) but not by vehicle (Veh) in PKCε-/- mice. *p<0.01 by two-tailed, unpaired t-test. Data shown are mean ± SEM values from 7 to10 animals.
PKCε-/- mice also showed greater tail-flick analgesia than PKCε+/+ mice (Figure 1c). A two-factor ANOVA showed an effect of genotype (F (1.58) = 9.552, p<0.0031) and dose (F(3,58) = 2.66. p<0.0001) with a strong trend towards an interaction between these factors (F(3, 58)= 2.66, p<0.056). The log EC50 for WIN55,212-2-induced analgesia in PKCε-/- mice (-0.32 ± 0.1 mg/kg) was significantly greater (p<0.01) than the log EC50 in PKCε+/+ 50 mice (0.0006 ± 0.09). These results indicate that WIN55,212-2 is a more potent analgesic in PKCε-/- mice than in PKCε+/+ mice. Because the tail flick assay is carried out for a maximum of 10 sec, it is not possible to measure efficacy. The analgesia produced by an ED84 dose WIN55,212-2 in PKCε-/- mice was blocked by SR141716A (Figure 1d), indicating that this response was mediated through CB1.
WIN55,212-2 produced locomotor suppression that was greater in PKCε-/- mice compared with wild type littermates (Figure 1e). A two-factor ANOVA showed an effect of genotype (F(1,46) = 11.03, p<0.0018) and dose (F(3,46) = 10.06, p< 0.0001). There was no interaction between these factors (F(3,46) = 1.484, p>0.05). The log EC50 values were similar (p = 0.33) for PKCε-/- mice (-0.76 ± 1.74 mg/kg) and PKCε+/+ mice (-0.3 ±0.27 mg/kg). Locomotor suppression produced by the maximally effective dose of WIN55, 212-2 (Figure 1f) was reversed by SR141716A. These results indicate that deletion of PKCε enhances hypothermic, analgesic, and locomotor inhibitory responses to WIN55,212-2.
The PKCε inhibitor εV1-2 increases WIN55,212-2-induced analgesia and hypothermia in PKCε+/+ mice
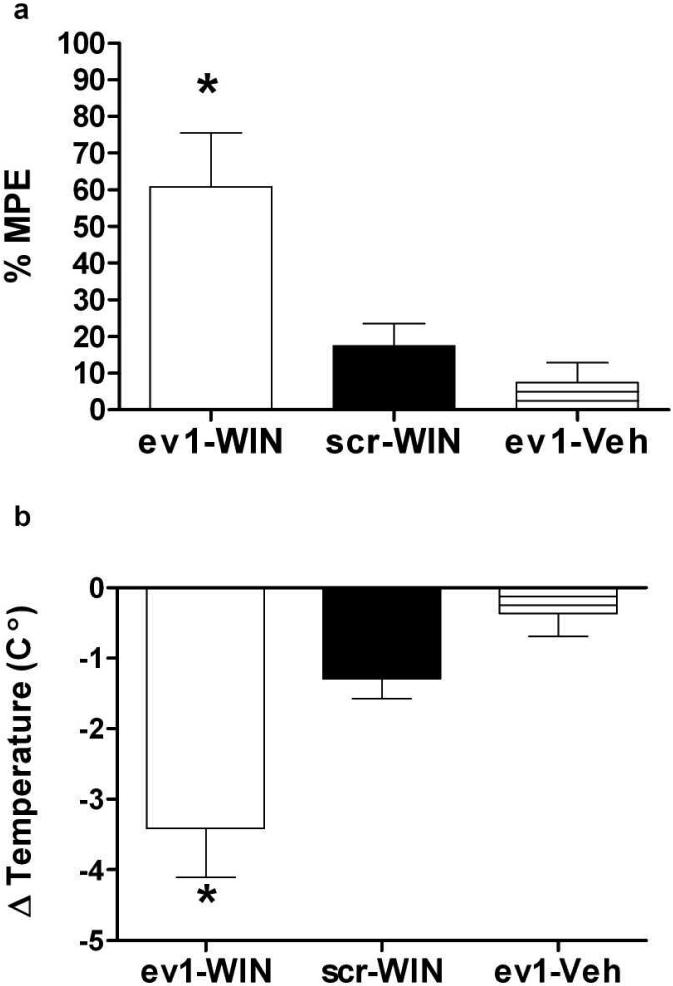
To confirm that the increased behavioral response to WIN55,212-2 observed in PKCε-/- mice was due to the absence of PKCε and not to an effect on development, we examined whether phenotypes in PKCε-/- mice could be produced in wild type mice by treatment with a PKCε inhibitor. For this experiment we injected the PKCε translocation inhibitor εV1-2 bilaterally into the lateral ventricles of PKCε+/+ mice and then measured their analgesic and hypothermic responses after treatment with 1 mg/kg WIN55,212-2. To facilitate transport of the peptide across cell membranes, we used εV1-2 conjugated to the sequence YGRKKRRQRRR, derived from the HIV Tat protein (Chen et al., 2001). The specificity of this peptide inhibitor for PKCε has been described previously (Gray et al., 1997; Johnson et al., 1996). We found that, similar to PKCε-/- mice, wild type mice treated with Tat-εV1-2 showed greater WIN55,212-2-induced tail-flick analgesia and hypothermia than PKCε+/+ mice treated with the control peptide, Tat-scrambled-εV1-2, or with Tat-εV1-2 followed by vehicle instead of WIN55,212-2 (Figures 2a and b). These findings indicate that the phenotypes we observed in PKCε-/- mice are due to absence of PKCε rather than to effects of PKCε deletion on development.
