Abstract
Chronic administration of antidepressant drugs produce changes in neuroplasticity and behavior in rodents, effects that may be associated with the slow emergence of clinical therapeutic effects. Because of uncertainty over the effects of chronic antidepressant treatments in mice, these experiments compared the regulation of neurogenesis, mobilization of neurotrophins, and behavior produced by chronic antidepressant treatments between two inbred mouse strains, MRL/MpJ and C57BL/6J. The MRL/MpJ strain is associated with enhanced wound healing and tissue regeneration, whereas C57BL/6J mice are commonly used for behavioral studies. Proliferation and survival of hippocampal progenitor cells were measured using flow cytometry, a new platform that rapidly quantifies BrdU incorporation. Hippocampal cell proliferation was significantly increased following chronic administration of fluoxetine (5, 10 mg/kg; i.p. b.i.d.) or desipramine (5, 10 mg/kg; i.p. b.i.d.) for 21 days in MRL/MpJ mice, but not in C57BL/6J mice. Hippocampal progenitor cells born prior to chronic antidepressant treatments were not affected in either mouse strain. Protein levels of brain-derived neurotrophic factor (BDNF) in MRL/MpJ mice were elevated significantly in the frontal cortex, hippocampus, and amygdala following chronic fluoxetine treatment, but increased only in the frontal cortex by chronic desipramine. In contrast, BDNF levels in C57BL/6J mice were decreased in all regions except for the amygdala after chronic fluoxetine, and were decreased in the brain stem after chronic desipramine. Novelty-induced hypophagia was used to examine a behavioral effect produced by chronic antidepressant treatment. MRL/MpJ mice chronically administered fluoxetine or desipramine had significantly shorter latencies to consume food when exposed to a novel environment than untreated mice, whereas there were no effects on the behavior of C57BL/6J mice. In conclusion, robust effects of chronic antidepressant treatments on hippocampal cell proliferation and BDNF levels paralleled the ability of these drugs to produce changes in NIH behavior in MRL/MpJ, but none of these effects were produced in C57BL/6J mice. The greater responsiveness of MRL/MpJ mice may be important for drug discovery, for genetic studies and for understanding the neural mechanisms underlying the physiological and behavioral effects of chronic antidepressant treatments.
Keywords: antidepressants, brain-derived neurotrophic factor (BDNF), flow cytometry (FACS), neurogenesis, novelty-induced hypophagia (NIH), strain differences
INTRODUCTION
The primary effect of most currently available pharmacologic antidepressants is to enhance the transmission of brain monoamine systems, principally the neurotransmitters serotonin and norepinephrine (Frazer, 2001). One of their major clinical limitations is that 2-6 weeks of treatment is usually necessary before therapeutic effects develop, even though monoaminergic signaling is affected shortly after the beginning of treatment. The temporal onset of clinical antidepressant effects has led to the hypothesis that the ultimate causes of therapeutic effects of chronic antidepressant drug administration arise from molecular and cellular adaptations requiring persistent drug exposure (Nestler et al., 2002; Duman and Monteggia, 2006).
Two specific mechanisms by which chronic antidepressant treatments may produce their effects are increases of adult hippocampal neurogenesis (Dranovsky and Hen, 2006) and mobilization of neurotrophic factors (Duman and Monteggia, 2006). Chronic, but not acute, administration of different classes of antidepressant treatments to rats have been shown to increase adult hippocampal neurogenesis (Malberg et al., 2000; Manev et al., 2001). Similarly, chronic administration to rats of antidepressant treatments from different classes have been reported to commonly increase the expression of brain-derived neurotrophic factor (BDNF) mRNA in the hippocampus (Nibuya et al., 1995; Coppell et al., 2003; Dias et al., 2003) and frontal cortex (Nibuya et al., 1995), as well as BDNF protein levels in the frontal cortex (Altar et al., 2003; Balu et al., 2008).
Studies of antidepressants in mice are important because of the ability to establish the generality of findings, the reduced costs of drug discovery, and the development of targeted genetic modifications. However, chronic antidepressants have produced inconsistent results in mice. Behavioral effects of antidepressants in mice in the forced swim test or tail suspension test vary according to genetic background (Lucki et al., 2001; Crowley et al., 2005). Although chronic antidepressant drug administration has increased hippocampal neurogenesis in 129SvEv mice following imipramine and fluoxetine (Santarelli et al., 2003) or mixed 129SvEv and C57BL/6 mice following desipramine (Gur et al., 2007), similar effects were not produced in the other mouse strains (Holick et al., 2007, 2008; Miller et al., 2008). Chronic, but not acute, treatment with imipramine or desipramine elevated BDNF mRNA levels in the hippocampus and frontal cortex in C57BL/6J mice (Tsankova et al., 2006) and mice with a mixed genetic background (129SvEv × C57BL/6) (Conti et al., 2002). However, few studies have examined the effects of chronic antidepressant treatments on BDNF protein levels in the mouse brain.
There are few behavioral tests in rodents that are responsive only following the chronic administration of antidepressant drugs. Behavioral hyponeophagia paradigms, that measure reduction of the consumption of a palatable food by exposure to a novel environment, have been shown to respond to antidepressant treatments only following chronic administration (Bechtholt et al., 2007; Dulawa and Hen, 2005; Merali et al., 2003). Novelty-induced hypophagia (NIH), which does not require food deprivation, is sensitive to chronic antidepressant treatments in a number of mouse strains (Merali et al., 2003; Dulawa et al., 2004; Gur et al., 2007). Using novelty suppression of feeding (NSF), a version of the test requiring food deprivation, the behavioral effects of chronic antidepressants in the NSF paradigm were related to increased hippocampal neurogenesis in studies demonstrating that ablation of adult neurogenesis by hippocampal-directed x-irradiation blocked the antidepressant behavioral response in mice (Santarelli et al., 2003) and rats (Jiang et al., 2005).
