Introduction
Diabetes is a complicated disease, which results from impairment in insulin secretion and/or function. Insulin is secreted by beta-cells in the pancreatic islets of Lanherhars when sugar levels increase (for example, after food intake). When insulin synthesis is disrupted, high unutilized glucose level leads to diabetes development. There is a growing unmet need in diabetes community to assess the level of surviving beta-cell non-invasively. Recent advances in this area include application of various imaging techniques (magnetic resonance imaging, MRI, positron emission tomography, PET and optical imaging techniques) in combination with proteomics and molecular biology techniques to identify specific imaginable beta-cell markers. Clearly, the biggest difficulties in imaging beta-cell mass originate from their low abundance in the pancreas (the islets of Langerhans represent only 2–3% of pancreatic tissue and are dispersed throughout the pancreas). In spite of these challenges some progress has been made in imaging of endogenous and transplanted islet mass, islet vasculature, islet apoptosis and infiltration of immune cells during diabetes progression. Several examples of these studies are discussed below (also reviewed in (1)).
Imaging of targeted beta-cells
One of the challenges in imaging beta-cell mass is in identifying beta-cell specific markers and designing highly specific ligands to these markers which could be further combined with imaging reporter(s). One of the examples of such study came from Samli et al, where the attempt had need made to identify beta-cell specific peptide from phage-display peptide libraries (2). While this study identified potential candidate peptides with beta-cell specificity, they only bound to beta-cells in healthy animals and did not recognize isles in diabetic ZDF rats. This could present a problem while imaging beta-cell mass during diabetes progression. In addition, since this study only utilized phage-associated peptides, the ability of isolated peptide to target beta-cells is still to be demonstrated. However, this study represented an interesting and promising approach in identifying targeting beta-cell specific peptides. In the same line of work is another study, utilizing beta-cell specific antibody K14D10 for beta-cell immunomagnetic purification (3). In this context one would expect that this antibody could be potentially utilized as imaging ligand for PET imaging. However, another group, which studied K14D10 Fab fragments for this purpose discovered that their specificity toward beta-cells was rather low though the affinity was comparable to that of native antibody (4).
Targeting of beta-cell specific markers has been also achieved by using the IC2 antibody shown to be beta-cell specific among islet cells (5–7). After intravenous injection of 111In-labeled antibody into healthy and streptozotocin (STZ)-induced diabetic mice there was a clear difference in signal intensity of imaged excised pancreases (8). This study will be further extended to imaging in vivo in various models of diabetes.
An alternative approach to imaging beta-cell mass was proposed by the group from Columbia University. Here, they focused on vesicular monoamine transporter 2 (VMAT2), which is expressed by beta-cells but is absent from the exocrine pancreas. It was targeted by dihydrotetrabenazine (DTBZ), which is already in clinical use for the positron emission tomography (PET) imaging of central nervous system (CNS) disorders. In their preliminary data the group showed the utility of this approach for imaging beta-cell mass in STZ-induced diabetic Lewis rats (9) and in rodent model of spontaneous type 1 diabetes (the BB-DP rat) (10). However, further whole body biodistribution studies in non-human primates failed to show any significant accumulation of the tracer in the pancreas (11), therefore raising serous concern regarding the application of this approach in diabetic patients. The latest attempt to use 18F as a radiotracer for DTBZ produced results similar to that obtained in rodents with 11C-labeled compound with the uptake of about 5% of injected dose (12). At this point it is unclear if substituting isotopes could produce any meaningful result in terms of biodistribution of these compounds in larger animals.