Figure 2.
A peptide inhibitor of PKCε increases the response to WIN55,212-2. (a) Wild type mice pretreated with Tat-εV1-2 (ev1) showed greater tail-flick analgesia than mice pretreated with the Tat-scrambled-εV1-2 peptide (scr) followed by WIN55,212-2 (1 mg/kg) or mice pretreated with Tat-εV1-2 followed by vehicle (Veh) (F(2, 130) = 5.63, p<0.02; *p<0.05 compared with sc-WIN or ev1-Veh by Tukey test). (b) Wild type mice pretreated with the Tat-εV1-2 peptide showed greater WIN55,212-2 (1 mg/kg)-induced hypothermia than mice pretreated with Tat-scrambled-εV1-2 followed by WIN55,212-2 (1 mg/kg) or mice pretreated with Tat-εV1-2 followed by vehicle (F(2, 130) = 6.11, p=0.01; *p<0.05 compared with sc-WIN or ev1-Veh by Tukey test). Data shown are mean ± SEM values from of 3 to 7 animals per treatment.
PKCε-/- mice show normal sensitivity to CP55,940 in vivo
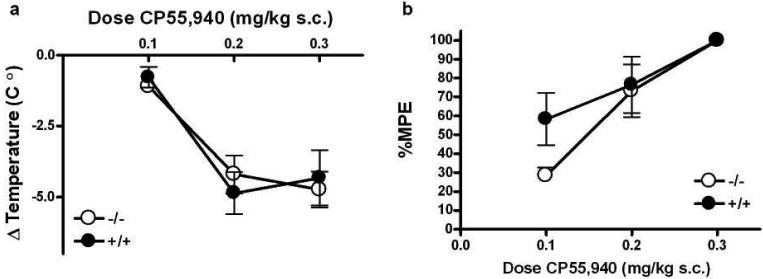
To determine if PKCε-/- mice showed increased sensitivity to a CB1 agonist that is structurally dissimilar to WIN55,21-2 (Compton et al., 1992), we examined CP55,940-induced hypothermia and analgesia. Unlike WIN55,212-2, CP55,940 produced a similar level of hypothermia in both genotypes (Figure 3a). ANOVA showed no main effects of genotype (F(1,24) = 0.00, p>0.05) or dose (F(2,24) = 20.69, p<0.0001), and no interaction between the factors (F(2,24) = 0.43, p>0.05). Likewise, for CP55,940-induced analgesia, there was no main effect of genotype (F(1,23) = 1.84, p>0.05) or dose (F(2,23) = 15.87, p<0.0001) and no interaction between these factors (F(2,23) = 1.31, p>0.05).
Figure 3.
PKCε-/- mice do not show increased responses to the CB1 agonist CP55,940. The hypothermic (a) and analgesic (b) effects of CP55,940 were similar in PKCε-/- and PKCε+/+ mice. Data shown are mean ± SEM values from 4 to 5 animals per treatment for each genotype.