Because of the uncertainty of the effects of chronic antidepressant drug treatments in mice, this study compared the response to chronic antidepressant drug treatments between two mouse strains, MRL/MpJ and C57BL/6J. The MRL/MpJ mouse strain was selected for their enhanced wound healing and regenerative response to injury (Clark et al., 1998; Leferovich et al., 2001; Heber-Katz et al., 2004) compared to C57BL/6J mice, the control strain for these studies. Moreover, MRL/MpJ mice displayed increased proliferation in the neurogenic subventricular zone compared to other mouse strains (Baker et al., 2006). C57BL/6J mice are commonly used in behavioral and pharmacological studies and constitute a portion of the MRL/MpJ genetic background. The effects of chronically administered fluoxetine and desipramine on the proliferation and survival of hippocampal progenitors cells, BDNF protein levels in different brain regions, and behavior in the NIH test were measured in both strains. The effects of chronic antidepressant drug treatments on hippocampal neurogenesis were measured in this study using flow cytometry, a technique that measures BrdU incorporation in cells more rapidly and objectively than immunohistochemistry. The augmented cytogenic, neurotrophic and behavioral responses to chronic antidepressant drug treatments of MRL/MpJ mice compared with C57BL/6 mice suggest them to be a suitable platform for antidepressant drug discovery based on chronic treatment effects.
MATERIAL AND METHODS
Animals
Adult male C57BL/6J and MRL/MpJ (Jackson Laboratories, Bar Harbor, ME, USA) were 8-9 weeks old at the beginning of all studies. The animals (non-littermates) were housed in groups of five in polycarbonate cages and maintained on a 12-h light/dark cycle (lights on at 07:00 hours) in a temperature (22°C)- and humidity-controlled colony. The animals were given free access to food and water. Animal procedures were conducted in accordance with the guidelines published in the NIH Guide for Care and Use of Laboratory Animals and all protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Drug Treatments
MPJ/MpJ and C57BL/6J mice were administered intraperitoneal (i.p.) injections of saline (0.9% NaCl), fluoxetine hydrochloride (Anawa, Zurich; 2.5, 5, 10 mg/kg), or desipramine hydrochloride (Sigma St. Louis, MO; 2.5, 5, 10 mg/kg) twice daily for 21 days (n = 10-15/group). All of the mice in each cage received the same drug treatment. The doses were calculated according to the base weight of the drug and administered in a volume of 10 ml/kg.
To measure the effects of chronic antidepressant drug treatments on hippocampal cell proliferation, animals were injected with 5-bromo-deoxyuridine (BrdU; 100 mg/kg i.p.; Roche Applied Sciences Indianapolis, IN) once daily during the last 4 days of antidepressant treatment (one hour after the morning antidepressant injection) and were sacrificed 24 hours after the last drug treatment. To measure the effects of antidepressants on hippocampal cell survival, mice were first loaded with BrdU (100 mg/kg i.p.) for four days and chronic antidepressant treatment for 21 days was initiated 24 hours after the last BrdU injection (Malberg et al., 2000). Mice were sacrificed 24 hours after the last antidepressant treatment. BrdU was dissolved in warm physiological saline and injected (i.p.) in a volume of 10 ml/kg.
BrdU Incorporation Using Flow Cytometry
The majority of the experiments studying the effects of chronic antidepressant drug treatments on neurogenesis measured BrdU incorporation in the hippocampus using flow cytometry. This method is more rapid and objectively quantitative than immunohistochemistry and produces a pattern of experimental results that are similar to those reported using immunohistochemistry (Bilsland et al., 2006).
Mice were decapitated, their brains quickly removed, and dissected on ice. The right hippocampal lobe was removed, placed in Hank's Balanced Salt Solution (HBSS, Gibco Grand Island, NY) and finely minced. Prior studies showed no differences between the right and left lobes of the hippocampus. An enzymatic cocktail (0.5 ml, 1 mg/ml papain, Roche Applied Sciences Indianapolis, IN; 0.1 M L-cysteine, Sigma St. Louis, MO) was added to that tube and incubated in a dry heat block at 37°C for 15 min. An equal amount of Hibernate-A (Brain Bits Springfield, IL) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco Grand Island, NY) was added to each tube to stop the enzymatic digestion. The tissue was mechanically triturated to form a single-cell suspension and then centrifuged at 300 × g for 5 min.
The supernatant was removed and the resultant cell suspension was stained using the FITC BrdU Flow Kit (BD Biosciences San Jose, CA). The cells were fixed and permeabilized by resuspension in 100 μL of Cytofix/Cytoperm buffer at room temperature for 30 min, followed by the addition of 1 ml of wash buffer. The samples were spun at 300 × g for 5 min, and the supernatant was aspirated. The cells were further permeabilized by resuspension in 100 μL of Cytoperm Plus buffer on ice for 10 min. After washing and centrifuging, the cells were refixed in 100 μL of Cytofix/Cytoperm buffer at room temperature for 5 min. The cells were then resuspended in 100 μL of DNAse (30 μg; stock from kit was diluted in DPBS (Ca2+/Mg2+ free) containing 0.1 mM CaCl2 and 10 mM MgCl2) in a dry heat block at 37°C for one hour. Following washing and centrifuging, the cells were labeled with 50 μL of FITC-conjugated anti-BrdU (1:50 dilution) in the dark, at room temperature for 20 min. After the samples were washed, they were labeled with 20 μL of the nuclear marker, 7-AAD, at room temperature in the dark. The cells were then resuspended in staining buffer (PBS, 3% FBS, 0.09% sodium azide). Prior to analysis, cells were filtered through a cell strainer cap (30 μm) to remove debris. The data was collected the same day on a BD FACS Canto system at the University of Pennsylvania Flow Cytometry Core Facility and analyzed using Winlist 6.0 software.
BrdU Incorporation Using Immunohistochemistry
Because flow cytometry has not been used frequently to measure neurogenesis, the effects of chronic antidepressant treatments on hippocampal cell proliferation were compared directly with immunohistochemistry. Separate cohorts of MRL/MpJ mice were treated with saline or FLX (5 mg/kg) or with saline or DMI (5 mg/kg) for 21 days. The brains were bisected with the left hemisphere used for immunohistochemistry and the right hippocampus used for flow cytometry. The left hemispheres were post-fixed in 4% paraformaldehyde for 7 days. The brains were then transferred to a 30% sucrose solution for 5 days, at which point the brains were removed and stored at -80°C until they were sliced.
