Abstract
Systemic administration of MK-801, an NMDA receptor antagonist, impairs reversal learning in weanling rats (Chadman, Watson, & Stanton, 2006). The brain systems responsible for this effect are not known in either adult or young animals. This study tested the hypothesis that hippocampal NMDA receptors are engaged in weanling-age rats during spatial discrimination reversal training in a T-maze. In Experiment 1, 26-day-old Long-Evans rats (P26) showed a dose-related impairment on this task following bilateral intrahippocampal administration of either 2.5 or 5.0 μg MK-801 or saline vehicle during the reversal training phase only. In Experiment 2, P26 rats were trained on the same task, but received intrahippocampal MK-801 (2.5 μg) during acquisition, reversal, both, or neither. MK-801 failed to impair acquisition, ruling out nonspecific “performance effects” of the drug. MK-801 impaired reversal irrespective of drug treatment during acquisition. NMDA receptor antagonism in the hippocampus is sufficient to account for the previously reported effects of systemic MK-801 on reversal of T-maze position discrimination.
Keywords: Development, Reversal Learning, Hippocampus, MK-801, Rat, Spatial Learning
The N-methyl-D-Aspartate (NMDA) receptor subtype of glutamate receptors plays a substantial role in neural physiology, synaptic plasticity, and behavioral learning and memory. These roles include, but are not limited to, the molecular/cellular basis of short- and long-term memory formation, the induction and maintenance of long-term potentiation (LTP), as well spatial learning and memory that depends upon the hippocampus (Morris, Anderson, Lynch, & Baudry, 1986; O’Keefe & Nadel, 1978; Shapiro, 2001).
NMDA receptors are heavily concentrated in the hippocampal formation, cortex, and striatum (Wong, Kemp, Priestley, Knight, Woodruff, & Iversen, 1986; Wong, Knight, & Woodruff, 1988). These same brain regions are essential for spatial learning and memory, contextual memory, and higher-order cognitive learning tasks in adult animals. It is currently unknown if these same brain regions are involved in reversal learning during early ontogeny.
The NMDA receptor subunit representation is not the same across the lifespan. The mRNA transcripts of all NMDA receptor subunits peak by post-natal day (P) 20 (except NR2D, which peaks at P7, and then decreases to adult levels) (Monyer, Burnashev, Laurie, Sakmann, & Seeburg, 1994). The most apparent changes occur by the end of the 3rd postnatal week of life (P21). The NR2B and NR2D subunits have mRNA expression that is first detected during prenatal development across most brain regions at embryonic day (E) 14 and then expression declines in adulthood. NR2A and NR2C subunits are barely detectable at birth, and mRNA transcripts are first expressed post-natally (approximately P7) and increase into adulthood, with NR2A expression highest in forebrain regions and NR2C particularly in the cerebellum (Monyer, et al., 1994). A similar pattern of expression of NMDA receptor subunits is seen across mouse development (Vallano, 1998). In general, the contribution of NR2B decreases across development, while NR2A has an increased contribution to NMDA receptor function (Cull-Candy, Brickley, & Farrant, 2001). In addition to these changes in receptor subunit expression across development, expression of LTP in CA1 pyramidal cells also changes with maturation; such that LTP first occurs by P5 and reaches maximal response around P15 (Harris & Teyler, 1984; Teyler, Perkins, & Harris, 1989). Hippocampal dendritic spine formation follows a similar timeline in developing rats (Bourne & Harris, 2008; Harris, 1999). In contrast with these neurobiological studies, much less is known about the role NMDA receptors play in the ontogeny of reversal learning and the neural substrates underlying this form of learning. Rats as young as P7–15 can acquire and reverse a position habit using suckling as a reward (Green & Stanton, 1989; Kenny & Blass, 1977). Reversal learning of a discrimination is cognitively more demanding than initial acquisition of a discrimination (Dias et al., 1997). Reversal learning is dependent upon three behavioral processes: 1) memory of the initially acquired response, 2) learned suppression of this initially acquired response, 3) learning the new (competing) response. A small number of studies have investigated the role of NMDA in learning in developing animals (Chadman, et al., 2006; Griesbach & Amsel, 1998; Griesbach, Hu, & Amsel, 1998; Highfield, Nixon, & Amsel, 1996). NMDA-receptor involvement was confirmed in both spatial and olfactory reversal learning and nonspatial working memory in weanling rats. Previous work in our lab has demonstrated that NMDA-receptor antagonism with MK-801 impaired spatial reversal performance in P21–30 rats (Chadman, et al., 2006). The role different brain regions play in this reversal learning deficit in young rats is currently unknown.
Two experiments examined the effect of NMDA receptor antagonism within the hippocampus on spatial discrimination reversal learning in weanling rats. Experiment 1 evaluated different doses of MK-801 to determine for the first time whether antagonism of hippocampal NMDA receptors impairs spatial reversal learning in P26 rats. Experiment 2 sought to determine if the MK-801 impairment was specific to the reversal learning phase relative to the acquisition phase by administering MK-801 before acquisition, reversal, both, or neither. Based on prior studies with systemically administered MK-801, we predicted that the highest dose of MK-801 would lead to the greatest impairment and the lowest dose of MK-801 would modestly impair reversal learning performance (Experiment 1). We also predicted that this effect would be selective for the reversal learning phase, sparing acquisition performance, eliminating the potential role of “performance” or state-dependent learning effects (Experiment 2).
Experiment 1
Systemically administered MK-801 selectively impairs reversal learning in weanling rats (Chadman, et al., 2006). In adult rats and mice, the hippocampal system plays a role in spatial learning (Morris, 2006; Morris, Moser, Riedel, Martin, Sandin, Day, & O’Carroll, 2003; Shapiro, 2001; Wang & Cai, 2006) and more specifically in discrimination reversal learning (Bardgett, Boeckman, Krochmal, Fernando, Ahrens, & Csernansky, 2003; Oliveira, Bueno, Pomarico, & Gugliano, 1997). However, the role of the hippocampus in reversal learning has not been examined in developing rats. Experiment 1 was designed to evaluate whether an infusion of MK-801 into the dorsal hippocampus would impair reversal learning in weanling rats, and whether the MK-801 impairment was dose-dependent.
Three treatment groups received 8 blocks of spatial discrimination training (4 blocks in acquisition, and 4 in reversal). All groups received bilateral infusions in dorsal hippocampus (0.5 μl per side). Separate groups were administered a high dose of MK-801 (5.0 μg per side); an intermediate dose (2.5 μg per side); or vehicle (sterile saline). If hippocampal receptors are involved in the effects of systemic MK-801 administration seen previously, then the present experiment should reveal dose-related impairment of reversal learning.
Method
Subjects
Thirty-one (16 female, 15 male) Long-Evans rat pups derived from 21 litters served as subjects. Litters were housed in the laboratory vivarium with ad lib access to food and water on a 12:12 hr light-dark cycle (onset at 0700 hr). Litters were culled to 8 pups (usually 4 males and 4 females) on P3 (date of birth is P0). A subset of the pups from the 21 litters were used for Experiment 1, the remaining pups were assigned to other ongoing studies. The pups were weaned on P21 and housed with same-sex littermates until P23. Weaned pups had uninterrupted access to food and water until the onset of behavioral procedures. The surgical procedure began on approximately P23 (range: 22–23). Subjects were housed individually in cages following surgery for the duration of the experiment. Pups were allowed to recover from surgery for 1 day before the onset of deprivation (see Procedure below). The average weight at deprivation for subjects was 59.8 ± 1.04 g (range: 51.0 – 77.0 g). ANOVA performed on the deprivation weight data that did not reveal differences among subjects between treatment groups (F < 0.61).
One pup was discarded from analysis because it did not consume the reward following drug administration. Of the remaining 30 pups, 7 pups were excluded from further analysis following histological analysis of cannula placement. These pups were excluded due to incorrect cannula placements in the corpus callosum or overlying cortex rather than the dorsal hippocampus. Data from the remaining 23 pups are reported below.