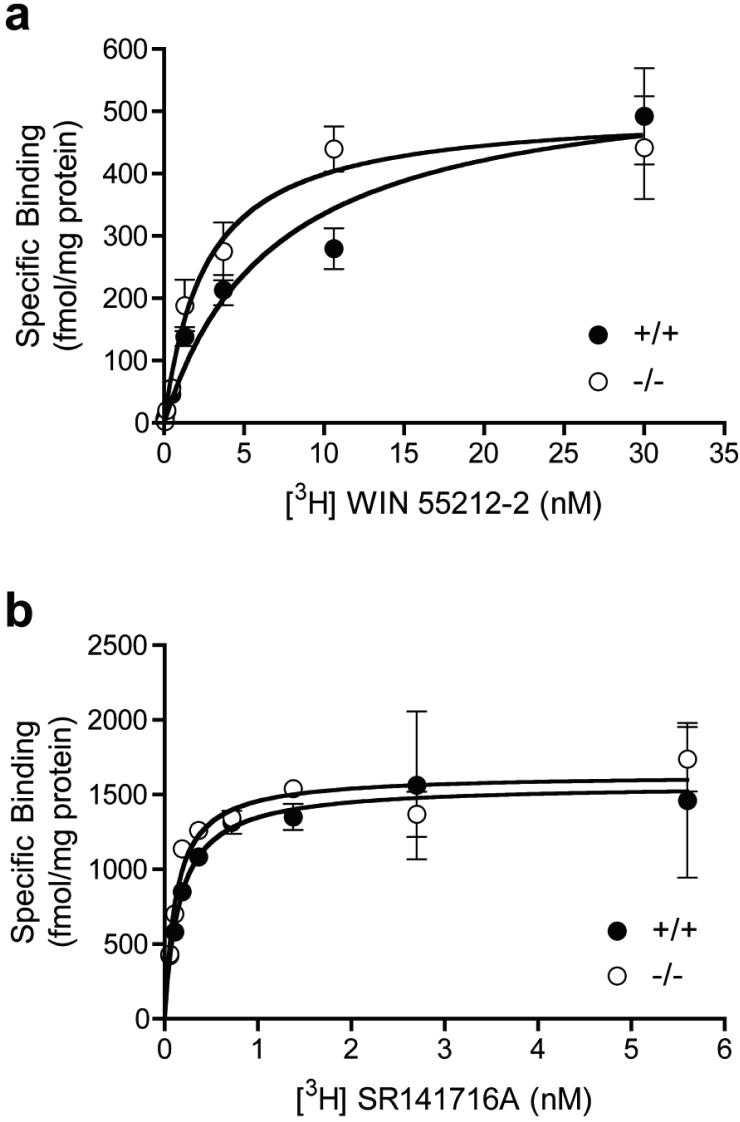
[3H]WIN55,212-2 binding affinity is greater in PKCε-/- whole brain membranes
We isolated whole brain membranes from PKCε-/- and PKCε+/+ mice to assess equilibrium saturation binding of the CB1 agonist WIN55,212-2 and the inverse agonist/antagonist SR141716A. The maximal number of binding sites (Bmax) for [3H]WIN55,212-2 were analyzed by nonlinear regression analysis and found to be similar (p>0.05) in PKCε-/- (357.6 ± 49.6, n=9) and PKCε+/+ (454.4 ± 57.8, n=12) membranes, but the equilibrium dissociation constant (Kd) was significantly lower (p<0.01) in PKCε-/- membranes (2.49 ± 0.38, n=9) than in PKCε+/+ membranes (4.53 ± 0.6, n=12). Figure 4a shows a representative [3H]WIN55,212-2 binding curve for PKCε-/- and PKCε+/+ membranes. In contrast, for the inverse agonist/antagonist [3H]SR141716A, there were no differences (p>0.05) in either the Bmax for PKCε-/- (1778 ± 281.4, n=4) and PKCε+/+ membranes (1646 ± 178.0, n=4) or the Kd for binding of PKCε-/- (0.14 ± 0.03, n=4) and PKCε+/+ (0.13 ± 0.02, n=4) membranes. Figure 4b shows a representative [3H]SR141716A binding curve for each genotype.
Figure 4.
WIN55,212-2 binding affinity is increased in whole brain membranes from PKCε-/- mice. (a) Representative saturation binding of [3H]WIN55,212-2 in PKCε-/- and PKCε+/+ whole brain membranes. (b) Representative saturation binding of [3H]SR141716A in PKCε-/- and PKCε+/+ whole brain membranes. Data shown are mean ± SEM values from 2 experiments conducted in triplicate.
G protein regulation of CB1 receptor affinity for [3H]WIN55,212-2 is not altered in PKCε-/- mice
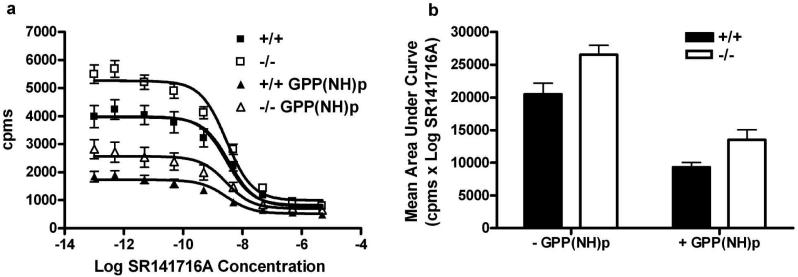
Agonist binding to G protein coupled receptors (GPCRs) promotes receptor coupling to G proteins, which in turn increases receptor affinity for agonist (Maguire et al., 1976). Therefore, we considered whether increased affinity of PKCε-/- brain membranes for [3H]WIN55,212-2 was caused by an increase in the proportion of high affinity receptors resulting from G protein-mediated effects on agonist affinity. The proportion of receptors in the high affinity state can be measured by conducting displacement binding experiments in the presence or absence of saturating concentrations of a GTP analog such as GPP(NH)p (Jiang et al., 2001; Maguire et al., 1976). A high concentration (100μM) of GPP(NH)p uncouples the G protein-receptor complex thereby eliminating the high affinity binding state for agonist.