Coronal sections (40 μm) from the entire rostrocaudal extent of the dentate gyrus were cut from a single hemisphere on a cryostat at -20°C. Every 6th slice was mounted in groups of 10-12 per slide (Superfrost plus), dried overnight, and processed for BrdU using peroxidase methods. BrdU staining and quantification was performed according to methods outlined in (Shors et al., 2007). Cells stained for BrdU were counted in the combined SGZ and GCL areas, avoiding cells in the outermost focal plane. The number of counted cells was multiplied by 12 (number of intervening slices x number of hemispheres) to give an estimate of the total number of BrdU labeled cells per hippocampus. Only animals with 8-14 countable sections were included for analysis. Harmonic means were generated for each subject to adjust for the number of tissue sections.
When the results of the DMI study were analyzed, we saw that there was a large increase in the basal levels of BrdU incorporation in MRL/MpJ mice compared to the companion FLX study done earlier and also to the characterization studies done previously in the laboratory. A second DMI comparison between FACS and IHC was done several months later and this study also measured an increased baseline rate of proliferation and more modest drug response. After contacting Jackson Laboratories, it was learned that the housing conditions of the MRL/MpJ mice were changed prior to the DMI studies (around October, 2007, Dr. Peter Kelmenson, personal communication), and that the time of this change corresponded to the date when the MRL/MpJ mice started showing higher rates of BrdU incorporation. The results of the two DMI studies were combined.
BDNF Protein
Twenty-four hours following the last antidepressant treatment, mice were decapitated and their brains quickly removed for dissection into the following regions: hippocampus, frontal cortex, amygdala, and brain stem. Each region was flash frozen in isopentane and placed in -80° C until analysis. BDNF protein levels were quantified using a commercially available sandwich ELISA kit (Promega, Madison, WI). The tissue was homogenized in 0.75 mL of lysis buffer (100 mM PIPES pH 7.0, 500 mM NaCl, 2 mM EDTA, 0.1% sodium azide, 2% bovine serum albumin, 0.2% Triton X-100, 5 μg/mL aprotinin, 0.1μg/mL pepstatin A, 0.5 μg/mL antipain). The homogenate was centrifuged at 14,000 × g for 30 min at 4°C. The supernatant was removed and the amount of BDNF protein was analyzed by ELISA in duplicate samples according to the manufacturer's instructions. BDNF levels were normalized to the wet tissue weight.
Novelty-induced Hypophagia
Mice were housed in pairs upon arrival in the animal facility in polycarbonate cages (11 × 7 × 5 in). They were allowed to acclimate to these conditions for one week prior to training. The training consisted of daily sessions (15 min for C57BL/6J mice and 5 min for MRL/MpJ mice) in which mice were given access to a highly palatable food (peanut butter chips; Nestle, Glendale, CA) delivered in a clear plastic petri dish. Opaque, black, plastic dividers (7 × 5 in) were placed inside each cage to separate the mice during the training and home cage testing periods. Mice were acclimated to the dividers for 1 h before the introduction of food. Although training sessions were conducted during daylight hours, the room was illuminated by red light to facilitate ingestion. The latency to approach and initiate ingestion and the amount of food consumption during the session were measured. For C57BL/6J mice, a stable mean latency to approach the food was obtained by the 9th day, such that the variability of means over 3 sessions was less than 20%. For MRL/MpJ mice, this criterion was reached by the 4th day. Mice that did not meet this criterion were not continued in the study. Training was then suspended after the stability criterion was reached.
Mice received injections with 0.9% saline (n = 10), desipramine (5 mg/kg; n = 9), or fluoxetine (5 mg/kg; n = 10) twice daily for 21 d. The doses were chosen because they produced the peak effect on cell proliferation in the MRL/MpJ mice. During days 18-20 of treatment, mice were re-exposed to the peanut butter chips under the same home cage training conditions described previously, except that the room was illuminated in visible fluorescent light. Testing in the home and novel environments occurred on the last 2 d of treatment, 1 h after their injection. Testing in the novel environment occurred in a different room from where the animals were trained. Mice were removed from their home cage and placed in an empty, clear polycarbonate cage (11 × 7 × 5 in) without bedding. The novel cage was placed on a table with white cardboard paper placed underneath and on two sides of the cage. Bright illumination was placed directly overhead (60W light bulb). There was no acclimation period prior to testing in the novel environment. The novel test session was videotaped and the latency to ingestion and the amount consumed during test sessions were scored later.
Data Analysis
Measurement of BrdU incorporation using flow cytomtery, BDNF protein levels, and NIH latencies between saline-injected controls and antidepressant treatments were compared using one-way analysis of variance (ANOVA). Dunnett's post-hoc test was used to compare the means of individual treatment groups to the common control group. Unpaired two-tailed Student's t-test was used to compare results between flow cytometry and manual counting. The results from the two DMI comparisons were not significantly different from each other and were therefore combined for analysis. Linear regression analysis was performed to determine the correlation between methods. For all tests, P < 0.05 was considered statistically significant.