Non-targeted methods for beta-cell imaging
Estimation of beta-cell mass using Manganese (Mn)-enhanced MRI was attempted as a tool to image beta-cell functionality in cell culture and isolated islets (13). Mn2+ accumulates in beta cells in proportion to the glucose concentration presented to the cells, presumably via Ca2+ channels. Similar to calcium, extracellular Mn was taken up by glucose-activated beta-cells resulting in 200% increase in MRI contrast enhancement, versus non-activated cells. Similarly, glucose-activated islets showed an increase in MRI contrast up to 45% (Fig. 1). Although glucose-stimulated Ca influx was depressed in the presence of 100 microM Mn, no significant effect was seen at lower Mn concentrations. Moreover, islets exposed to Mn showed normal glucose sensitivity and insulin secretion. These results demonstrate a link between image contrast enhancement and beta-cell activation in vitro, and provide the basis for future noninvasive in vivo imaging of islet functionality and beta-cell mass.
Figure 1.

Mn2+-enhanced T1-weighted contrast of rat pancreatic islets. Two capillary tubes containing pancreatic-islets were incubated for 30 minutes in the presence of 25 mM MnCl2. The tubes were incubated in 5 mM glucose (left) and 16.7 mM glucose (right). The image was acquired at 500 MHz with TR = 400 ms, TE = 7.2 ms, slice thickness = 100 mm, field-of-view = 4.8 mm × 2.4mm, acquisition matrix = 128 × 64, and number of averages = 64. Reprinted from Gimi et al. (2006) with kind permission from Cognizant Communication Corp.
Another way to measure endogenous beta-cell mass is to engineer cells with reporter genes to retain radioisotopes which could be imaged by PET or SPECT. One of the first studies demonstrating this was performed by the group from Lawson Institute (Canada) where INS-1 832/13 and aTC1–6 cells were stably transfected with a herpes simplex virus type 1-thymidine kinase-green fluorescent protein (HSV1-thymidine kinase-GFP) fusion construct (tkgfp) (14). Cells expressing these genes could be readily identified after transplantation under the kidney capsule using SPECT/CT.
Studies in pancreatic islet cell biology could utilize various techniques including laser-scanning microscopy, which allowed for repetitive in vivo imaging of islet vascularization. Speier et al. (15) reported on the application of this technique for imaging of pancreatic islets using the anterior chamber of the eye as a natural body window. In this work the investigators transplanted mouse islets into the anterior chamber of the eye using injection through the cornea. This technique allowed for simultaneous imaging of GFP-expressing islets and islet vascular network after intravenous injection of Texas red-conjugated dextran. Interestingly, this transplantation site allowed for blood glucose normalization in streptozotocin-treated mice. In addition, imaging of cytoplasmic free Ca2+ concentration was possible in this model paving the way to deep investigation of islet cell signal transduction mechanisms under physiological and pathological conditions. Furthermore, the possibility of non-invasive imaging of beta cell death was assessed using Annexin V-APC conjugate, which revealed strong staining of apoptotic nuclei in islets treated with alloxan. Overall, this study presented a new platform for noninvasive and longitudinal studies of pancreatic islet cell biology in vivo (15).
Clearly, imaging pancreatic islets pre-labeled with either reporter gene or exogenous contrast agent prior to transplantation seems to be the most advanced area of beta-cell imaging due to the simplicity of the pre-labeling procedure. Normally, for labeling with contrast agents isolated pancreatic islets are incubated for several hours in the presence of the agent, washed and used for transplantation. As of today two type of contrast agents have been used for islet labeling, both being non-specific to beta-cells. One of them, Gd-based contrast agent GdHPDO3A is a neutral, highly hydrophilic contrast agent for MRI currently used in clinical practice. In this study it was used for labeling pancreatic islets followed by imaging of transplanted grafts (16). Gd-labeled islets seemed to maintain their function and were visible up to 65 days after transplantation by in vivo MRI.
However, the majority of the studies on imaging of transplanted islets were performed using iron oxide nanoparticles as labeling agents for islet visualization. We and others have shown that labeling of pancreatic islets with superparamagnetic nanoparticles does not impair islet viability, morphology and function (17–19). As mentioned above, labeling of pancreatic islets by iron oxide nanoparticles was non-specific towards beta-cell.