To determine whether the absence of PKCε produces a greater proportion of CB1 receptors that exist in a high affinity binding state for [3H]WIN55,212-2, we performed GPP(NH)p shift analysis using SR141716A as the displacing ligand. To determine the shift, we first analyzed binding using nonlinear regression analysis with a one-site competition model. As expected from our results for saturation binding, in the absence of GPP(NH)p, maximal binding of 2 nM [3H]WIN55,212-2 was greater in PKCε-/- membranes than in PKCε+/+ membranes (p < 0.0001), and there was no difference between the genotypes in log IC50 values for displacement of binding by SR141716A (p=0.73). We then analyzed the effect of GPP(NH)p within each genotype and found that for PKCε-/- membranes, maximal binding was greater in the absence of GPP(NH)p than in its presence (p < 0.0001), whereas the log IC50 values were similar (p=0.98) for each condition. Likewise, for PKCε+/+ membranes, maximal binding was greater in the absence of GPP(NH)p than in its presence (p < 0.0001), but the log IC50 values were similar (p=0.69) for each condition.
The analysis above indicated that GPP(NH)p reduces binding in both genotypes. To determine whether the degree to which GPP(NH)p reduced binding was different between the genotypes, we measured the areas under the binding curves and compared them by ANOVA with factors for genotype and treatment with GPP(NH)p . This analysis revealed that GPP(NH)p decreased binding to a similar extent in membranes from both genotypes (Figure 5b). There was a significant effect of genotype (F(1,10) = 14.04, p<0.004) and GPP(NH)p treatment (F(1,10) = 14.04, p<0.0001) and no interaction between these factors (F(1,10) = 0.43, p=0.53), These results indicate that increased affinity of [3H]WIN55,212-2 for CB1 in PKCε-/- membranes is not due to an increase in the proportion of receptors that exist in a high affinity state, resulting from an enhanced effect of G protein coupling on agonist affinity.
Figure 5.
The proportion of CB1 receptors in a high affinity state for [3H]WIN55,212-2 was not increased in PKCε-/- membranes (a) Competitive binding of [3H]WIN55,212-2 and SR141716A in the presence and absence of GPP(NH)p showed that 2 nM [3H]WIN55,212-2 binding was increased in PKCε-/- membranes compared with PKCε+/+ membranes and that GPP(NH)p reduced binding in both genotypes. (b) Analysis of mean values for the area under the curves for competitive [3H]WIN55,212-2 binding showed similar effect of GPP(NH)p in both genotypes. Data shown are mean ± SEM values from 6 experiments conducted in triplicate.
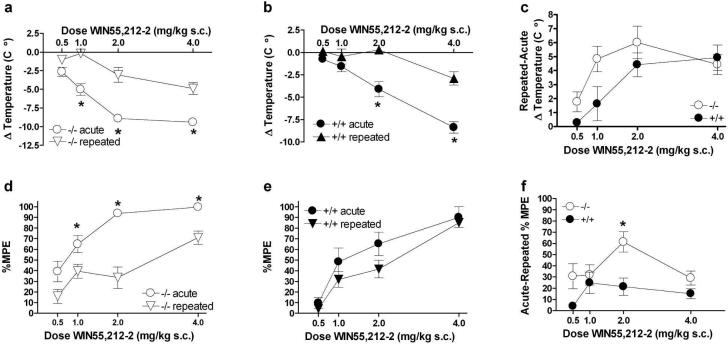
PKCε-/- mice show increased behavioral tolerance to WIN55,212-2
WIN55,212-2 produces robust tolerance in vivo (Maldonado, 2002; Sim-Selley & Martin, 2002). Our data showing that WIN55,212-2 binding and WIN55,212-2-induced behaviors are increased in PKCε-/- mice suggest that CB1 receptor signaling is increased in PKCε-/- mice. Therefore, we predicted that tolerance to WIN55,212-2 would also be increased in PKCε-/- mice. To test this hypothesis, we examined hypothermic and analgesic responses after repeated, equipotent WIN55,212-2 dosing. PKCε-/- mice developed tolerance to WIN55, 212-2-induced hypothermia after repeated treatment (Figure 6a). Analysis of these data by two-factor ANOVA showed main effects of treatment (F(1,68) = 90.47, p< 0.0001) and dose (F(3,68) = 33.95, p< 0.0001) and a significant interaction between these factors (F(3,68) = 4.18, p< 0.01). Likewise, PKCε+/+ mice also displayed tolerance to WIN55,212-2-induced hypothermia (Figure 6b) and two-way ANOVA also showed main effects of treatment (F(1,50) = 38.6, p< 0.0001) and dose (F3,50) = 27.91, p<0.0001), with an interaction between these factors (F(3,50) = 6.26, p<0.001). Comparison of the magnitude of tolerance to hypothermia revealed that PKCε-/- mice showed increased tolerance when compared with PKCε+/+ littermates (Figure 6c). Two-way ANOVA showed main effects of genotype (F(1,55) = 4.756, p<0.05) and dose (F(3,55) = 7.31, p<0.001) without a significant interaction between these factors (F(3,55) = 1.43, p> 0.05).