Surgery
Our surgical procedure for weanling rat intrahippocampal cannula implantation has been described previously (see Watson, Herbert, & Stanton, 2008). Commercially-obtained cannulas (Plastics One, Roanoke, VA) were implanted bilaterally under stereotaxic guidance in the brains of weanling rats under ketamine/xylazine anesthesia (52.2–60.9 mg/kg ketamine/7.8–9.1 mg/kg xylazine in a 0.7 – 0.85 ml/kg injection volume). Buprenorphine (0.03 mg/kg in a volume of 0.05 ml/100 gm) was also administered subcutaneously to alleviate pain during and following the surgical procedure. The dorsal skull surface was exposed and small holes were drilled in the skull based on stereotaxic coordinates adjusted empirically from an atlas of the developing rat brain (Sherwood & Timiras, 1970). Guide cannulas were bilaterally implanted in the dHPC (AP + 2.6 mm, ML ± 2.3 mm, DV −1.8 mm). All AP and ML coordinates were based on interaural coordinates as measured from the horizontal zero plane, such that the ear bars and incisor bar were set to zero (Sherwood & Timiras, 1970). The cannula assembly was secured to the hooks implanted in the skull with dental acrylic at the end of surgery (Gilbert & Cain, 1980; Stanton & Freeman, 1994). Dummy cannulas were inserted into the guide cannula to prevent obstruction until infusions were made. Following antibiotic ophthalmic ointment application, rats were then returned to their home cages with food and water. Rats were monitored and kept warm until they recovered from anesthesia. Rats received 1 day of recovery prior to the deprivation procedure that started the T-maze protocol. This amount of recovery time has been found sufficient for weanling/juvenile rats having undergone stereotaxic surgery (Freeman, Rabinak, & Campolattaro, 2005; Watson, et al., 2008).
Drugs
The experiment involved administration of the non-competitive NMDA-receptor antagonist, dizocilpine (MK-801). MK-801 was purchased commercially from Tocris (Ellisville, MO). It was dissolved in sterile saline. (Treatment doses are described under drug infusion procedure below.)
Drug infusion procedure
The drug infusion procedure was performed as described by Watson et al. (2008). Five minutes prior to the start of each position habit training session, the awake rats were bilaterally intrahippocampally infused. Vehicle infusions were made before the acquisition session to all treatment groups, and MK-801 or vehicle was administered before the reversal session. Rats were gently held while the dummy cannulas were removed and an injection cannula was lowered through each guide cannula extending 1 mm below the tip. The injection cannula was connected to polyethylene tubing attached to a 10 μL Hamilton syringe mounted on a microinfusion pump. MK-801 was dissolved in sterile saline at a concentration of either 5 or 10 μg per μL and delivered at a rate of 0.5 μL per minute for 1 minute, for a total volume of 0.5 μL per side. This volume delivered either 2.5 or 5.0 μg of MK-801 per side, for the two concentrations of MK-801, respectively. The same volume of saline was used for the control infusions. One minute after infusion, the injection cannula were removed and replaced with the dummy cannulas. Rats were gently held during the entire infusion procedure (~ 2 min) to facilitate the microinfusions, while minimizing tangles in the tubing of the infusion pump apparatus.
Apparatus
As described by Freeman and Stanton (1991), subjects were trained in one of four Plexiglas T-mazes scaled to the size of weanling rats. Briefly, the T-mazes consisted of three equal length arms: a left and a right choice arm that were perpendicular to the start arm. The start arm was separated from a central choice point and the choice point was separated from the choice arms by pneumatically-operated doors.
Computer-controlled syringe pumps that dispensed the light cream reward (“Half & Half,” Cumberland Dairy, Rosenhayn, NJ) were connected to small metal cups that were located at the ends of both the left and right choice arms. When subjects broke a photoelectric beam placed in front of the feeding cup, the computer recorded the latency to make the response, lowered the maze doors, and when appropriate, activated the syringe pump (and delivered .07 ml light cream). Boxes of clear Plexiglas with hinged lids were used to house subjects between trials.
Design
Spatial discrimination reversal training consisted of a total of 96 trials over two sessions (4 12-trial blocks per session, Freeman & Stanton, 1991). The experimental design was a 3 (treatment) × 2 (phase) × 4 (trial blocks) mixed factorial design. (Sex, maze, and direction of acquisition were additional factors, but since they failed to reveal effects in preliminary ANOVAs that separately analyzed for each factor, 3 × 2 × 4 ANOVAs were performed; see Data Analysis below.) The rewarded goal arm, maze, and sex were counterbalanced across treatment groups. Littermates were assigned to treatment groups such that a maximum of 1 male and 1 female per litter contributed to a given group.
Procedure
All subjects underwent the following procedure of deprivation, maze acclimation, and training. The spatial discrimination and reversal procedures have been described in great detail elsewhere (Chadman, et al., 2006; Freeman & Stanton, 1991; Pagani, Brown, & Stanton, 2005). Briefly, subjects were weaned on P21, received cannula implantation on P23, were deprived on P24, acclimated on P25, and trained on P26.
During the deprivation procedure, subjects were deprived of food and water approximately 16 hr prior to initial T-maze exposure (usually between 1600 and 1700 hr). The pups were weighed to the nearest gram, tail-marked for identification, and a metal spoon was secured to one side of the cage. About 0.1 ml of the light cream reward was infused directly into each animal’s mouth and an additional 1 ml of cream was placed in the spoon. Pre-exposure to the Half & Half minimized taste-related neophobia when subjects were presented with the reward later during acclimation and training.
During acclimation training, pups learned to consume the light cream from the reward cups at the end of each choice arm during two goal-box training sessions and then to run in the maze during a forced-run session that followed. No intrahippocampal infusions occurred during maze acclimation.
During the spatial discrimination reversal sessions, infusions occurred 5 minutes before each session as described above. Subjects were trained in squads of four animals, with two pups assigned to each maze. Subjects were given a choice of maze arm such that reward was contingent upon choosing the correct arm (either right or left, counterbalanced across subsets of subjects). The subject was returned to its ITI box at the end of the trial. The pups were run in rotation such that the ITI for a given pup was the trial time for the other pup in the squad (~ 30 seconds). After acquisition, a 5 hour intersession interval was imposed to maintain adequate motivation and to eliminate the behavioral effects of morning drug administration (Chadman, et al., 2006) before the reversal session began. Reversal training was identical to acquisition in terms of procedure, except the subjects were rewarded for entrance into the opposite goal arm (i.e. if in acquisition the rewarded arm was the left; in reversal, the rewarded arm was right). The 48 trials in each training session were divided into 4 blocks of 12 trials to evaluate changes in performance within training sessions. At the end of the afternoon session, subjects were returned to ad libitum access to food and water.
Histological Analysis
The histological analysis procedure was performed as described by Watson et al. (2008). Briefly, within an average of 36.4 ± 3.12 hours (range: 24–72 hours) after completion of behavioral testing, pups were deeply anesthetized with an intraperitoneal injection of a ketamine/xylazine cocktail following a 0.5 μL injection of 2% pontamine skyblue dye solution through each guide cannula to show the position of the internal cannula tip. Animals were perfused intracardially with saline followed by formalin; brains were removed and post-fixed; and the following day, brains were placed in 30% sucrose in 10% buffered formalin. After the brains sank, coronal sections (40 μm) were taken using a cryostat (Leica CM3050 S), mounted on slides, and then counterstained with Neutral Red (1%). Slides were examined under a microscope for cannula tip placement.
Data Analysis
Body weight, choice run latencies, and the total percent of correct trials per block were collected for each subject during testing sessions. These measures were subjected to analysis of variance (ANOVA), as well as post-hoc paired comparisons (Newman-Keuls). The between-groups variables used in the analysis were sex (male or female), maze (1–4), treatment (2.5 μg MK-801, 5.0 μg MK-801, or saline), and direction of acquisition (left or right). The within-group variables were phase (acquisition or reversal) and blocks (4 blocks of 12 trials). No effect of sex, maze, or direction, was found in preliminary ANOVAs separately examining each factor, so the reported ANOVAs were combined across these factors.
Additional measures were also used to analyze the type of errors made during reversal training, from the total errors calculation. Total errors were categorized into either perseverative errors or regressive errors (Chadman, et al., 2006; Dias, Robbins, & Roberts, 1997; Ragozzino, Ragozzino, Mizumori, & Kesner, 2002). These previous studies have shown that blockade or inactivation of specific brain regions can affect the types of errors made during reversal learning. Perseverative errors provide a measure of the ability of the subject to shift away from a previously reinforced discrimination in acquisition (i.e. more perseverative errors imply an inability to shift away from the reinforced direction in acquisition). While, regressive errors provide a measure of the ability to effectively learn and maintain a new discrimination in reversal (i.e. more regressive errors imply an inability to learn the new discrimination in reversal). Perseverative errors were defined as incorrect choices 3 or more times in consecutive blocks of 4 trials. Fewer than 3 errors were classified as regressive errors. Trials to criterion (10 correct responses in 12 trials) were also calculated (Chadman, et al., 2006).