However, since 60–80% of the cells in the islets are beta-cells, the overall majority of labeled cells in the islet are usually beta-cells. Visualization of magnetically labeled transplanted islets was shown in a mouse model under the kidney capsule and after intrahepatic injection. In both cases transplanted pancreatic islets could be detected using in vivo MRI. Intraportal infusion is the only method pursued in human trials and proven to lead to insulin independence. However, one of the most critical points that need to be resolved is the viability and long-term functionality of the transplant. In order to speed up the transition of our imaging approach into clinic, we adopted the FDA-approved commercially available contrast agent Feridex, which is used clinically for liver imaging. Human pancreatic islets or islet clusters labeled with Feridex could be easily visualized on T2* images as distinct foci of signal loss in the liver parenchyma (Fig. 2). Though this method of islet cell labeling is non-specific, it allows for longitudinal tracking of transplanted islets.
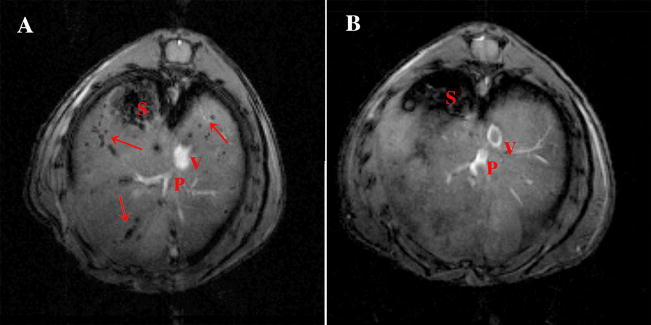
Figure 2.
In vivo imaging of intrahepatically transplanted human islets. A: Representative images of NOD.scid mice with transplanted islets. On in vivo images, Feridex-labeled islets appeared as signal voids scattered throughout the liver. B: Non-labeled islets were not detectable using the same imaging parameters. S – stomach, P – portal vein, V – superior vena cava. Reprinted from Evgenov et al. (2006) with kind permission from the American Diabetes Association.
A separate set of markers that could be utilized for beta-cell imaging is based on the unique physiology of beta-cell. Specifically, the zinc content in the pancreatic beta cell is one of the highest of the body. It appears to be an important component of insulin-secreting cells since insulin is stored inside secretory vesicles as a solid hexamer bound with two Zn2+ ions per hexamer. Zinc is also known to play an important role in the mechanisms behind insulin secretion. Therefore beta-cells express specialized zinc transporters, which can be targeted (20, 21). On the other hand, zinc itself could serve as a marker of beta-cell mass and could be imaged using novel chelate-based contrast agent.
Though not directly intended for beta-cell imaging, Meade group synthesized a novel Zn(II)-activated gadolinium (Gd)-based MRI contrast agent in which the coordination geometry of the complex rearranges upon binding of Zn(II) (22). In the absence of Zn(II) water is restricted from binding to a chelated Gd(III) ion by coordinating acetate arms resulting in a low relaxivity which increases significantly upon addition of Zn(II).
Another group from MIT synthesized water-soluble porphyrins as a dual-function molecular imaging platform for MRI and fluorescence zinc sensing (23). In the metal-free form this molecule showed superb fluorescence “turn-on” properties with high selectivity for zinc among other divalent metal ions. If the metal (manganese) is added to the porphyrin structure, the compound switches its function to generate an MRI contrast. In the presence of zinc, the relaxivity of this compound in aqueous solution is significantly altered, which makes it a promising zinc MRI sensor. Future studies will unravel whether either compound could be suitable for beta-cell imaging.
Another approach applied by Malaisse’s group involved labeled sugars, since beta cells are sensitive to changes in blood glucose. A range of tracers based on radiolabeled glucose has been explored (24–27) with various successes. The most encouraging so far have been studies involving the ketoheptose D-mannoheptulose. This compound is taken up by the cells mainly through the GLUT-2 receptor, which is exclusively expressed on hepatocytes and insulin-producing beta-cells and, therefore, displays discriminating capacity between pancreatic islets and exocrine pancreas. Furthermore, its accumulation in pancreatic islets is proportional to beta-cell mass and can be utilized to assess metabolic status in diabetic animals. Though its accumulation in the liver is relatively high, mannoheptulose has been proposed as one of the more promising candidate tracers (28–31).