Figure 6.
PKCε-/- mice show enhanced tolerance to WIN55,212-2-induced hypothermia and analgesia following equipotent, twice daily injections of WIN55,212-2 for 6 days. (a) PKCε-/- mice showed tolerance to WIN55,212-2 induced hypothermia (*p< 0.001 by Bonferroni post-hoc test). (b) PKCε+/+ mice also showed tolerance to WIN55,212-2-induced hypothermia (*p< 0.001 by Bonferroni post-hoc test). (c) The magnitude of tolerance to WIN55,212-2-induced hypothermia was greater in PKCε-/- compared with PKCε+/+ mice. (d) PKCε-/- mice showed tolerance to WIN55,212-2-induced analgesia (*p<0.05 by Bonferroni post-hoc test). (e) PKCε+/+ mice did not show significant tolerance to WIN55,212-2-induced analgesia. (f) The magnitude of chronic tolerance to WIN55,212-2 induced analgesia was greater in PKCε-/- compared with PKCε+/+ mice (*p< 0.05 by Bonferroni post-hoc test). Data represent mean ± standard error of 5 to 9 mice per genotype.
PKCε-/- mice also displayed tolerance to WIN55,212-2 analgesia following repeated treatment (Figure 6d) and two-way ANOVA showed main effects of treatment (F(1,69) = 45.88, p<0.0001) and dose (F(3,69) = 23.2, p<0.0001), with an interaction between these factors (F(3,69) = 3.06, p<0.05). However, although there was a trend toward an effect, repeated treatment of PKCε+/+ mice (Figure 6e) did not produce statistically significant analgesic tolerance when compared with acute treatment by two-way ANOVA (F(1,53) = 3.49, p<0.067). There was a main effect of dose (F(3,53) = 22.59, p<0.0001) but there was no interaction between treatment and dose (F(3,53) = 0.45, p>0.05). As with hypothermia, the magnitude of tolerance to WIN55,212-2-induced analgesia was greater in PKCε-/- mice when compared with PKCε+/+ mice (Figure 6f), with main effects of genotype (F(1,63) = 5.02, p<0.05) and dose (F(3,63) = 2.979, p<0.05) and a significant interaction between these factors (F(3,63) = 4.023, p<0.01).
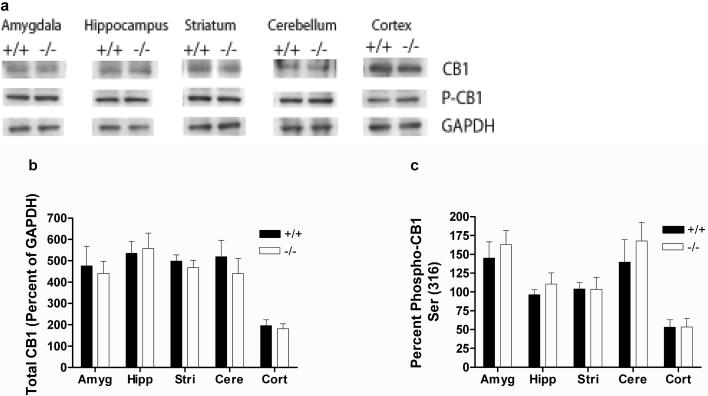
Ser-317 phosphorylation is not altered in PKCε-/- mice
Ser 317 of rat CB1 is a locus for PKC-dependent phosphorylation of heterologously expressed CB1 receptors in At-t20 cells, and phosphorylation of this site by PKC decreases WIN55,212-2 signaling (Garcia et al., 1998). Because our results indicated that PKCε regulates effects of WIN55,212-2 at brain CB1 receptors, we evaluated cortical, amygdalar, cerebellar, hippocampal and striatal membranes for CB1-pSer317 immunoreactivity. The antibody we used was generated against an epitope containing Ser 316 of the human CB1 receptor; this residue is equivalent to Ser 317 of the rat and mouse receptor. We found that, in all brain areas examined, both the amount of total CB1 immunoreactivity (Figure 7a and b) and the percentage of anti-phospho-CB1 (Ser 317) immunoreactivity (Figure 7a and c) were similar between the genotypes (p>0.05 by two-tailed, unpaired t-tests). These results suggest that PKCε regulates brain CB1 receptor signaling through phosphorylation of a site other than Ser 317 of mouse CB1.
Figure 7.