Results and Discussion
Histology – Cannula Placement
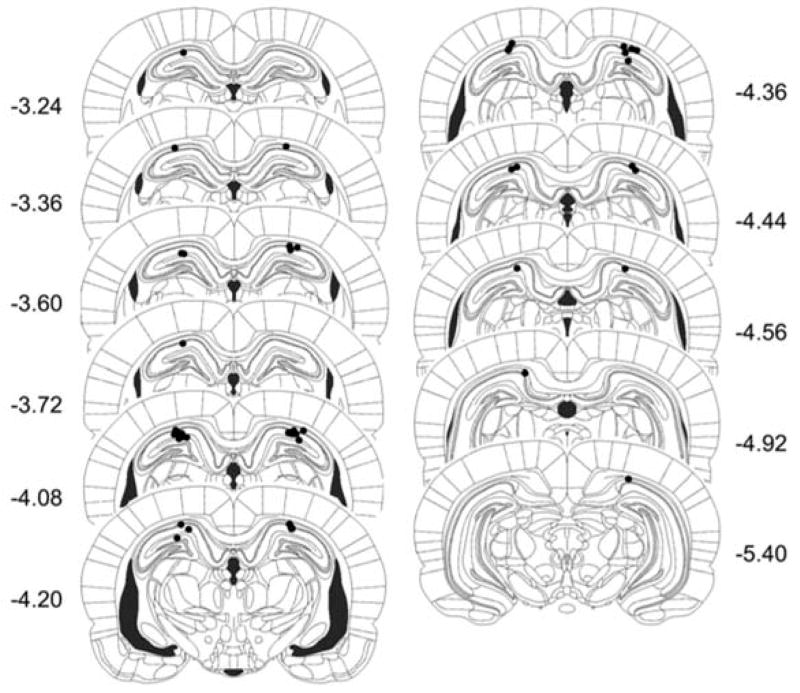
Rats were included in the analysis if the cannulas were located within or around the borders of the dorsal aspect of the hippocampus (7 excluded, 23 included; see included cannula placements in Figure 1). Thus, there were 8 animals in the saline group, 9 animals in the 2.5 μg MK-801 dosed group, and 6 animals in the 5.0 μg MK-801 group.
Figure 1.
Schematic representation of cannula placements targeted to the dHPC in Experiment 1. Coordinates represent distances posterior to Bregma (Paxinos & Watson, 2005).
Body Weight
Body weights at the start of Session 1, were 48.8 ± 1.40 g, 51.7 ± 2.20 g, 49.7 ± 1.58 g for the saline, 2.5 μg, and 5.0 μg MK-treated animals, respectively. ANOVA showed that body weight did not differ across treatment (F < 0.71).
Behavioral Effect of Intrahippocampal Administration of MK-801
Latency
A 3 (treatment) × 2 (phase) × 4 (trial blocks) repeated measures ANOVA performed on the latency data revealed small, but significant, main effects of phase, F (1, 20) = 13.24, p <.002; blocks, F (3, 60) = 8.97, p < .0001; and Phase × Blocks, F (3, 60) = 5.73, p < .002 but no main effects or interactions involving treatment (all F’s < 2.00). In general, reversal latencies were faster than acquisition latencies (Acq: 3.999 ± 0.174 s; Rev: 2.993 ± 0.072 s, respectively), and latency improved across blocks, especially in Blocks 1–3 in reversal (B1: 3.359 ± 0.236 s; B2: 2.960 ± 0.097 s; and B3: 2.857 ± 0.085 s, respectively) relative to acquisition (B1: 4.157 ± 0.274 s; B2: 4.737 ± 0.508 s; and B3: 3.738 ± 0.309 s, respectively). There was no evidence that the drug decreased motivation to perform the task.
Percent Correct Choice
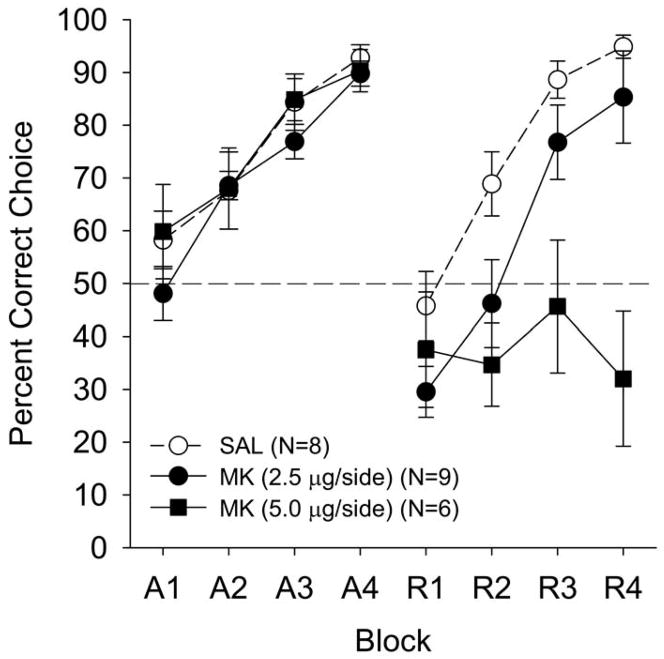
The percent correct choice data are shown as a function of drug treatment (Saline, 2.5 μg, and 5.0 μg MK-801 treated animals), and 12-trial blocks in Figure 2. Performance was greatly impaired in the 5.0 μg MK-801 treatment group during the reversal phase relative to the 2.5 μg MK-801 and vehicle treated animals. The saline-administered subjects readily acquired the reversal task. In contrast, performance of the 2.5 μg MK-801 group was moderately impaired and the 5.0 μg MK-801 group never performed above chance levels throughout training. There were no differences in acquisition performance (as expected because all groups received SAL in acquisition).
Figure 2.
Mean (±SE) percentage of correct responses for the three treatment groups beginning on P26 in Experiment 1 as a function of training phase (acquisition or reversal), 12-trial blocks, and dose. During the reversal phase only, the intrahippocampal treatment groups were vehicle (SALINE: open circles), or one of two drug doses (2.5 μg MK-801: closed circles; 5.0 μg MK-801: closed squares). All treatment groups received saline infusions during acquisition. Dashed line at 50 percent indicates chance performance.
A 3 (treatment) × 2 (phase) × 4 (blocks) mixed factorial ANOVA yielded main effects for treatment, F (2, 20) = 8.58, p < .002; phase, F (1, 20) = 22.47, p < .0001; blocks, F (3, 60) = 46.07, p < .0001; Blocks × Treatment, F (6, 60) = 3.52, p < .005; Phase × Treatment, F (2, 20) = 8.56, p < .002. More importantly, a Phase × Blocks × Treatment interaction was found, F (6, 60) = 2.61, p < .026. In general, the 2.5 μg MK-801 and 5.0 μg MK-801 treatment groups showed poor performance relative to the vehicle treated group only during reversal and this impairment emerged across blocks of reversal training. Newman-Keuls post-hoc analysis of the Phase × Treatment interaction revealed that the 5.0 μg MK-treated animals (37.46 ± 5.330 %) had a lower percent correct choice during reversal relative to the 2.5 μg MK- (59.47 ± 5.199 %) and saline-treated animals (74.53 ± 4.165 %) (p’s < .002), which marginally differed (p <.06). More importantly, Newman-Keuls post-hoc analysis of the Phase × Blocks × Treatment interaction revealed that the 5.0 μg MK-treated animals had a lower percent correct choice during Blocks 2–4 of reversal relative to saline-treated animals (p’s < .01); and Block 3–4 of reversal relative to the 2.5 μg MK-treated animals (p’s < .01). Finally, the 2.5 μg MK-treated group significantly differed from the SAL group in Block 2 (p < .01). Thus, a dose-response effect was found—such that the 5.0 μg MK-treated animals showed the most impaired performance throughout reversal training, with the moderately impaired 2.5 μg MK-treated animals surpassing the performance of the 5.0 μg MK-treated animals at the end of reversal training.