RESULTS
Effects of Chronic Fluoxetine and Desipramine Treatments on Hippocampal Cell Proliferation
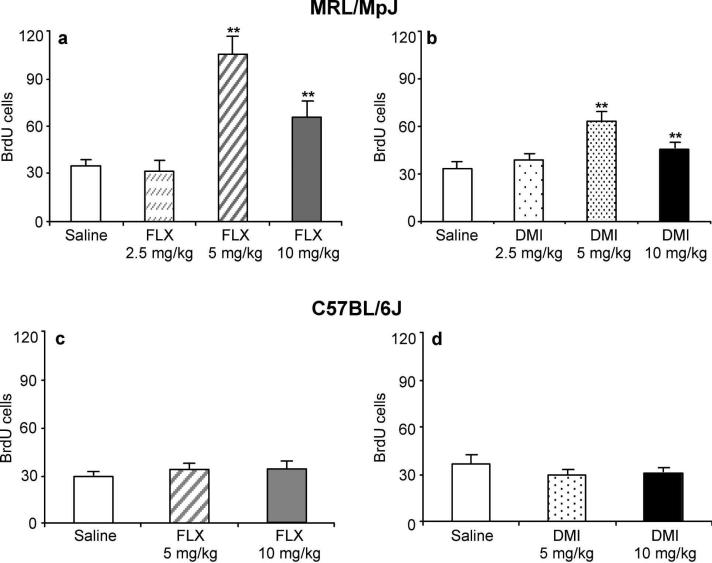
In MRL/MpJ mice, chronic administration of fluoxetine (Figure 1a) or desipramine (Figure 1b) for 21 days dose-dependently increased cell proliferation in the hippocampus as measured with flow cytometry (Figure 1a). The lowest dose (2.5 mg/kg) of fluoxetine or desipramine failed to elevate cell proliferation. A peak 3-fold elevation of proliferative activity was achieved with the 5 mg/kg dose of fluoxetine, while the highest dose tested (10 mg/kg) produced a 2-fold increase. The peak effect for desipramine, a 2-fold elevation of proliferation, was achieved with the 5 mg/kg dose, while the highest tested dose (10 mg/kg) produced a 50% increase. Contrary to the results obtained with MRL/MpJ mice, chronic treatment of C57BL/6J mice with fluoxetine (Figure 1c) or desipramine (Figure 1d), at any of the tested doses, failed to increase hippocampal cell proliferation.
Figure 1.
Chronic fluoxetine and desipramine treatments elevated cell proliferation in MRL/MpJ mice but not C57BL/6J mice. (a) MRL/MpJ mice were administered saline (n = 15) or fluoxetine (FLX; 2.5, 5, 10 mg/kg b.i.d.; n = 10/group) for 21 days. The 5 and 10 mg/kg doses of FLX increased cell proliferation (F(3,38) = 21.89, P < 0.0001) (b) MRL/MpJ mice were administered saline (n = 15) or desipramine (DMI; 2.5, 5, 10 mg/kg b.i.d.; n = 10/group) for 21 days. DMI (5 and 10 mg/kg) significantly increased cell proliferation (F(3,38) = 8.77, P < 0.001). (c) C57BL/6J mice were administered saline (n = 10) or fluoxetine (FLX; 5 or 10 mg/kg b.i.d.; n = 10/group) for 21 days. FLX did not alter cell proliferation (F(2,21) = 0.60, P = 0.56). (d) C57BL/6J mice were administered saline (n = 10) or desipramine (DMI; 5 or 10 mg/kg b.i.d.; n = 10/group) for 21 days. DMI did not increase cell proliferation (F(2,22) = 1.17, P = 0.32). Values are expressed as the number of BrdU positive cells per 10,000 7-AAD events. Bars represent mean values + 1 s.e.m. Asterisks (**) indicate groups that differed significantly from control (P < 0.005) according to Dunnett's test.
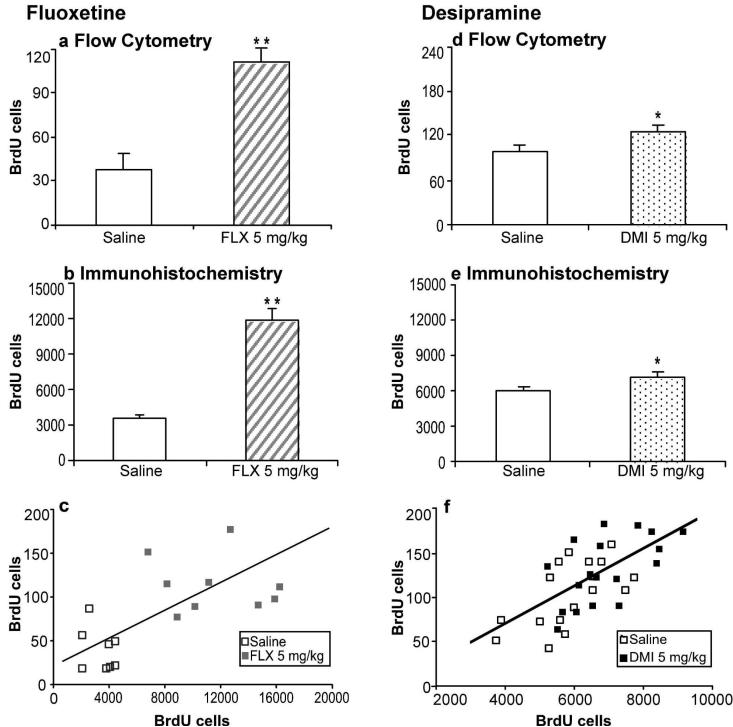
In order to validate the findings obtained by flow cytometry, two separate cohorts of MRL/MpJ mice were chronically treated with 5 mg/kg of fluoxetine or desipramine for 21 days. Hippocampal cell proliferation was measured in the same animal by bisecting the brain and quantifying BrdU incorporation by flow cytometry in one hippocampal lobe and immunohistochemistry on the contralateral hemisphere. The effects of fluoxetine (Figure 2a,b) and desipramine (Figure 2d,e) on cell proliferation were equivalent in magnitude for both methods. Moreover, there was a significant correlation between the results obtained with flow cytometry and manual counting in both the fluoxetine (Figure 2c; r(15) = 0.62, P = 0.007) and desipramine (Figure 2f; r(33) = 0.65, P < 0.001) experiments. The effect sizes were calculated for both drug treatments to compare the precision of the two methods of analysis. The effect size correlations (fluoxetine: flow cytometry = 0.77, immunohistochemistry = 0.85; desipramine: flow cytometry = 0.35, immunohistochemistry = 0.42) showed that immunocytochemistry was slightly more precise than flow cytometry by providing greater average differences between groups relative to the overall variance. Fluoxetine produced a similar magnitude of effect in MRL/MpJ mice between the first and second study (c.f., Figs. 1 and 2). The effect of desipramine was smaller in the second study than in the first, due to changes in rearing conditions for MRL/MpJ mice imposed by Jackson Laboratories around October, 2007 (D. Balu, personal communication).
Figure 2.