Phosphorylation of Ser-317 is not altered in PKCε-/- mice. (a) Representative western blots showing detection of 63 kDa bands by anti-CB1 and anti-phospho-CB1(Ser-316) antibodies, and detection of a 37 kDa band by an anti-GAPDH antibody in brain tissue from PKCε-/- and PKCε+/+ mice. (b) There were no genotypic differences in the levels of total CB1 immunoreactivity in the amygdala, hippocampus, striatum, cerebellum and cortex of PKCε-/- and PKCε+/+ mice. (c) There were also no genotypic differences in the level of phospho-CB1 (Ser 316) immunoreactivity in any of these brain regions. Data are mean ± SEM values from 4 separate animals per genotype.
Discussion
The principle finding of this study was that mice lacking PKCε exhibit increased sensitivity to the cannabinoid agonist WIN55,212-2. Inhibition of PKCε by a selective peptide inhibitor also increased sensitivity to WIN55,212-2 in wild type mice, confirming that the PKCε-/- phenotype is due to absence of PKCε signaling rather than a developmental abnormality. The increase in behavioral sensitivity appeared selective for WIN55,212-2 because there was no difference between PKCε-/- and PKCε+/+ mice in their response to the structurally unrelated CB1 agonist CP55,940. Saturation binding showed increased affinity for [3H] WIN55,212-2 in PKCε-/- membranes compared with PKCε+/+ membranes, whereas, the affinity for the antagonist/inverse agonist [3H] SR141716A was not different between genotypes. The increased affinity for WIN55,212-2 in PKCε-/- membranes was not due to increased G protein coupling favoring a high affinity state of the receptor for agonists. Rather it appeared due to a different mechanism that selectively affects receptor binding to WIN55,212-2.
Because WIN55,212-2 produces behavioral tolerance (Maldonado, 2002; Sim-Selley & Martin, 2002) and PKCε-/- mice show enhanced acute responses to WIN55,212, we predicted that PKCε-/- mice would also show greater tolerance than PKCε-+/+ littermates. Our behavioral data showing that the magnitude of hypothermic and analgesic tolerance was greater in PKCε-/- mice support this hypothesis. Taken together, these results indicate that deletion of PKCε increases CB1 receptor affinity for WIN55,212-2, which leads to an increased acute response and greater tolerance following repeated exposure.
We do not yet know the mechanism by which PKCε regulates the affinity of WIN55,212-2 for CB1. The results of our GPP(NH)p shift assay suggest that PKCε does not regulate the affinity WIN55,212-2 binding by altering G protein modulation of receptor affinity for agonist. Of note, lack of PKCε increased WIN55,212-2 binding but not SR1417116A binding, and increased WIN55,212-2 but not CP55,940 behavioral effects, indicating that PKCε regulation of CB1 is highly ligand selective. This is of interest since CB1 receptor ligands are structurally diverse and can produce different effects on CB1 signaling and trafficking, probably by binding to different sites on the receptor (Howlett, 2002; Martini et al., 2007). Indeed, evidence from computational and mutational studies indicates the presence of multiple ligand binding sites on CB1. For example, mutation of human CB1 at Thr 210 to alanine or isoleucine differentially alters the affinity of agonists and inverse agonists (D'Antona et al., 2006), whereas mutation of Lys 192 to alanine alters the binding of anandamide, CP55,940, HU-210 and SR141716A, but not of WIN55,212-2 (Chin et al., 1998; Hurst et al., 2002; Song & Bonner, 1996). In contrast, mutation of Ser 383 perturbs the helical structure of CB1 such that CP55,940 binding is eliminated but WIN55,212-2 and SR141716A binding are not (Kapur et al., 2007). Evidence from structural modeling and site-directed mutagenesis indicates that there is a binding pocket for WIN55,212-2 within a CB1 aromatic microdomain formed by transmembrane helices 3-4-5-6 (McAllister et al., 2003). Thus, PKCε may selectively regulate WIN55,212-2 binding by phosphorylating residues on CB1 that alter the structure of such a domain.
Although Ser 317 on CB1 was previously identified as a PKC phosphorylation site, our results indicate that PKCe does not phosphorylate this residue. It is possible that PKCε regulates receptor affinity for WIN55,212-2, by phosphorylating other residues on the receptor. It is also possible that PKCε acts by phosphorylating and regulating the function of a different protein that is involved in CB1 receptor signaling. For example, different CB1 agonists initiate differential post-endocytotic sorting via the G-protein-associated sorting protein (GASP1), which targets the receptor for recycling to the membrane or for degradation (Martini et al., 2007). PKCε might regulate receptor sorting and recycling, thereby altering WIN55,212-2-stimulated CB1 signaling and its influence on behavior. Future analyses of PKCε mediated phosphorylation of the CB1 receptor and its associated signaling proteins should reveal the sites at which PKCε acts.