Multiple attempts to utilize pharmacologic agents for beta-cell imaging have been made. One of the most studied marker included sulfonylurea receptor ligands as imaging agents. Insulin secretion is regulated by the membrane potential of the beta-cell, which depends on the activity of ATP-sensitive K+ channels in the plasma membrane. These channels are composed of a small inwardly rectifying K+ channel subunit (Kir6.1or Kir6.2) and a sulfonylurea receptor (SUR) (32). SURs represent the target for hypoglycemic sulfonylureas such as glyburide, tolbutamide, a well- known group of antidiabetic agents which have been in clinical use for years (33). Three sulfonylurea receptors (SUR1, SUR2A, and SUR2B) have been cloned. SUR1 is expressed in very high numbers at the internal face of the plasma membrane (34) of the pancreatic islet cells but not in the exocrine part of the pancreas. Logically, it was proposed that a radio-labeled glyburide or tolbutamide derivative could serve as tracers for imaging pancreatic islet cell mass. Various labeled glyburide and tolbutamide derivatives have been tested (21, 35–37). For various reasons, including overall radiochemical yield, binding affinity, deposition time at the receptor, uptake in the liver, plasma protein binding and others a lot of these agents have been found unsuitable for in vivo evaluations.
Alternative tracers have been designed based on a non-sulfonylurea hypoglycemic drug, named repaglinide, which functions similarly to glyburide by inhibiting potassium efflux from beta cells. This drug is considered superior than glyburide because of its enhanced absorption and elimination profile, as well as its high affinity for SUR1. Initial biodistribution experiments and in vitro experiments with 18F and 11C-labeled repaglinide and its metoxy analog suggest that its improved uptake by the pancreas makes repaglinide and its derivatives a promising candidate for in vivo imaging by nuclear methods (38, 39).
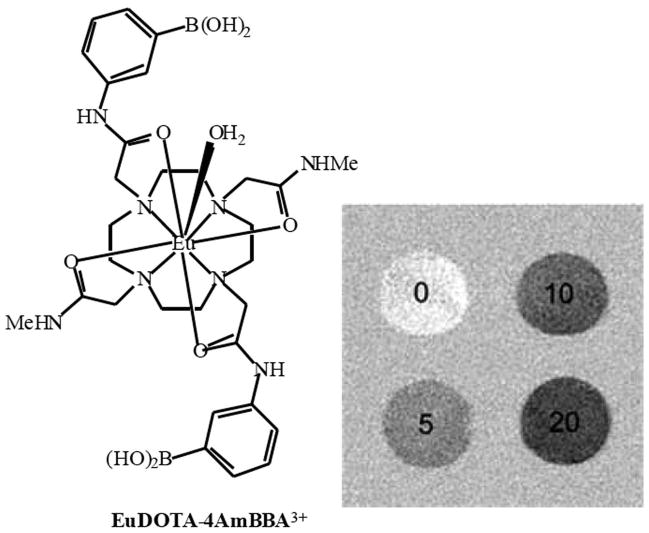
Beta-cell imaging is a fast developing field with new techniques and approaches being introduced continuously. As such, chemical exchange saturation transfer (CEST) technique and its derivative PARACEST could be used for evaluating the concentration of molecules such as glucose in vivo with high sensitivity. Very encouraging results were obtained with the glucose-sensitive Europium-based PARACEST agent, EuDOTA-4AmBBA. MR images of phantoms containing different amounts of glucose showed clear differences in signal intensity between the four samples (Fig. 3). Furthermore, the sensitivity of this system for differences in glucose concentration was within the physiologically relevant range (5mM), opening up new possibilities for the quantification of metabolites in tissue (40, 41).
Figure 3.