Comparison of BrdU incorporation in the hippocampus measured by flow cytometry and immunohistochemistry following chronic antidepressant treatments. One cohort of MRL/MpJ mice (a-c) was administered saline (n = 9; open bars) or fluoxetine (FLX; 5 mg/kg b.i.d.; n = 9; striped bars), while another (d-f) was administered saline (n = 17; open bars) or desipramine (DMI; 5 mg/kg b.i.d.; n = 18; dotted bars) for 21 days. (a, d) Cell proliferation was measured in one hippocampal lobe by flow cytometry. Values are expressed as the number of BrdU positive cells per 10,000 7-AAD events. FLX (t(18) = 5.94, P < 0.001) and DMI (t(33) = 2.12, P = 0.04) significantly increased cell proliferation. (b, e) The contralateral hemisphere was sectioned and BrdU-labeled cells in the hippocampus were counted using immunohistochemistry. Values are expressed as the number of BrdU positive cells per hippocampus. FLX (t(15) = 6.47, P < 0.001) and DMI (t(33) = 2.66, P = 0.01) significantly increased cell proliferation. Bars represent mean values + 1 s.e.m. Asterisks indicate groups that differed significantly from saline (* P < 0.05, ** P < 0.005), according to unpaired Student's t-test. (c, f) Scatter plots showing the correlation between results obtained from individual mice using flow cytometry and immunohistochemistry (FLX: r(15) = 0.62, P = 0.007; (r(33) = .65, P < 0.001).
Effects of Chronic Fluoxetine and Desipramine Treatments on BDNF Protein Levels
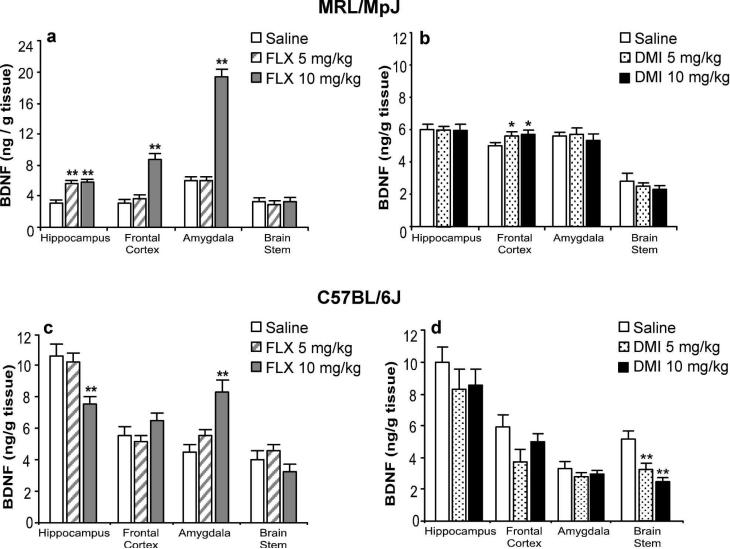
Chronic treatment of MRL/MPJ mice with fluoxetine elevated BDNF protein levels in several brain regions (Figure 3a). In the hippocampus, both the 5 mg/kg and 10 mg/kg doses significantly increased BDNF protein levels by two-fold. In the frontal cortex, the lower dose of fluoxetine elevated BDNF levels by 20% (n.s.), while the higher dose caused a significant three-fold elevation in BDNF protein. Fluoxetine at 10 mg/kg also increased BDNF levels in the amygdala by three-fold. Neither dose of fluoxetine affected BDNF protein levels in the brain stem. Chronic treatment of MRL/MpJ mice with 5 and 10 mg/kg of desipramine (Figure 3b) significantly elevated BDNF protein levels in the frontal cortex by 15%, without effect in any of the other examined brain regions.
Figure 3.
Differential regulation of BDNF protein levels by chronic fluoxetine and desipramine treatments in MRL/MpJ mice and C57BL/6J mice. (a) MRL/MpJ and (c) C57BL/6J mice were administered saline (open bar, n = 10) or fluoxetine (FLX, n = 10/group) at 5 (striped bar) or 10 mg/kg (grey bar) b.i.d. for 21 days. In MRL/MpJ mice, FLX increased BDNF levels in the hippocampus (F(2,25) = 52.62, P < 0.001), frontal cortex (F(2,25) = 44.81 P < 0.001), and amygdala (F(2,25) = 155.08, P < 0.001). In C57BL/6J mice, FLX decreased BDNF levels significantly in the hippocampus (F(2,25) = 7.40, P < 0.003) and increased BDNF levels in the amygdala (F(2,25) = 14.04, P < 0.001). All other regional comparisons were not statistically significant. (b) MRL/MpJ and (d) C57BL/6J mice were administered saline (open bars, n = 10) or desipramine (DMI, n = 10/group) at 5 (dotted bars) or 10 (black bar) mg/kg b.i.d. for 21 days. In MRL/MpJ mice, DMI increased BDNF levels only in the frontal cortex (F(2,27) = 4.33, P = 0.02). In C57BL/6J mice, DMI decreased BDNF levels in the brain stem (F(2,27) = 11.36, P < 0.001). All other regional comparisons were not statistically significant. Values are expressed as the amount of BDNF protein per gram of tissue. Bars represent mean values + 1 s.e.m. Asterisks indicate groups that differed significantly from saline treatment (* P < 0.05, ** P < 0.005) according to Dunnett's test.
Chronic treatment of C57BL/6J mice with fluoxetine or desipramine produced different effects on BDNF protein in various brain regions (Figure 3c). At the 10 mg/kg dose, fluoxetine caused an almost 2-fold elevation of BDNF levels in the amygdala, while BDNF levels were reduced by 30% in the hippocampus. Fluoxetine at 5 mg/kg did not affect BDNF levels in any of the examined brain regions. In contrast to the results with MRL/MpJ mice, chronic treatment of C57BL/6J mice with desipramine (Figure 3d) at both doses, did not increase BDNF protein levels in any region of the forebrain, but selectively reduced BDNF levels by 50% in the brainstem.