The studies we present here provide the first evidence for modulation of brain CB1 receptor signaling by a specific member of the PKC family. PKCε-/- mice show enhanced responses to morphine (Newton et al., 2007), and to ethanol and other drugs that are positive allosteric modulators of GABAA receptors (Hodge et al., 1999). They also show increased GABA release in the central amygdala (Bajo et al., 2008). The endogenous cannabinoid system exhibits cross talk with endogenous opioid systems (Robledo et al., 2008) and inhibition of GABA release is one of the downstream effects of cannabinoid signaling (Lovinger, 2008). We do not know if effects of PKCε on opioid or GABAergic systems contribute to the cannabinoid phenotypes we have observed in this study. However, given the specificity of the phenotypes we observed for WIN55,212-2 and their inhibition by SR141716A, we believe that they are related to direct effects of PKCε on CB1 receptor signaling.
Abnormal function of the endogenous cannabinoid system has been implicated in many pathologies, including pain, affective and neurodegenerative disorders, inflammation, obesity and metabolic dysfunction (Di Marzo, 2008). The development of CB1 agonists to treat these disorders has been limited by psychoactive side effects and the high degree of tolerance these compounds produce (De Vry et al., 2004; Gonzalez et al., 2005; Martin et al., 2004; Rubino et al., 2005). Our finding that the effect of PKCε is selective for WIN55,212-2 is intriguing because it indicates that responses to specific CB1 agonists can be differentially regulated. Thus, it may be possible to alter affinity and tolerance to individual CB1 receptor agonists without affecting the action of other ligands, thereby providing a unique therapeutic strategy that could reduce side effects and the development of drug tolerance.
Acknowledgements
These studies were supported by fellowship DA019308 from the NIDA to M.J.W., and by grant AA013588 from the NIAAA and funds provided by the State of California for medical research on alcohol and substance abuse through the University of California to R.O.M.
Footnotes
Disclosure/Conflicts of Interest The authors have no conflict of interest.
References
- Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–614. [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A. 2008;105:8410–5. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–6. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–13. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- Chen L, Wright LR, Chen CH, Oliver SF, Wender PA, Mochly-Rosen D. Molecular transporters for peptides: delivery of a cardioprotective epsilonPKC agonist peptide into cells and intact ischemic heart using a transport system, R(7) Chem Biol. 2001;8:1123–9. doi: 10.1016/s1074-5521(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Chin CN, Lucas-Lenard J, Abadji V, Kendall DA. Ligand binding and modulation of cyclic AMP levels depend on the chemical nature of residue 192 of the human cannabinoid receptor 1. J Neurochem. 1998;70:366–73. doi: 10.1046/j.1471-4159.1998.70010366.x. [DOI] [PubMed] [Google Scholar]
- Choi DS, Wang D, Dadgar J, Chang WS, Messing RO. Conditional rescue of protein kinase C epsilon regulates ethanol preference and hypnotic sensitivity in adult mice. J Neurosci. 2002;22:9905–11. doi: 10.1523/JNEUROSCI.22-22-09905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992;263:1118–26. [PubMed] [Google Scholar]
- Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1998;285:1150–6. [PubMed] [Google Scholar]
- D'Antona AM, Ahn KH, Kendall DA. Mutations of CB1 T210 produce active and inactive receptor forms: correlations with ligand affinity, receptor stability, and cellular localization. Biochemistry. 2006;45:5606–17. doi: 10.1021/bi060067k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR, Kuhl E, Eckel G. Behavioral effects of cannabinoids show differential sensitivity to cannabinoid receptor blockade and tolerance development. Behav Pharmacol. 2004;15:1–12. doi: 10.1097/00008877-200402000-00001. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–55. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1992. [Google Scholar]
- Garcia-Navarro S, Marantz Y, Eyal R, Kalina M, Disatnik MH, Mochly-Rosen D, Ben-Menahem D, Reiss N, Naor Z. Developmental expression of protein kinase C subspecies in rat brain-pituitary axis. Mol Cell Endocrinol. 1994;103:133–8. doi: 10.1016/0303-7207(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Garcia DE, Brown S, Hille B, Mackie K. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J Neurosci. 1998;18:2834–41. doi: 10.1523/JNEUROSCI.18-08-02834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–33. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–18. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Gray MO, Karliner JS, Mochly-Rosen D. A selective epsilon-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxiainduced cell death. J Biol Chem. 1997;272:30945–51. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–26. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Characterization and localization of cannabinoid receptors in brain: an in vitro technique using slide-mounted tissue sections. NIDA Res Monogr. 1991;112:129–45. [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68-69:619–31. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl 1):345–58. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Basavarajappa BS, Vadasz C, Kunos G, Rodriguez de Fonseca F, Colombo G, Serra S, Parsons L, Koob GF. Ethanol, endocannabinoids, and the cannabinoidergic signaling system. Alcohol Clin Exp Res. 2002;26:565–74. [PubMed] [Google Scholar]