CEST images of phantoms containing 10 mM EuDOTA-4AmBBA plus either 0, 5, 10 or 20 mM glucose, which was obtained by subtracting the image obtained by saturating at 50 ppm from that at 30 ppm. Reprinted from Zhang et al. (2003) with kind permission from the American Chemical Society.
In conclusion, beta-cell imaging is a long-sought goal of diabetes researches, and with the development of various modalities and approaches for sensing beta-cell mass there is hope for millions of diabetic patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lin M, Lubag A, McGuire M, et al. Advances in molecular imaging of pancreatic beta cells. Front Biosci. 2008;13:4558–4575. doi: 10.2741/3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samli K, McGuire M, Newgard C, Johnston S, Brown K. Peptide-mediated targeting of the islets of Langerhans. Diabetes. 2005;54:2103–2108. doi: 10.2337/diabetes.54.7.2103. [DOI] [PubMed] [Google Scholar]
- 3.Hadjivassiliou V, Green M, Green I. Immunomagnetic purification of beta-cells from rat islets of Langerhans. Diabetologia. 2000;43:1170–1177. doi: 10.1007/s001250051509. [DOI] [PubMed] [Google Scholar]
- 4.Hampe C, Wallen A, Schlosser M, Ziegler M, Sweet I. Quantitative evaluation of a monoclonal antibody and its fragment as potential markers for pancreatic beta cell mass. Exp Clin Endocrinol Diabetes. 2005;113:381–387. doi: 10.1055/s-2005-865716. [DOI] [PubMed] [Google Scholar]
- 5.Brogren C, Hirsch F, Wood P, Poussier P. Production and characterization of a monoclonal islet cell surface autoantibody from the BB rat. Diabetologia. 1986;29:330–333. doi: 10.1007/BF00452071. [DOI] [PubMed] [Google Scholar]
- 6.Buschard K, Brogren C, Ropke C, Rygaard J. Antigen expression of the panceatic beta-cells is dependent on their functional state, as shown by a specific, BB rat monoclonal antibody IC2. Acta pathologica, microbiologica et immunologica Scandinavica. 1988;96:342–346. doi: 10.1111/j.1699-0463.1988.tb05313.x. [DOI] [PubMed] [Google Scholar]
- 7.Aaen K, Rygaard J, Josefsen K, et al. Dependence of antigen expression on functional state of beta-cells. Diabetes. 1990;39:697–701. doi: 10.2337/diab.39.6.697. [DOI] [PubMed] [Google Scholar]
- 8.Moore A, Bonner-Weir S, Weissleder R. Non-invasive in vivo measurement of beta-cell mass in mouse model of diabetes. Diabetes. 2001;50:2231–2236. doi: 10.2337/diabetes.50.10.2231. [DOI] [PubMed] [Google Scholar]
- 9.Simpson N, Souza F, Witkowski P, et al. Visualizing pancreatic β cell mass with [11C]DTBZ. Nuc Med Biol. 2006;33:855–864. doi: 10.1016/j.nucmedbio.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souza F, Simpson N, Raffo A, et al. Longitudinal noninvasive PET-based β cell mass estimates in a spontaneous diabetes rat model. J Clin Invest. 2006;116:1506–1513. doi: 10.1172/JCI27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murthy R, Harris P, Simpson N, et al. Whole body [11C]-dihydrotetrabenazine imaging of baboons: biodistribution and human radiation dosimetry estimates. Eur J Nucl Med Mol Imaging. 2007 doi: 10.1007/s00259-007-0648-2. [DOI] [PubMed] [Google Scholar]
- 12.Kung M, Hou C, Lieberman B, et al. In vivo imaging of β-cell mass in rats Using 18F-FP-(+)-DTBZ: a potential PET ligand for studying diabetes mellitus. J Nucl Med. 2008;49:1171–1176. doi: 10.2967/jnumed.108.051680. [DOI] [PubMed] [Google Scholar]