Error-type Analysis
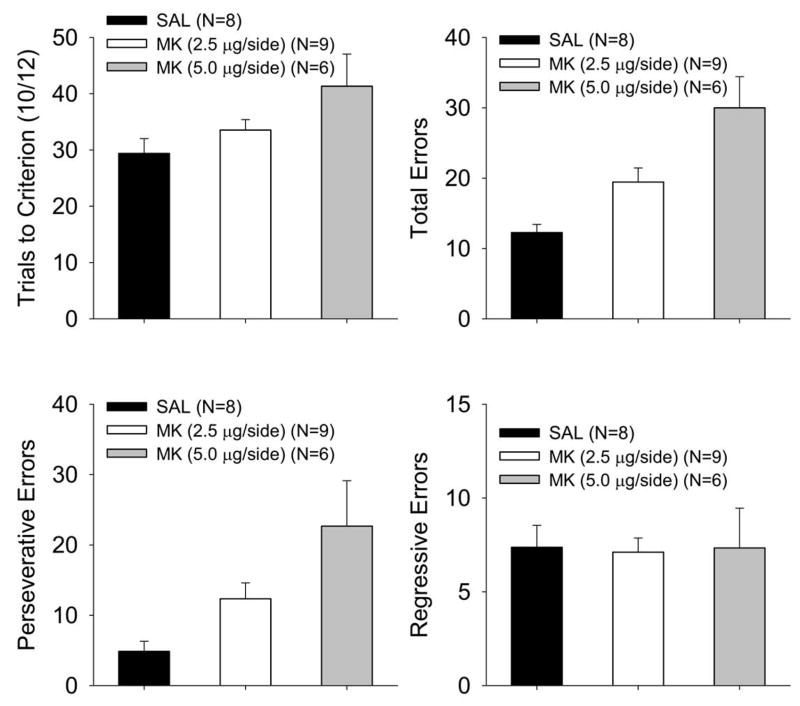
In order to characterize the types of errors made after MK-801 infusion in reversal (Figure 3), independent ANOVAs were performed on the trials to criterion (TTC), total errors, perseverative errors, and regressive errors (see Method, Data Analysis). For the TTC measure a marginal main effect of treatment was found, F (2, 20) = 3.05, p <.07. For the total errors and perseverative errors, a main effect of treatment was found [total: F (2, 20) = 11.34, p < .0005; persev: F (2, 20) = 6.25, p <.008]. The effect on total and perseverative errors was due to an increased number of errors made by the 5.0 μg MK-treated animals, relative to the 2.5 μg MK- and saline-treated animals, which marginally differed in total errors (p < .06) and did not differ in perseverative errors (p < .14). No effect of treatment was found for regressive errors, F < 0.01. To confirm that these findings were robust across parametric and nonparametric statistical models, Kruskal-Wallis tests confirmed treatment effects for total errors (p < .002), perseverative errors (p < .006), and regressive errors (p < .79). This analysis confirms the effects of ANOVA in all cases except TTC, where the marginal effect (p < .07) proved significant in the Kruskal-Wallis test (p < .05).
Figure 3.
Analysis of trials to criterion (TTC) and error types during reversal for P26 rats as a function of dose of MK-801 in Experiment 1. Mean (±SE) for TTC (A), total errors (B), perseverative errors (C), and regressive errors (D).
Experiment 2
Experiment 1 clearly established that intrahippocampal NMDA-receptor antagonism dose-dependently impairs reversal learning performance in weanling rats. Experiment 2 was designed to determine whether this MK-801-induced impairment is specific to reversal learning. Experiment 2 evaluated the effect of MK-801 administration on acquisition of T-maze discrimination, as well as the effect of a change in drug condition across acquisition and reversal phases (state-dependent learning). Changes in contextual cues typically improve reversal performance in weanling rat pups (Moye, Brasser, Palmer, & Zeisset, 1992; Pagani, et al., 2005). The state-dependent learning hypothesis therefore predicts greater performance impairment when MK-801 is administered before both learning phases, than when it is administered before reversal only. Internal drug-related cues might create a stimulus change between phases, serving to release rats from proactive interference between acquisition and reversal, thereby improving performance during the reversal phase. MK-801 administration does not state-dependently impair reversal performance in weanling rats when the drug is given systemically (Chadman, et al., 2006). Thus, we predicted that intrahippocampal MK-801 administration would not produce state-dependent learning effects.
The lowest effective dose of MK-801 from the previous experiment was used in the present study. Four treatment groups received 8 blocks of T-maze training (4 12-trial blocks in acquisition and 4 in reversal). All groups received bilateral infusions in dorsal hippocampus (0.25 μl per side). MK-801 (2.5 μg per side) was administered before either acquisition or reversal learning phases, before both learning phases, or before neither session. We predicted that if hippocampal NMDA receptors are specifically involved in reversal learning—then intrahippocampal MK-801 administration will not impair acquisition, but will impair reversal regardless of drug treatment during acquisition. State-dependent learning effects would appear as enhanced reversal in the groups that experienced a change in drug condition across the acquisition and reversal phases.
Method
The methods, apparatus, etc. in Experiment 2 were the same as those detailed for Experiment 1 except where noted below.
Subjects
Fifty-nine (29 female, 30 male) Long-Evans rat pups derived from 16 litters served as subjects. The surgical procedure began on P23 for all subjects. The average weight at deprivation for subjects was 60.2 ± 0.93 g (range: 46.0 – 76.0 g). A 2 (acquisition treatment) × 2 (reversal treatment) factorial ANOVA was performed on the deprivation weight data that did not reveal differences among subjects in different treatment groups (F’s < 1.65). No more than one same-sex littermate was assigned to a given experimental group.
Four pups were discarded from analysis due to experimental error. Of the remaining 55 pups, 5 were excluded from further analysis following histological analysis of cannula placement. These pups were excluded due to incorrect cannula placements in the corpus callosum or overlying cortex rather than the dorsal hippocampus (see Results below). Data from the remaining 50 pups are reported below.
Surgery
The surgical details for cannula implantation are the same as previously described with the exception that guide cannulas were bilaterally implanted in the dHPC (AP + 2.6 mm, ML ± 2.3 mm, DV −1.8 (n=2) or −2.0 mm (n=48)). The DV coordinate of −2.0 mm was used instead of −1.8 mm in Experiment 1 in order to reduce the number of cannula placements that were too dorsal.
Design
The experimental design was a 2 (acquisition treatment) × 2 (reversal treatment) × 2 (phase) × 4 (trial blocks) mixed factorial design. (Sex, maze, and direction of acquisition were additional factors, but since they failed to reveal effects in separate ANOVAs, 2 × 2 × 2 × 4 ANOVAs were performed; see Data Analysis below.) The acquisition treatment × reversal treatment factorial yielded four main groups, designated SAL-SAL, MK-SAL, SAL-MK and MK-MK (where acquisition treatment appears before the hyphen, and reversal drug treatment appears after the hyphen).
Drug infusion procedure
The drug infusion procedure was the same as Experiment 1, except that MK-801 was dissolved in sterile saline at a concentration of 10 μg per μL. MK-801 was delivered at a rate of 0.25 μL per minute for 1 minute, for a total volume of 0.25 μL per side. This volume delivered 2.5 μg of MK-801 per side. All other procedures were the same as detailed in Experiment 1.