Effects of Chronic Antidepressant Treatment on the Survival of Hippocampal Progenitors in the MRL/MpJ and C57BL/6J Mouse Strains
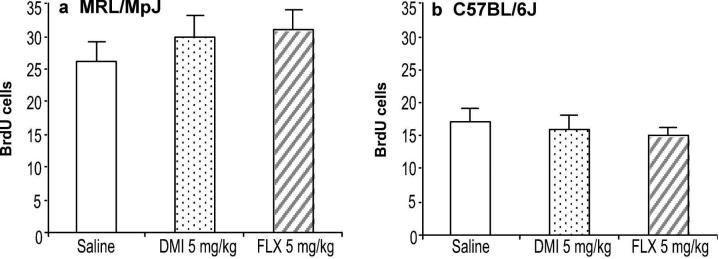
The effects of antidepressant drug treatments on cell survival were examined by administering BrdU prior to chronic treatment for 21 days. For these experiments, only the 5 mg/kg dose of fluoxetine and desipramine, which produced the peak effects on cell proliferation, were tested. However, neither chronic administration of fluoxetine nor desipramine altered the survival of newly born hippocampal progenitors in either the MRL/MpJ strain (Figure 4a) or C57BL/6J strain (Figure 4b).
Figure 4.
Chronic treatments with fluoxetine or desipramine did not affect the survival of newly born hippocampal progenitor cells. Cells were labeled by administration of BrdU (100 mg/kg i.p. for 4 days) prior to the initiation of chronic treatment. (a) MRL/MpJ mice or (b) C57BL/6J mice (n = 10 mice/group) were administered saline (open bar), fluoxetine (FLX; 5 mg/kg b.i.d.; striped bar), or desipramine (DMI; 5 mg/kg b.i.d.; dotted bar) for 21 days. Values for ANOVA were not statistically significant (P > 0.05). Values are expressed as the number of BrdU positive cells per 10,000 7-AAD events. Bars represent mean values + 1 s.e.m.
Effects of Chronic Antidepressant Treatments on Novelty-Induced Hypophagia Behavior
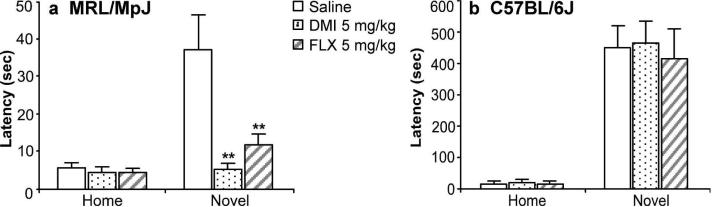
Exposure of MRL/MpJ mice to a novel environment increased their latency to consume the peanut butter chips 6-fold compared to their home cage latency times of 5 sec, to a mean value of 37 sec (Figure 5a). Chronic treatment of these mice with fluoxetine (5 mg/kg) or desipramine (5 mg/kg) significantly reduced the consumption latencies to 11 and 5 sec, respectively (Figure 5a). As compared to the home cage, consumption in the novel environment was suppressed by 50%. However, there were no differences in home cage or novel cage consumptions between the drug treatment groups (data not shown).
Figure 5.
Chronic treatments with fluoxetine and desipramine reduced reactivity to novelty in the NIH test in MRL/MpJ mice but not in C57BL/6J mice. (a) MRL/MpJ mice (n = 7-9) or (b) C57BL/6J mice (n = 7-9) were administered saline (open bar), desipramine (DMI; 5 mg/kg b.i.d.; dotted bar), or fluoxetine (FLX; 5 mg/kg; striped bar) for 21 days. The chronic antidepressant treatments reduced the latency to initiate feeding in a novel environment for MRL/MpJ mice (F(2,25) = 8.13, P = 0.002) but not for C57BL/6J mice (F(2,20) = 0.10, P = .90). The antidepressant treatments did not alter home cage feeding for both strains. Values are expressed as the latency (sec) to consume peanut butter chips in either the home cage (Home) or a novel environment (Novel). Bars represent mean values + 1 s.e.m. Asterisks (**) indicate groups that differed significantly from saline (P < 0.005) according to Dunnett's test.
During training, C57BL/6J mice were given more training sessions and a longer exposure time to the food than MRL/MpJ mice in order to develop stable home cage response latencies. C57BL/6J mice had stable home cage latencies of 13 sec, compared to MRL/MpJ mice that were stable at 5 sec. Exposure to the novel environment produced an increase in approach latency and decrease in food consumption (data not shown) in the C57BL/6J mice. Approach latencies were increased to a greater extent than MRL/MpJ mice (Figure 5b). Unlike the MRL/MpJ mice, chronic antidepressant drug treatments did not reduce their latency to eat in the novel cage (Figure 5b). The drug treatments also did not alter their home cage or novel cage consumptions (data not shown).
DISCUSSION
The results of the present study demonstrated the existence of important strain differences in the responses of mice to chronic administration of antidepressant drugs. MRL/MpJ mice demonstrated robust increases in hippocampal cell proliferation, increased cortico-limbic BDNF protein levels, and diminished reactions to novelty in the NIH test, whereas C57BL/6J mice did not show significant changes in these parameters after the same treatments. The background strain is critical for producing typical effects of antidepressants following acute (Lucki et al., 2001; Crowley et al., 2005) or chronic (Holick et al., 2008; Miller et al., 2008) treatment. The results of the present study emphasize the importance of strain differences for measuring the effects of antidepressants requiring chronic drug administration. MRL/MpJ mice were studied because they are known distinctively for enhanced wound healing and tissue regeneration after injury (Clark et al., 1998; Leferovich et al., 2001; Heber-Katz et al., 2004). The present study demonstrated exaggerated neuroplastic responses in parallel with behavioral changes to chronic antidepressant drug treatments in these mice. Identification of genetic and neural substrates responsible for these responses in MRL/MpJ mice could provide important information concerning the presently unknown mechanisms that regulate the chronic effects of antidepressants, and that may be more closely associated with their ultimate clinical therapeutic effects.