- Hurst DP, Lynch DL, Barnett-Norris J, Hyatt SM, Seltzman HH, Zhong M, Song ZH, Nie J, Lewis D, Reggio PH. N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-p yrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Mol Pharmacol. 2002;62:1274–87. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci U S A. 2001;98:3577–82. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Gray MO, Karliner JS, Chen CH, Mochly-Rosen D. An improved permeabilization protocol for the introduction of peptides into cardiac myocytes. Application to protein kinase C research. Circ Res. 1996;79:1086–99. doi: 10.1161/01.res.79.6.1086. [DOI] [PubMed] [Google Scholar]
- Kapur A, Hurst DP, Fleischer D, Whitnell R, Thakur GA, Makriyannis A, Reggio PH, Abood ME. Mutation studies of Ser7.39 and Ser2.60 in the human CB1 cannabinoid receptor: evidence for a serine-induced bend in CB1 transmembrane helix 7. Mol Pharmacol. 2007;71:1512–24. doi: 10.1124/mol.107.034645. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–60. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–96. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Lambert SM, Childers SR. Modification of guanine nucleotide-regulatory components in brain membranes. I. Changes in guanosine 5'-triphosphate regulation of opiate receptor-binding sites. J Neurosci. 1984;4:2755–63. doi: 10.1523/JNEUROSCI.04-11-02755.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008:435–77. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Maguire ME, Van Arsdale PM, Gilman AG. An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. Mol Pharmacol. 1976;12:335–9. [PubMed] [Google Scholar]
- Maldonado R. Study of cannabinoid dependence in animals. Pharmacol Ther. 2002;95:153–64. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Martin BR, Sim-Selley LJ, Selley DE. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci. 2004;25:325–30. doi: 10.1016/j.tips.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, Freissmuth C, Whistler JL. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. Faseb J. 2007;21:802–11. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Rizvi G, Anavi-Goffer S, Hurst DP, Barnett-Norris J, Lynch DL, Reggio PH, Abood ME. An aromatic microdomain at the cannabinoid CB(1) receptor constitutes an agonist/inverse agonist binding region. J Med Chem. 2003;46:5139–52. doi: 10.1021/jm0302647. [DOI] [PubMed] [Google Scholar]
- Nakazi M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:19–24. doi: 10.1007/s002109900147. [DOI] [PubMed] [Google Scholar]
- Newton PM, Kim JA, McGeehan AJ, Paredes JP, Chu K, Wallace MJ, Roberts AJ, Hodge CW, Messing RO. Increased response to morphine in mice lacking protein kinase C epsilon. Genes Brain Behav. 2007;6:329–38. doi: 10.1111/j.1601-183X.2006.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Messing RO, Hodge CW. Reduced operant ethanol self-administration and in vivo mesolimbic dopamine responses to ethanol in PKCepsilon-deficient mice. Eur J Neurosci. 2000;12:4131–40. doi: 10.1046/j.1460-9568.2000.00297.x. [DOI] [PubMed] [Google Scholar]
- Olive MF, Messing RO. Protein kinase C isozymes and addiction. Mol Neurobiol. 2004;29:139–54. doi: 10.1385/mn:29:2:139. [DOI] [PubMed] [Google Scholar]
- Reggio PH, McGaughey GB, Odear DF, Seltzman HH, Compton DR, Martin BR. A rational search for the separation of psychoactivity and analgesia in cannabinoids. Pharmacol Biochem Behav. 1991;40:479–86. doi: 10.1016/0091-3057(91)90350-b. [DOI] [PubMed] [Google Scholar]
- Robledo P, Berrendero F, Ozaita A, Maldonado R. Advances in the field of cannabinoid--opioid cross-talk. Addict Biol. 2008;13:213–24. doi: 10.1111/j.1369-1600.2008.00107.x. [DOI] [PubMed] [Google Scholar]
- Rubino T, Forlani G, Vigano D, Zippel R, Parolaro D. Ras/ERK signalling in cannabinoid tolerance: from behaviour to cellular aspects. J Neurochem. 2005;93:984–91. doi: 10.1111/j.1471-4159.2005.03101.x. [DOI] [PubMed] [Google Scholar]
- Saito N, Itouji A, Totani Y, Osawa I, Koide H, Fujisawa N, Ogita K, Tanaka C. Cellular and intracellular localization of epsilon-subspecies of protein kinase C in the rat brain; presynaptic localization of the epsilon-subspecies. Brain Res. 1993;607:241–8. doi: 10.1016/0006-8993(93)91512-q. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-b enzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther. 2002;303:36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- Song ZH, Bonner TI. A lysine residue of the cannabinoid receptor is critical for receptor recognition by several agonists but not WIN55212-2. Mol Pharmacol. 1996;49:891–6. [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav. 2005;81:360–8. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]