- 13.Gimi B, Leoni L, Oberholzer J, et al. Functional MR microimaging of pancreatic beta-cell activation. Cell Transplant. 2006;15:195–203. doi: 10.3727/000000006783982151. [DOI] [PubMed] [Google Scholar]
- 14.Tai J, NguyEn B, Wells R, et al. Imaging of gene expression in live pancreatic islet cell lines using dual-isotope SPECT. J Nucl Med. 2008;49:94–102. doi: 10.2967/jnumed.107.043430. [DOI] [PubMed] [Google Scholar]
- 15.Speier S, Nyqvist D, Cabrera O, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biancone L, Crich S, Cantaluppi V, et al. Magnetic resonance imaging of gadolinium-labeled pancreatic islets for experimental transplantation. NMR In Biomedicine. 2007;20:40–48. doi: 10.1002/nbm.1088. [DOI] [PubMed] [Google Scholar]
- 17.Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med. 2006;12:144–148. doi: 10.1038/nm1316. [DOI] [PubMed] [Google Scholar]
- 18.Evgenov NV, Medarova Z, Pratt J, et al. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes. 2006;55:2419–2428. doi: 10.2337/db06-0484. [DOI] [PubMed] [Google Scholar]
- 19.Tai JH, Foster P, Rosales A, et al. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes. 2006;55:2931–2938. doi: 10.2337/db06-0393. [DOI] [PubMed] [Google Scholar]
- 20.Fujibayashi Y, Saji H, Yomoda I, Suzuki KH, Torizuka K, Yokoyama A. A new approach toward a pancreas-seeking zinc radiopharmaceutical. I. Accumulation of 65Zn-amino acid and aminopolycarboxylic acid complexes in pancreatic tissue slices. Eur J Nucl Med. 1986;11:484–487. doi: 10.1007/BF00252794. [DOI] [PubMed] [Google Scholar]
- 21.Shiue CY, Schmitz A, Schirrmacher R, Shiue GG, Alavi AA. Ptential approaches for beta cell imaging with PET and SPECT. Curr Med Chem - Immun, Endoc & Metab Agents. 2004;4:271–280. [Google Scholar]
- 22.Major J, Parigi G, Luchinat C, Meade T. The synthesis and in vitro testing of a zinc-activated MRI contrast agent. Proc Nat Acad Sci USA. 2007;104:13881–13886. doi: 10.1073/pnas.0706247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Lovejoy K, Jasanoff A, Lippard S. Water-soluble porphyrins as a dual-function molecular imaging platform for MRI and fluorescence zinc sensing. Proc Nat Acad Sci USA. 2007;104:10780–10785. doi: 10.1073/pnas.0702393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malaisse WJ, Damhaut P, Ladriere L, Goldman S. Fate of 2-deoxy-2-[18F]fluoro-D-glucose in hyperglycemic rats. Int J Mol Med. 2000;6:549–552. doi: 10.3892/ijmm.6.5.549. [DOI] [PubMed] [Google Scholar]
- 25.Malaisse WJ, Damhaut P, Malaisse-Lagae F, Ladriere L, Olivares E, Goldman S. Fate of 2-deoxy-2-[18F]fluoro-D-glucose in control and diabetic rats. Int J Mol Med. 2000;5:525–532. doi: 10.3892/ijmm.5.5.525. [DOI] [PubMed] [Google Scholar]
- 26.Malaisse WJ, Ladriere L, Malaisse-Lagae F. Pancreatic fate of 6-deoxy-6-[125I]iodo-D-glucose: in vivo experiments. Endocrine. 2000;13:95–101. doi: 10.1385/ENDO:13:1:95. [DOI] [PubMed] [Google Scholar]
- 27.Malaisse WJ, Ladriere L, Sener A. Pancreatic fate of 6-deoxy-6-[125I]iodo-D-glucose: in vitro experiments. Endocrine. 2000;13:411–416. [PubMed] [Google Scholar]
- 28.Malaisse WJ. On the track to the beta-cell. Diabetologia. 2001;44:393–406. doi: 10.1007/s001250051635. [DOI] [PubMed] [Google Scholar]