The existence of neurogenesis throughout the mammalian lifespan in particular brain regions has now been accepted. Adult hippocampal neurogenesis has been associated with a number of diseases, such as depression, schizophrenia, epilepsy, Parkinson's disease, and Alzheimer's disease (Balu and Lucki, 2008; Thompson et al., 2008; Zhao et al., 2008). As a result, there is much interest in studying the effects of various pharmacologic treatments on adult or developmental hippocampal neurogenesis. Hippocampal neurogenesis is regulated by a variety of neurotransmitters and hormones, and pharmacological regulation of neurogenesis can lead to the discovery of novel medications for these disorders. The most common method for measuring cell genesis in brain tissues involves marking DNA synthesis by administering the thymidine analog BrdU and then counting the number of BrdU-labeled cells using immunohistochemistry. However, the intensive labor and time required to perform these studies has made hippocampal neurogenesis an impractical target for screening novel compounds in animals. The present study used flow cytometry to quantify BrdU incorporation into hippocampal cells after administering chronic antidepressant drug treatments and demonstrated significant drug and strain differences in cell proliferation (see also (Bilsland et al., 2006; Shankaran et al., 2006). This technique circumvents many technical limitations associated with immunohistochemistry, and provides the speed and automated analysis necessary to facilitate drug discovery and understand the mechanisms underlying drug effects on neurogenesis. The enhanced analytical power of flow cytometry was associated with slightly inferior precision, as compared to immunohistochemistry, which may reflect the ability to topographically restrict counting labeled cells to the dentate gyrus. Future advances of cell phenotyping using flow cytometry, double-labeling BrdU-positive cells with antibodies for neural, glial and developmental markers, will enable more specific morphogenic changes produced by chronic antidepressant drug treatments to be identified rapidly. Nevertheless, rapid screening can still identify those conditions requiring more detailed anatomical analysis.
The value of a rapid quantitative screening approach for measuring hippocampal cell proliferation was demonstrated in the present study. Both fluoxetine and desipramine showed an inverted U-shaped dose-response curve for increased proliferative activity in MRL/MpJ mice following chronic treatment. The inverted U-shaped curve for these antidepressants could be caused by their affinity for receptors unrelated to their primary effect of selectively blocking serotonin and norepinephrine transporters, respectively (Richelson, 2003). In contrast, C57BL/6J mice did not show an increase in cell proliferation. There have been mixed reports on the ability of antidepressants to regulate hippocampal neurogenesis in C57BL/6J mice. Other studies have also found that fluoxetine administered for 10 days (10 mg/kg i.p.) (Beauquis et al., 2006) or for 24 days (10, 16, or 25 mg/kg in the drinking water) (Navailles et al., 2008) failed to increase hippocampal cell proliferation. However, other studies reported that fluoxetine (10 mg/kg, i.p.) administered for 21 days (Lagace et al., 2007) or amitriptyline (in drinking water) given for 28 days (Caldarone et al., 2004) resulted in a 25% increase in hippocampal cell proliferation in C57BL/6J mice. It is unclear whether procedural differences in drug administration, BrdU loading protocols, or differences in levels of endogenous stress contributed to the different findings between laboratories. Differences in the response to chronic fluoxetine have been reported for other strains (c.f., Santarelli et al., 2003; Holick et al., 2008; Miller et al., 2008), but no strain showed as large as a response as the MRL/MpJ mice. Most of the cells in the adult hippocampus generated from amplifying progenitor cells become neurons (Encinas et al., 2006; Wang et al., 2008b).
Although the regulation of hippocampal cell proliferation by chronic antidepressant treatments is established, their ability to enhance the long-term survival of newly born neurons is less well defined. Treatment with desipramine or fluoxetine for 21 days did not enhance the survival of newly born progenitors in either the MRL/MpJ or C57BL/6J strain in this study, when BrdU was administered before the antidepressant treatments. A previous study showed that hippocampal cell survival in rats was not altered by chronic treatment with fluoxetine (5 mg/kg) for 14 days (Malberg et al., 2000). However, more recent studies demonstrated that fluoxetine given chronically to rats (5 mg/kg, i.p.) or 129SvEv mice (18 mg/kg, in drinking water) for 28 days was effective in prolonging cell survival (Nakagawa et al., 2002; Wang et al., 2008a). Treatments with other doses, longer durations, or BrdU-labeling protocols could show increased cell survival, and this remains to be determined. However, increased survival of newborn hippocampal cells was not produced under chronic treatment conditions that increased cell proliferation in MRL/MpJ mice.
Another important marker of chronic antidepressant treatments is their ability to increase levels of BDNF protein or mRNA, particularly in the hippocampus and frontal cortex (Duman and Monteggia, 2006). Although similar findings have been reported in the mouse (Conti et al., 2002; Song et al., 2006; Tsankova et al., 2006), BDNF was measured only at the mRNA level. In MRL/MpJ mice, chronic administration of fluoxetine increased BDNF protein levels in the hippocampus, frontal cortex, and amygdala, while desipramine elevated BDNF levels specifically in the frontal cortex, which is similar to effects reported recently in rats (Balu et al., 2008). In contrast, the chronic antidepressant treatments did not increase BDNF levels in the frontal cortex or hippocampus in C57BL/6J mice. Fluoxetine (10 mg/kg) increased BDNF in the amygdala and decreased BDNF in the hippocampus, while desipramine dose-dependently and selectively, reduced BDNF protein levels in the brain stem. Mobilization of neurotrophins following chronic antidepressant drug treatments has been considered to play a key role in promoting neuroplasticity and behavioral change (Duman and Monteggia, 2006; Sairanen et al., 2005). In the present study, the increased BDNF levels in MRL/MpJ mice was associated with increased hippocampal cytogenesis and changes in behavior, and is a candidate for driving these changes. In addition, the augmentation of other forms of synaptic plasticity following chronic antidepressant treatments, such as enhanced LTP (Wang et al., 2008b), circuit level activity (Airan et al., 2007) in the dentate gyrus of the hippocampus, and increased dendritic arborization of adult-born hippocampal neurons (Wang et al., 2008b), could be mediated, in part, by BDNF mobilization (Elmariah et al., 2005; Mamounas et al., 1995).
The differential effects of fluoxetine and desipramine in neuroplasticity and behavior between the two mouse strains could result from differences in pharmacokinetics. However, since these effects were produced by two pharmacologically distinct antidepressants, this explanation is unlikely.