Results and Discussion
Histology – Cannula Placement
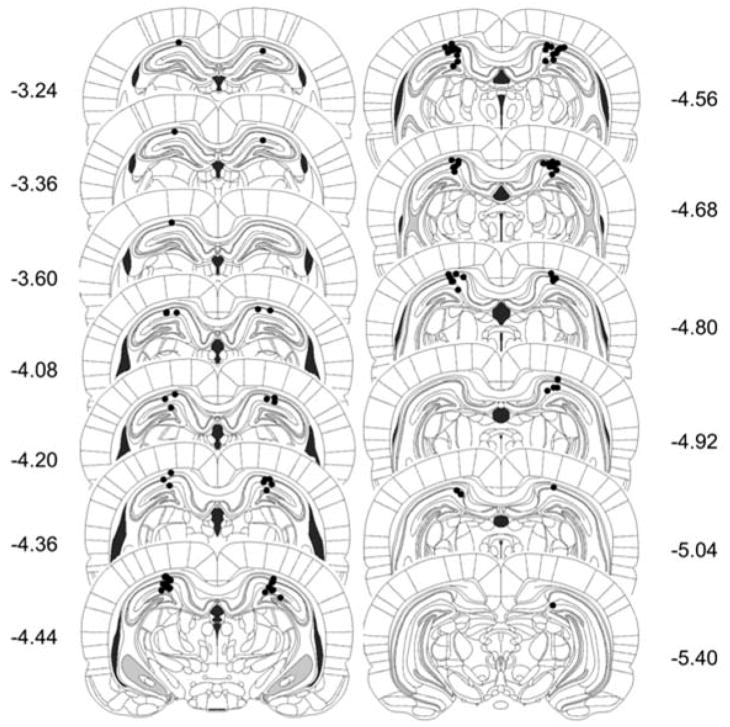
Rats were included in the analysis if the cannulas were located within or around the borders of the dorsal aspect of the hippocampus (5 excluded, 50 included; see included cannula placements in Figure 4). Thus, group sizes were as follows: SAL-SAL, n=13; SAL-MK, n=11; MK-SAL, n=12; and MK-MK, n=14.
Figure 4.
Schematic representation of cannula placements targeted to the dHPC in Experiment 2. Coordinates represent distances posterior to Bregma (Paxinos & Watson, 2005).
Body Weight
Body weights at Session 1 were 50.3 ± 1.96 g, 50.5 ± 1.41 g, 54.3 ± 1.80 g, and 51.4 ± 1.66 g for the SAL-SAL, SAL-MK, MK-SAL, and MK-MK treated animals, respectively. A 2 (acquisition treatment) × 2 (reversal treatment) mixed factorial ANOVA found no differences across these groups (F’s < 1.91).
Behavioral Effect of Intrahippocampal Administration of MK-801
Latency
A 2 (acquisition treatment) × 2 (reversal treatment) × 2 (phase) × 4 (trial blocks) repeated measures ANOVA performed on the latency data (see Table 1) revealed small, but significant, main effects of phase, F (1, 46) = 20.30, p <.0001; and blocks, F (3, 138) = 9.90, p < .0001; as well as interactions between Phase × Acquisition treatment, F (1, 46) = 7.51, p < .009; and Phase × Reversal treatment, F (1, 46) = 11.08, p < .002. As in Experiment 1, reversal latencies were faster than acquisition latencies (Acq: 3.841 ± 0.156 s; Rev: 3.087 ± 0.060 s, respectively) and latency improved across blocks (B1: 3.970 ± 0.184 s; B2: 3.771 ± 0.238 s; B3: 3.155 ± 0.129 s; and B4: 2.959 ± 0.060 s, respectively). Newman-Keuls analyses of the both the Phase × Acquisition treatment and Phase × Reversal treatment interaction revealed that the SAL group had slower latencies relative to the MK treated animals (p’s < .025). There was no evidence that the drug decreased motivation to perform the task as the latency effects did not mimic the effects on percent correct choice (see below).
Table 1.
Mean (+SE) of choice run latencies for the four treatment groups beginning on P26 in Experiment 2 as a function of training phase (acquisition or reversal), 12-trial blocks, and dose.
| ACQUISITION |
REVERSAL |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Block 1 | Block 2 | Block 3 | Block 4 | Block 1 | Block 2 | Block 3 | Block 4 | n | |
| SAL-SAL | 4.862 ± 0.676 | 4.108 ± 0.323 | 3.502 ± 0.215 | 3.402 ± 0.174 | 3.695 ± 0.381 | 3.694 ± 0.422 | 3.212 ± 0.382 | 2.832 ± 0.151 | 13 |
| SAL-MK | 5.887 ± 1.112 | 4.838 ± 0.580 | 4.311 ± 0.979 | 3.501 ± 0.328 | 2.998 ± 0.177 | 2.679 ± 0.058 | 2.680 ± 0.070 | 2.621 ± 0.060 | 11 |
| MK-SAL | 3.673 ± 0.208 | 3.356 ± 0.261 | 2.868 ± 0.088 | 2.809 ± 0.112 | 3.930 ± 0.312 | 3.658 ± 0.209 | 3.075 ± 0.130 | 2.859 ± 0.060 | 12 |
| MK-MK | 3.851 ± 0.209 | 4.905 ± 1.492 | 2.951 ± 0.091 | 2.960 ± 0.138 | 3.062 ± 0.097 | 2.868 ± 0.086 | 2.764 ± 0.059 | 2.720 ± 0.073 | 14 |
Percent Correct Choice
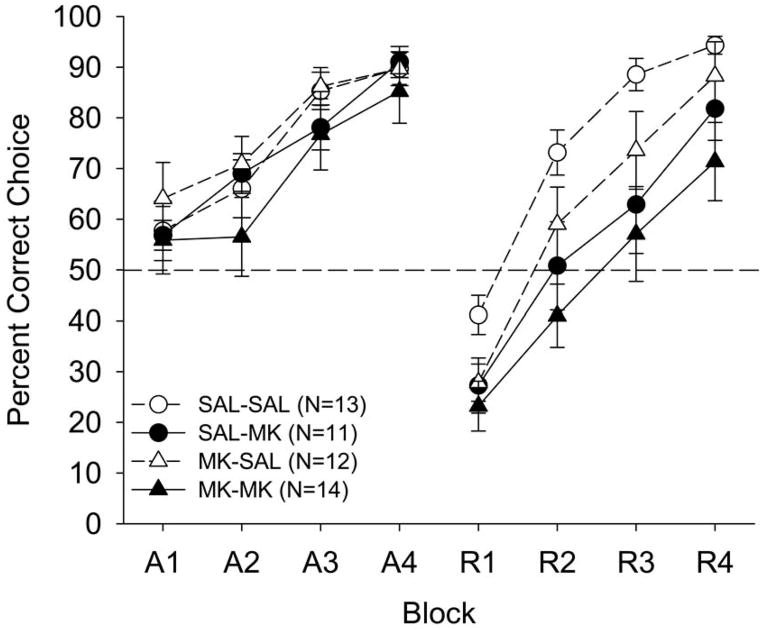
The percent correct choice data are shown as a function of drug treatment and 12-trial blocks in Figure 5. There were no differences in acquisition performance following MK-801 administration but, during reversal, MK-801 treated groups were impaired relative to vehicle-treated groups.
Figure 5.
Mean (±SE) percentage of correct responses for the four MK-801 (2.5 μg) treatment groups in Experiment 2 beginning on P26 as a function of training phase (acquisition or reversal), 12-trial blocks, and treatment. The intrahippocampal treatment groups were saline-saline (SAL-SAL: open circles), saline-MK-801 (SAL-MK: closed circles), MK-801-SAL (MK-SAL: open triangles), or MK-801-MK-801 (MK-MK: closed triangles). Dashed line at 50 percent indicates chance performance.
A 2 (acquisition treatment) × 2 (reversal treatment) × 2 (phase) × 4 (blocks) mixed factorial ANOVA yielded main effects for reversal treatment, F (1, 46) = 13.00, p < .0008; phase, F (1, 46) = 12.81, p < .0008; blocks, F (3, 138) = 159.55, p < .0001; and Phase × Blocks, F (3, 138) = 13.03, p < .0001. The reversal treatment main effect showed that the MK-treated (61.2 ± 2.11 %) rats had worse performance during reversal relative to the SAL-treated rats (72.3±1.78 %). The ANOVA did not reveal significant interactions involving acquisition treatment, but a marginal main effect of acquisition treatment was found, F (1, 46) = 3.39, p < .07, such that the MK-treated (63.7 ± 2.10 %) rats had worse performance in general relative to the SAL-treated rats (70.0 ± 1.81 %). To further characterize these treatment effects, separate 2 (acquisition treatment) × 2 (reversal treatment) × 4 (blocks) ANOVAs were run on each phase. During acquisition, only a main effects of blocks, F (3, 138) = 47.22, p < .0001, was found, indicating that all treatment groups acquired the position discrimination task at the same rate. During the reversal phase, ANOVA revealed main effects of reversal treatment, F (1, 46) = 9.33, p < .004, and blocks, F (3, 138) = 125.21, p < .0001, indicating that the drug impaired reversal performance (as in Experiment 1). During reversal, a marginal main effect of acquisition treatment was also found, F (1, 46) = 3.40, p < .07, which suggests that it may be hazardous to conclude that MK-801 treatment in acquisition failed entirely to influence performance during reversal (see General Discussion). No other main effects or interactions between treatment groups were significant in either the acquisition or reversal phase.