- 29.Ladriere L, Leclercq-Meyer V, Malaisse WJ. Assessment of islet beta-cell mass in isolated rat pancreases perfused with D-[(3)H]mannoheptulose. Am J Physiol Endocrinol Metab. 2001;281:E298–303. doi: 10.1152/ajpendo.2001.281.2.E298. [DOI] [PubMed] [Google Scholar]
- 30.Malaisse WJ, Doherty M, Kadiata MM, Ladriere L, Malaisse-Lagae F. Pancreatic fate of D-[3H] mannoheptulose. Cell Biochem Funct. 2001;19:171–179. doi: 10.1002/cbf.911. [DOI] [PubMed] [Google Scholar]
- 31.Malaisse WJ, Ladriere L. Assessment of B-cell mass in isolated islets exposed to D-[3H]mannoheptulose. Int J Mol Med. 2001;7:405–406. [PubMed] [Google Scholar]
- 32.Bryan J, Aguilar-Bryan L. Sulfonylurea receptors: ABC transporters that regulate ATP-sensitive K(+) channels. Biochim Biophys Acta. 1999;1461:285–303. doi: 10.1016/s0005-2736(99)00164-9. [DOI] [PubMed] [Google Scholar]
- 33.Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51 (Suppl 3):S368–376. doi: 10.2337/diabetes.51.2007.s368. [DOI] [PubMed] [Google Scholar]
- 34.Uhde I, Toman A, Gross I, Schwanstecher C, Schwanstecher M. Identification of the potassium channel opener site on sulfonylurea receptors. J Biol Chem. 1999;274:28079–28082. doi: 10.1074/jbc.274.40.28079. [DOI] [PubMed] [Google Scholar]
- 35.Ladriere L, Malaisse-Lagae F, Malaisse WJ. Uptake of tritiated glibenclamide by endocrine and exocrine pancreas. Endocrine. 2000;13:133–136. doi: 10.1385/ENDO:13:1:133. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz A, Shiue CY, Feng Q, et al. Synthesis and evaluation of fluorine-18 labeled glyburide analogs as beta-cell imaging agents. Nucl Med Biol. 2004;31:483–491. doi: 10.1016/j.nucmedbio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Schneider S, Feilen PJ, Schreckenberger M, et al. In vitro and in vivo evaluation of novel glibenclamide derivatives as imaging agents for the non-invasive assessment of the pancreatic islet cell mass in animals and humans. Exp Clin Endocrinol Diabetes. 2005;113:388–395. doi: 10.1055/s-2005-865711. [DOI] [PubMed] [Google Scholar]
- 38.Wangler B, Beck C, Shiue CY, et al. Synthesis and in vitro evaluation of (S)-2-([11C]methoxy)-4-[3-methyl-1-(2-piperidine-1-yl-phenyl)-butyl-carbam oyl]-benzoic acid ([11C]methoxy-repaglinide): a potential beta-cell imaging agent. Bioorg Med Chem Lett. 2004;14:5205–5209. doi: 10.1016/j.bmcl.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 39.Wangler B, Schneider S, Thews O, et al. Synthesis and evaluation of (S)-2-(2-[18F]fluoroethoxy)-4-([3-methyl-1-(2-piperidin-1-yl-phenyl)-butyl -carbamoyl]-methyl)-benzoic acid ([18F]repaglinide): a promising radioligand for quantification of pancreatic beta-cell mass with positron emission tomography (PET) Nucl Med Biol. 2004;31:639–647. doi: 10.1016/j.nucmedbio.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S, Trokowski R, Sherry AD. A paramagnetic CEST agent for imaging glucose by MRI. J Am Chem Soc. 2003;125:15288–15289. doi: 10.1021/ja038345f. [DOI] [PubMed] [Google Scholar]
- 41.Woods M, Zhang S, Sherry AD. Toward the design of MR agents for imaging beta-cell function. Curr Med Chem - Immun, Endoc & Metab Agents. 2004;4:349–367. doi: 10.2174/1568013043357338. [DOI] [PMC free article] [PubMed] [Google Scholar]