Reduction of hyponeophagia is one of the few behavioral responses produced by chronic antidepressant drug treatments (Merali et al., 2003; Dulawa and Hen, 2005), and was used to compare behavioral effects between these mouse strains. Chronic administration of fluoxetine or desipramine to MRL/MpJ mice, but not C57BL/6J mice, reduced their latency to consume food in the NIH paradigm. The divergent behavioral responses of the mouse strains paralleled their response to chronic antidepressant treatments on hippocampal cell proliferation and cortio-limbic BDNF levels, although the link between these factors is unclear (Dranovsky and Hen, 2006). Hippocampal neurogenesis appeared to be required for the behavioral effects of chronic antidepressant treatments in the NSF paradigm in 129SvEvTac mice because cessation of neurogenesis by x-irradiation prevented chronic antidepressant behaviors (Santarelli et al., 2003). However, hippocampal neurogenesis did not correlate with the ability of chronic fluoxetine to mediate NIH behavior in unperturbed BALB/cJ mice (Holick et al., 2007, 2008). Nevertheless, a causal link may still exist when changes in hippocampal neurogenesis occur and the moderation of NIH behavior following chronic antidepressant drug treatments, and future studies are needed to assess this relationship in the MRL/MpJ strain. Taken together with neurogenesis and BDNF mobilization, these results using markers of chronic, rather than acute, antidepressant drug activity highlight the potential utility of the MRL/MpJ mouse to test novel compounds for potential antidepressant activity.
ACKNOWLEDGEMENTS
This research was conducted by a National Center for Drug Discovery Group (NCDDG) in Mood Disorders established between the University of Pennsylvania and Wyeth Research and funded by USPHS grant MH72832. We are grateful for the critical comments and advice provided by Dr. Julie Blendy at the University of Pennsylvania and Drs. Lee Schechter, Robert Ring, and Zia Rahman at Wyeth Research and other members of the NCDDG. We thank Hank Pletcher of the University of Pennsylvania Flow Cytometry Center for his assistance in developing some of the techniques described in this paper. We also thank Gina Colaizzo for her excellent technical assistance.
Footnotes
DISCLOSURE/CONFLICTS OF INTEREST Dr. Lucki is a member of the Neuroscience Scientific Advisory Board for Wyeth Research, and has received research support from AstraZeneca Pharmaceuticals, Forest Laboratories and Epix Pharmaceuticals during the past 3 years. There are no other disclosures.
REFERENCES
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703–709. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Baker KL, Daniels SB, Lennington JB, Lardaro T, Czap A, Notti RQ, Cooper O, Isacson O, Frasca S, Jr., Conover JC. Neuroblast protuberances in the subventricular zone of the regenerative MRL/MpJ mouse. J Comp Neurol. 2006;498:747–761. doi: 10.1002/cne.21090. [DOI] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: Regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2008 doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and nonantidepressant drug treatments. Brain Res. 2008;1211:37–43. doi: 10.1016/j.brainres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauquis J, Roig P, Homo-Delarche F, De Nicola A, Saravia F. Reduced hippocampal neurogenesis and number of hilar neurones in streptozotocin-induced diabetic mice: reversion by antidepressant treatment. Eur J Neurosci. 2006;23:1539–1546. doi: 10.1111/j.1460-9568.2006.04691.x. [DOI] [PubMed] [Google Scholar]
- Bilsland JG, Haldon C, Goddard J, Oliver K, Murray F, Wheeldon A, Cumberbatch J, McAllister G, Munoz-Sanjuan I. A rapid method for the quantification of mouse hippocampal neurogenesis in vivo by flow cytometry. Validation with conventional and enhanced immunohistochemical methods. J Neurosci Methods. 2006;157:54–63. doi: 10.1016/j.jneumeth.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56:657–664. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppell AL, Pei Q, Zetterstrom TS. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44:903–910. doi: 10.1016/s0028-3908(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. Serotonergic and noradrenergic reuptake inhibitors: prediction of clinical effects from in vitro potencies. J Clin Psychiatry. 2001;62(Suppl 12):16–23. [PubMed] [Google Scholar]
- Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, Malberg JE, Blendy JA. cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci. 2007;27:7860–7868. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber-Katz E, Leferovich J, Bedelbaeva K, Gourevitch D, Clark L. The scarless heart and the MRL mouse. Philos Trans R Soc Lond B Biol Sci. 2004;359:785–793. doi: 10.1098/rstb.2004.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral Effects of Chronic Fluoxetine in BALB/cJ Mice Do Not Require Adult Hippocampal Neurogenesis or the Serotonin 1A Receptor. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- Leferovich JM, Bedelbaeva K, Samulewicz S, Zhang XM, Zwas D, Lankford EB, Heber-Katz E. Heart regeneration in adult MRL mice. Proc Natl Acad Sci U S A. 2001;98:9830–9835. doi: 10.1073/pnas.181329398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Uz T, Smalheiser NR, Manev R. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur J Pharmacol. 2001;411:67–70. doi: 10.1016/s0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- Merali Z, Levac C, Anisman H. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry. 2003;54:552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- Miller BH, Schultz LE, Gulati A, Cameron MD, Pletcher MT. Genetic regulation of behavioral and neuronal responses to fluoxetine. Neuropsychopharmacology. 2008;33:1312–1322. doi: 10.1038/sj.npp.1301497. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002;22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, Hof PR, Schmauss C. Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. J Comp Neurol. 2008;509:372–381. doi: 10.1002/cne.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelson E. Interactions of antidepressants with neurotransmitter transporters and receptors and their clinical relevance. J Clin Psychiatry. 2003;64(Suppl 13):5–12. [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Shankaran M, King C, Lee J, Busch R, Wolff M, Hellerstein MK. Discovery of novel hippocampal neurogenic agents by using an in vivo stable isotope labeling technique. J Pharmacol Exp Ther. 2006;319:1172–1181. doi: 10.1124/jpet.106.110510. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry. 2007;62:487–495. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav. 2006;83:186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Thompson A, Boekhoorn K, Van Dam AM, Lucassen PJ. Changes in adult neurogenesis in neurodegenerative diseases: cause or consequence? Genes Brain Behav. 2008;7(Suppl 1):28–42. doi: 10.1111/j.1601-183X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen Rt. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008a;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008b;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]