To confirm that these findings were robust across parametric and nonparametric statistical models, Kruskal-Wallis tests confirmed the non-significant treatment effects in acquisition performance averaged across blocks (p < .78) and significant treatment effects for reversal performance averaged across blocks (p < .006)—which were due to differences between the MK-MK and SAL-SAL groups (p < .005). No other comparisons between treatment groups were significant in either phase (p’s > .09). That treatment effects were found in reversal but not acquisition confirms that the drug interfered with processes related specifically to reversal learning rather than T-maze learning in general (Chadman et al., 2006).
MK-801 administration did not have a state dependent effect. As noted previously, changing contextual cues across acquisition versus reversal phases improves reversal performance (Pagani et al., 2005). However, previous MK-801 administration during acquisition did not improve performance during the reversal phase for the MK-SAL group relative to the SAL-SAL group. Previous saline exposure in the SAL-MK group did not enhance performance relative to the SAL-SAL group. Finally, there were no differences between a double (MK-MK) or a single (SAL-MK) MK-801 administration on reversal performance. Thus, state dependency learning effects, such as an alteration in context resulting from a change in drug cues, cannot explain our effects. Our main conclusion is that reversal learning, not initial acquisition, is more sensitive to NMDA-receptor antagonism confined to dorsal hippocampus.
Error-type Analysis
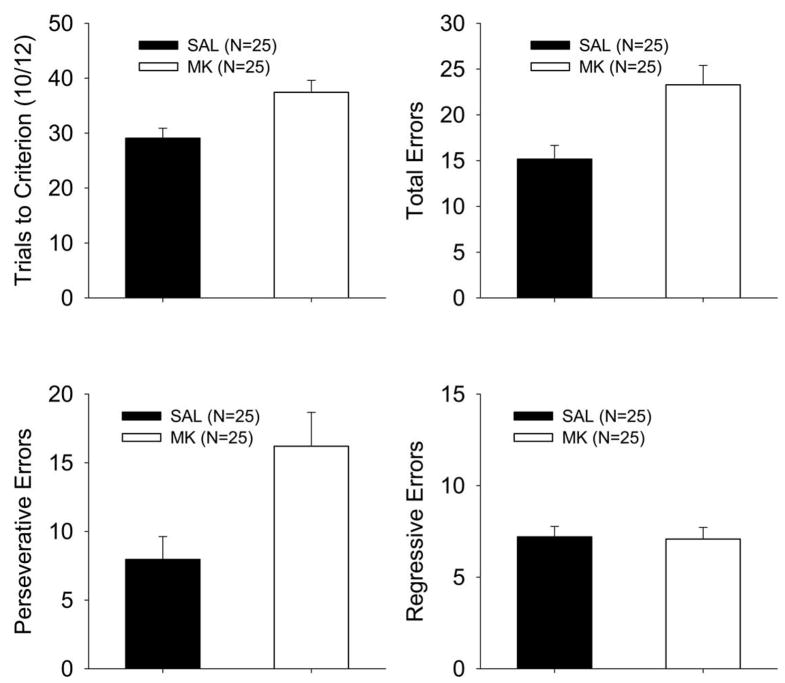
In order to further characterize the performance after MK-801 infusion during reversal training, independent ANOVAs were performed on the TTC, total errors, perseverative errors, and regressive errors data (Figure 6). Because there were no main or interactions effects involving acquisition treatment, data are shown pooled across this factor. For the TTC, total errors, and perseverative errors measure, a main effect of reversal treatment was found [TTC: F (1, 46) = 8.48, p < .006; total: F (1, 46) = 9.29, p < .004; persev: F (1, 46) = 7.06, p <.011]. No other main effects or interactions were significant (F’s < 3.36). The effect on TTC, total, and perseverative errors was due to an increased number of trials and errors by the MK-treated animals, relative to the saline-treated animals. No main effects or interactions were found for regressive errors, F’s < 0.62. MK-801 treatment during the reversal phase increased the amount of perseveration on the direction trained in acquisition, which generally impaired reversal performance relative to vehicle treated animals. As in Experiment 1, robustness of these results across statistical models was confirmed by nonparametric Kruskal-Wallis tests on the four treatment groups. These tests also revealed effects of reversal treatment on TTC [p < .013; mean ranks: SAL-SAL (15.2), SAL-MK (30.6), MK-SAL (24.3), and MK-MK (32.0)], total errors [p < .007; SAL-SAL (15.0), SAL-MK (29.5), MK-SAL (23.8), and MK-MK (33.7)], perseverative errors [p < .006; SAL-SAL (14.4), SAL-MK (28.8), MK-SAL (24.9), and MK-MK (33.7)], and regressive errors [p < .89; SAL-SAL (26.2), SAL-MK (27.9), MK-SAL (25.0), and MK-MK (23.4)]. For TTC, total errors, and perseverative errors significant comparisons were found between MK-MK and SAL-SAL treatment groups (p’s < .017); no other comparisons were significant (p’s > .059).
Figure 6.
Analysis of trials to criterion (TTC) and error types during reversal for P26 rats as a function of dose of MK-801 in Experiment 2. Differences were not found between a first (SAL-MK) or second (MK-MK) infusion of MK-801during reversal, thus the SAL-MK and MK-MK treatment groups were pooled into Group MK (open bars), and the saline groups (MK-SAL and SAL-SAL) were pooled into Group SAL (closed bars). Mean (±SE) for TTC (A), total errors (B), perseverative errors (C), and regressive errors (D).
General Discussion
Two experiments evaluated the effects of intrahippocampal NMDA-receptor antagonism on spatial discrimination reversal learning in developing rats. Experiment 1 demonstrated that hippocampal administration of MK-801 disrupted reversal learning performance in P26 rats. A dose-dependent effect was found such that 2.5 μg MK-801 moderately impaired reversal performance and 5.0 μg MK-801 severely impaired reversal learning. Experiment 2 determined that the MK-801 (2.5 μg) impairment was specific to the reversal learning phase. Interestingly, learning during the acquisition phase was completely intact following NMDA receptor antagonism. State-dependent learning effects, cannot explain the reversal learning impairment, because groups receiving a shift in drug treatment across acquisition and reversal phases did not differ significantly from their counterparts (matched for reversal drug) that did not experience a shift in drug treatment.
Ontogeny of spatial discrimination and reversal learning has been demonstrated in pre-and post-weanling rats between the ages of P7–30 (Green & Stanton, 1989; Kenny & Blass, 1977; Moye, et al., 1992; Pagani, et al., 2005; Smith & Bogomolny, 1983). Reversal learning, not initial acquisition, is NMDA-receptor dependent in post-weanling rat pups (Chadman, et al., 2006; Griesbach, et al., 1998). The reversal impairment in these studies was not due to disruption of the basic sensory, motor, or motivational processes necessary for position habit learning, as acquisition of the spatial discrimination was not impaired by systemic MK-801 administration. The current findings suggest that hippocampal effects of MK-801 are sufficient to account for the effects of systemic drug administration established previously (Chadman, et al., 2006). These findings extend our previous developmental work by showing, for the first time, that hippocampal function is specifically involved in reversal learning in weanling rats.
In developing animals, systemically administered MK-801, disrupts nonspatial working memory—patterned single alternation—in a runway (Griesbach & Amsel, 1998; Highfield, et al., 1996), olfactory discrimination and reversal in a Y-maze (Griesbach, et al., 1998), and spatial discrimination reversal in a T-maze (Chadman, et al., 2006). Amsel and colleagues reported that a moderate MK-801 dose (0.05 mg/kg) disrupted olfactory discrimination and its reversal in P22–28 rats. In addition to differences in drug administration routes (intrahippocampal vs. systemic), this finding contrasts with the current findings, in which hippocampal administration of MK-801 in Experiment 2 did not disrupt acquisition learning. Differences in task difficulty or sensory requirements between spatial and olfactory reversal learning may account for these behavioral effects. Spatial discrimination reversal learning was selectively impaired by systemic administration of a high dose of MK-801 (0.10 mg/kg, i.p.) before both acquisition and reversal learning phases in P21–30 rat pups without impairing acquisition of the position habit and there was no evidence that drug treatment in acquisition influenced reversal performance (Chadman, et al., 2006). The current findings with intrahippocampally administered MK-801 mirror these previous findings involving systemic administration in every respect, except that there was a marginally significant tendency for MK-801 treatment in acquisition to influence performance during the reversal session. Whether this difference across studies is reliable and what the basis for it might be remains unclear. In any case, there is converging evidence that MK-801 seems to interfere with processes related specifically to reversal learning rather than position habit learning in general (Chadman et al., 2006). There is also agreement that impairment of reversal by systemically or intrahippocampally administered MK-801 is not due to state-dependent learning effects.
In this set of studies, acquisition of spatial position discrimination did not appear to depend on NMDA receptor systems in the hippocampus. However, a clear role for hippocampal NMDA receptors in the reversal learning phase was established (Experiment 2). The dye infusion in Experiment 2 was restricted to the dorsal hippocampus and did not spread to extrahippocampal areas, suggesting that reversal performance was disrupted by specific targeting of the hippocampus, not surrounding areas.
Thus far, there are no studies that have directly evaluated the effects of intrahippocampal infusion of NMDA receptor antagonists on spatial reversal learning in developing or adult rats. In adult rats and mice, the systemic MK-801 administration studies (Bardgett, et al., 2003; Murray, Ridley, Snape, & Cross, 1995), in conjunction with lesion studies, have clearly established a role for the hippocampal system in spatial discrimination reversal learning (Bardgett, et al., 2003; Kimble, 1968; Kimble & Kimble, 1965; Oliveira, et al., 1997). Systemic MK-801 administration also impairs other forms of spatial learning in adult rats, including Morris Water Maze learning and reversal (Ahlander, Misane, Schott, & Ogren, 1999), spatial serial reversal (van der Meulen, Bilbija, Joosten, de Bruin, & Feenstra, 2003), radial arm maze learning (Caramanos & Shapiro, 1994; Shapiro & O’Connor, 1992; Woodside, Borroni, Hammonds, & Teyler, 2004), conditional visuo-spatial learning (Murray & Ridley, 1997), cognitive-set shifting (Stefani & Moghaddam, 2005a; Stefani & Moghaddam, 2005b), spontaneous alternation in a radial arm maze (Homayoun, Stefani, Adams, Tamagan, & Moghaddam, 2004), and spatial delayed alternation (Hauber, 1993; Locchi, Dall’olio, Gandolfi, & Rimondini, 2007; Verma & Moghaddam, 1996). The results from these studies combined provide support for the well-accepted theory of the role of the hippocampus in spatial learning tasks. The present findings extend this role to weanling rats and to spatial reversal learning (see also Watson, et al., 2008).
Debate over theories of hippocampal function have dominated the field of the neurobiology of learning and memory for nearly half a century (Morris, 2007). These theories include declarative memory theory (Squire, 2004), cognitive map theory (O’Keefe & Nadel, 1978), configural-association theory (Rudy & Sutherland, 1995), relational-processing theory (Eichenbaum & Cohen, 2001), contextual theory (Bouton, 2004; Fanselow, 2000; Good & Honey, 1991), and neurobiological theory (Morris, 2006). Considering the wealth data available on behavioral functions of the hippocampus (the current results included), it is a difficult challenge for any one theory to adequately cover all the findings (for review see Morris, 2007). The challenge presented by the present findings is to explain the role of the hippocampus in reversal but not acquisition of T-maze discrimination. Of recently proposed theories, a promising one is predicted ambiguity theory, which encompasses configural, relational-processing, and contextual theories of hippocampal function (Morris, 2007). Each of these sub-theories emphasize the fact that a stimulus can mean one thing in one situation but something else in another (Morris, 2007). For example, it is possible that the role that the hippocampus plays during the initial learning phase in spatial discrimination reversal is “incidental” and not necessary for behavioral performance; however, when the contingencies change during the reversal learning phase, the hippocampal processes are “intentional” or “contingent” and thus essential for learning the task (Good, de Hoz, & Morris, 1998). Additional empirical work is required to fully characterize what hippocampal-dependent cognitive processes contribute to this reversal deficit. The present findings suggest that these processes operate as early as P26 in the rat.
Although the present findings indicate that hippocampal NMDA receptors play a role in T-maze reversal, it should be kept in mind that other brain systems also likely play a role in reversal learning and other complex learning tasks (i.e. set-shifting). For example, NMDA receptor-dependent prefrontal cortical contribution to discrimination learning and set-shifting has been demonstrated in adult rats in a modified plus-maze (Stefani & Moghaddam, 2005a). In addition to medial prefrontal cortex—lesion, temporary inactivation, and infusion of NMDA receptor antagonists into the dorsal striatum impair reversal learning in adult rats in a modified plus-maze (Ragozzino, 2007).
Learning problems, including deficits in spatial working memory, behavioral flexibility, and executive function are central to neurodevelopmental diseases, such as schizophrenia, fetal alcohol syndrome, and autism (Coldren & Halloran, 2003; Kodituwakku, 2007; Steele, Minshew, Luna, & Sweeney, 2007). NMDA receptor hypofunction may be one underlying cause of the deficits seen in schizophrenia and autism (Carlsson, 1998; Laruelle, Kegeles, & Abi-Dargham, 2003; Rowland, Astur, Jung, Bustillo, Lauriello, & Yeo, 2005). The current finding that the dorsal hippocampus is necessary for spatial reversal learning during development may be relevant to animal models of autism and schizophrenia.
Acknowledgments
This research was supported in part by the University of Delaware, NIH grants 1-R01-AA11945 and 1-PO1-HD35466 to MES, and NRSA fellowship, F31 MH079635 to DJW. The authors would like to thank Jennifer Burr, Jenna Cohen, and Andrea Kaiser for technical assistance. Correspondence concerning this article should be addressed to Deborah J. Watson, Department of Psychology, University of Delaware, Newark, DE 19716, dwatson@psych.udel.edu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlander M, Misane I, Schott PA, Ogren SO. A behavioral analysis of the spatial learning deficit induced by the NMDA receptor antagonist MK-801 (dizocilpine) in the rat. Neuropsychopharmacology. 1999;21(3):414–26. doi: 10.1016/S0893-133X(98)00116-X. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Boeckman R, Krochmal D, Fernando H, Ahrens R, Csernansky JG. NMDA receptor blockade and hippocampal neuronal loss impair fear conditioning and position habit reversal in C57Bl/6 mice. Brain Research Bulletin. 2003;60(1–2):131–42. doi: 10.1016/s0361-9230(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annual Review of Neuroscience. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning and Memory. 2004;11(5):485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Caramanos Z, Shapiro ML. Spatial memory and N-methyl-D-aspartate receptor antagonists APV and MK-801: memory impairments depend on familiarity with the environment, drug dose, and training duration. Behavioral Neuroscience. 1994;108(1):30–43. doi: 10.1037//0735-7044.108.1.30. [DOI] [PubMed] [Google Scholar]
- Carlsson ML. Hypothesis: is infantile autism a hypoglutamatergic disorder? Relevance of glutamate - serotonin interactions for pharmacotherapy. Journal of Neural Transmission. 1998;105(4–5):525–35. doi: 10.1007/s007020050076. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Watson DJ, Stanton ME. NMDA receptor antagonism impairs reversal learning in developing rats. Behavioral Neuroscience. 2006;120(5):1071–83. doi: 10.1037/0735-7044.120.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldren JT, Halloran C. Spatial reversal as a measure of executive functioning in children with autism. Journal of Genetic Psychology. 2003;164(1):29–41. doi: 10.1080/00221320309597501. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Current Opinion in Neurobiology. 2001;11(3):327–35. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. Journal of Neuroscience. 1997;17(23):9285–97. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection. Oxford: Oxford University Press; 2001. [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research. 2000;110(1–2):73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Rabinak CA, Campolattaro MM. Pontine stimulation overcomes developmental limitations in the neural mechanisms of eyeblink conditioning. Learning and Memory. 2005;12(3):255–9. doi: 10.1101/lm.91105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Stanton ME. Fimbria-fornix transections disrupt the ontogeny of delayed alternation but not position discrimination in the rat. Behavioral Neuroscience. 1991;105(3):386–395. doi: 10.1037//0735-7044.105.3.386. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Cain DP. Electrode implantation in infant rats for kindling and chronic brain recording. Behavioural Brain Research. 1980;1(6):553–5. doi: 10.1016/0166-4328(80)90010-8. [DOI] [PubMed] [Google Scholar]
- Good M, de Hoz L, Morris RG. Contingent versus incidental context processing during conditioning: dissociation after excitotoxic hippocampal plus dentate gyrus lesions. Hippocampus. 1998;8(2):147–59. doi: 10.1002/(SICI)1098-1063(1998)8:2<147::AID-HIPO7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Good M, Honey RC. Conditioning and contextual retrieval in hippocampal rats. Behavioral Neuroscience. 1991;105(4):499–509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- Green RJ, Stanton ME. Differential ontogeny of working memory and reference memory in the rat. Behavioral Neuroscience. 1989;103(1):98–105. doi: 10.1037//0735-7044.103.1.98. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Amsel A. Immediate and long-term effects of neonatal MK-801 treatment on nonspatial learning. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(19):11435–9. doi: 10.1073/pnas.95.19.11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach GS, Hu D, Amsel A. Effects of MK-801 on vicarious trial-and-error and reversal of olfactory discrimination learning in weanling rats. Behavioural Brain Research. 1998;97(1–2):29–38. doi: 10.1016/s0166-4328(98)00015-1. [DOI] [PubMed] [Google Scholar]
- Harris K. Structure, development, and plasticity of dendritic spines. Current Opinion in Neurobiology. 1999;9(3):343–8. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Harris K, Teyler T. Developmental onset of long-term potentiation in area CA1 of the rat hippocampus. Journal of Physiology. 1984;346:27–48. doi: 10.1113/jphysiol.1984.sp015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W. Clozapine improves dizocilpine-induced delayed alteration impairment in rats. Journal of neural transmission General section. 1993;94(3):223–33. doi: 10.1007/BF01277027. [DOI] [PubMed] [Google Scholar]
- Highfield DA, Nixon K, Amsel A. The NMDA antagonist MK-801 affects nonspatial learning in preweanling rats. Behavioral Neuroscience. 1996;110(2):300–4. doi: 10.1037//0735-7044.110.2.300. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacology. 2004;29(7):1259–69. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Kenny JT, Blass EM. Suckling as incentive to instrumental learning in preweanling rats. Science. 1977;196(4292):898–9. doi: 10.1126/science.860121. [DOI] [PubMed] [Google Scholar]
- Kimble DP. Hippocampus and internal inhibition. Psychol Bull. 1968;70(5):285–95. doi: 10.1037/h0026470. [DOI] [PubMed] [Google Scholar]
- Kimble DP, Kimble RJ. Hippocampectomy and response perseveration in the rat. Journal of Comparative and Physiological Psychology. 1965;60(3):474–6. doi: 10.1037/h0022550. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neuroscience and Biobehavioral Reviews. 2007;31(2):192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Annals of the New York Academy of Sciences. 2003;1003:138–58. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- Locchi F, Dall’olio R, Gandolfi O, Rimondini R. Water T-maze, an improved method to assess spatial working memory in rats: Pharmacological validation. Neuroscience Letters. 2007;422(3):213–6. doi: 10.1016/j.neulet.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morris RG. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. European Journal of Neuroscience. 2006;23(11):2829–46. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Morris RG. Theories of hippocampal function. In: Andersen P, Morris RG, Amaral D, Bliss T, O’Keefe J, editors. The hippocampus book. Oxford: Oxford University Press; 2007. [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319(6056):774–6. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O’Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2003;358(1432):773–86. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye TB, Brasser SM, Palmer L, Zeisset C. Contextual control of conflicting associations in the developing rat. Developmental Psychobiology. 1992;25(3):151–64. doi: 10.1002/dev.420250302. [DOI] [PubMed] [Google Scholar]
- Murray TK, Ridley RM. The effect of dizocilpine (MK-801) on conditional discrimination learning in the rat. Behavioural Pharmacology. 1997;8(5):383–8. [PubMed] [Google Scholar]
- Murray TK, Ridley RM, Snape MF, Cross AJ. The effect of dizocilpine (MK-801) on spatial and visual discrimination tasks in the rat. Behavioural Pharmacology. 1995;6(5 And 6):540–549. [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Oxford University Press; 1978 . [Google Scholar]
- Oliveira MG, Bueno OF, Pomarico AC, Gugliano EB. Strategies used by hippocampal- and caudate-putamen-lesioned rats in a learning task. Neurobiology of Learning and Memory. 1997;68(1):32–41. doi: 10.1006/nlme.1996.3761. [DOI] [PubMed] [Google Scholar]
- Pagani JH, Brown KL, Stanton ME. Contextual modulation of spatial discrimination reversal in developing rats. Developmental Psychobiology. 2005;46(1):36–46. doi: 10.1002/dev.20041. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy of Sciences. 2007;1121:355–75. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral Neuroscience. 2002;116(1):105–15. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Astur RS, Jung RE, Bustillo JR, Lauriello J, Yeo RA. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology. 2005;30(3):633–9. doi: 10.1038/sj.npp.1300642. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: an appraisal and reconfiguration. Hippocampus. 1995;5(5):375–89. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- Shapiro M. Plasticity, hippocampal place cells, and cognitive maps. Archives of Neurology. 2001;58(6):874–81. doi: 10.1001/archneur.58.6.874. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, O’Connor C. N-methyl-D-aspartate receptor antagonist MK-801 and spatial memory representation: working memory is impaired in an unfamiliar environment but not in a familiar environment. Behavioral Neuroscience. 1992;106(4):604–12. doi: 10.1037//0735-7044.106.4.604. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Timiras PS. A stereotaxic atlas of the developing rat brain. Berkeley: University of California Press; 1970. [Google Scholar]
- Smith GJ, Bogomolny A. Appetitive instrumental training in preweanling rats: I. Motivational determinants. Developmental Psychobiology. 1983;16(2):119–28. doi: 10.1002/dev.420160205. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiology of Learning and Memory. 2004;82(3):171–7. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH., Jr Eyeblink conditioning in the infant rat: an animal model of learning in developmental neurotoxicology. Environmental Health Perspectives. 1994;2(102 Suppl):131–9. doi: 10.1289/ehp.94102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele SD, Minshew NJ, Luna B, Sweeney JA. Spatial working memory deficits in autism. Journal of Autism and Developmental Disorders. 2007;37(4):605–12. doi: 10.1007/s10803-006-0202-2. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behavioral Neuroscience. 2005a;119(2):420–8. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biological Psychiatry. 2005b;57(4):433–6. doi: 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Perkins ATt, Harris K. The development of long-term potentiation in hippocampus and neocortex. Neuropsychologia. 1989;27(1):31–9. doi: 10.1016/0028-3932(89)90088-2. [DOI] [PubMed] [Google Scholar]
- Vallano ML. Developmental aspects of NMDA receptor function. Critical Reviews in Neurobiology. 1998;12(3):177–204. doi: 10.1615/critrevneurobiol.v12.i3.20. [DOI] [PubMed] [Google Scholar]
- van der Meulen JA, Bilbija L, Joosten RN, de Bruin JP, Feenstra MG. The NMDA-receptor antagonist MK-801 selectively disrupts reversal learning in rats. Neuroreport. 2003;14(17):2225–8. doi: 10.1097/00001756-200312020-00018. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. Journal of Neuroscience. 1996;16(1):373–9. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behavioural Brain Research. 2006;175(2):329–36. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Herbert MR, Stanton ME. NMDA receptor involvement in spatial delayed alternation in developing rats. Behavioral Neuroscience. 2008 doi: 10.1037/a0013633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(18):7104–8. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EH, Knight AR, Woodruff GN. [3H]MK-801 labels a site on the N-methyl-D-aspartate receptor channel complex in rat brain membranes. Journal of Neurochemistry. 1988;50(1):274–81. doi: 10.1111/j.1471-4159.1988.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Woodside BL, Borroni AM, Hammonds MD, Teyler TJ. NMDA receptors and voltage-dependent calcium channels mediate different aspects of acquisition and retention of a spatial memory task. Neurobiology of Learning and Memory. 2004;81(2):105–14. doi: 10.1016/j.nlm.2003.10.003. [DOI] [PubMed] [Google Scholar]