Abstract
Atherosclerosis, the primary cause of heart disease and stroke is initiated in the vascular endothelium, and risk factors for its development include environmental exposure to persistent organic pollutants. Caveolae are membrane microdomains involved in regulation of many signaling pathways, and in particular in endothelial cells. We tested the hypothesis that intact caveolae are required for coplanar PCB77-induced up-regulation of monocyte chemoattractant protein-1 (MCP-1), an endothelium-derived chemokine that attracts monocytes into sub-endothelial space in early stages of the atherosclerosis development. Atherosclerosis-prone LDL-R−/− mice (control) or caveolin-1−/−/-LDL-R−/− mice were treated with PCB77. PCB77 induced aortic mRNA expression and plasma protein levels of MCP-1 in control, but not caveolin-1−/−/LDL-R−/− mice. To study the mechanism of this effect, primary endothelial cells were used. PCB77 increased MCP-1 levels in endothelial cells in a time- and concentration-dependent manner. This effect was abolished by caveolin-1 silencing using siRNA. Also, MCP-1 up-regulation by PCB77 was prevented by inhibiting p38 and c-Jun N-terminal kinase (JNK), but not ERK1/2, suggesting regulatory functions via p38 and JNK MAPK pathways. Finally, pretreatment of endothelial cells with the aryl hydrocarbon receptor (AhR) inhibitor α-naphthoflavone (α-NF) partially blocked MCP-1 up-regulation. Thus, our data demonstrate that coplanar PCB77 can induce MCP-1 expression by endothelial cells and that this effect is mediated by AhR, as well as p 38 and JNK MAPK pathways. Intact caveolae are required for these processes both in vivo and in vitro. This further supports a key role for caveolae in vascular inflammation induced by persistent organic pollutants.
Keywords: Endothelial cells; 3,3′,4,4′-tetrachlorobiphenyl (PCB77); caveolin-1; monocyte chemoattractant protein (MCP-1)
Introduction
Endothelial activation is one of the earliest events in the development of atherosclerosis (Ross, 1999). Exposure to circulating persistent organic pollutants, such as polychlorinated biphenyls (PCBs), can facilitate this process (Hennig et al., 2002). Coplanar PCBs, for example 3,3′, 4,4′-tetrachlorobiphenyl (PCB77), bind to the aryl hydrocarbon receptor (AhR) in endothelial cells, causing up-regulation of cytochrome P450 1A1 (CYP1A1) (Toborek et al., 1995). Subsequent uncoupling of CYP1A1 by PCB77 leads to overproduction of reactive oxygen species (ROS) (Schlezinger et al., 2006), activation of oxidative stress-sensitive signaling pathways, and up-regulation of inflammatory mediators (Slim et al., 1999).
Monocyte chemoattractant protein-1 (MCP-1) is an endothelium-derived chemokine that plays an essential role in the recruitment of leukocytes to the site of injury during inflammation. The recruitment of monocytes into the artery wall, followed by their differentiation into macrophages and foam cells, is also one of the earliest events in the pathology of atherosclerosis (Packard and Libby, 2008). It has been reported that AhR ligands, for example polycyclic aromatic hydrocarbon benzo[a]pyrene (B[a]P) (Knaapen et al., 2007) or the strongest known AhR ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Vogel et al., 2007), have the ability to induce MCP-1 production. Understanding mechanisms of MCP-1 up-regulation by coplanar PCBs would help to dissect mechanisms responsible for the increased incidence of atherosclerosis observed after PCB77 treatment in vivo (Arsenescu et al., 2008), as well as epidemiological evidence implicating PCBs in increased cardiovascular risk in exposed populations (Gustavsson and Hogstedt, 1997; Goncharov et al., 2008).
Many endothelial functions, including signal transduction, seem to be regulated through caveolae (Frank et al., 2003; Mineo and Shaul, 2006), which are 50–100 nm membrane microdomains enriched in cholesterol and sphingolipids, as well as its major structural protein caveolin-1 (Sargiacomo et al., 1993). Interestingly, a significant reduction in the size of atherosclerotic lesions has been observed in ApoE−/− mice deficient in caveolin-1 (Frank et al., 2004). Recent evidence from our laboratory implicate caveolae as a regulatory platform involved in endothelial activation by environmental contaminants, namely B[a]P (Oesterling et al., 2008) and coplanar PCBs (Lim et al., 2007; Lim et al., 2008). Caveolin-1 was required for eNOS activation by PCB77 (Lim et al., 2007), and AhR binding to caveolin-1 seems to play a role in up-regulation of downstream AhR targets including CYP1A1 (Lim et al., 2008).
Thus, the current study was designed to test the hypothesis that functional caveolae are required for MCP-1 up-regulation by coplanar PCB77 in endothelial cells. Our data provide clear evidence that PCB77 increases MCP-1 expression through AhR signaling and that caveolin-1 is a possible biomarker of inflammatory events mediated through AhR.
Materials and Methods
Materials and chemicals
The inhibitors α-naphthoflavone, N-acetyl-L-cysteine, SB203580 and PD98059, as well as sterile, endotoxin-tested, dimethyl sulfoxide (DMSO), were purchased from Sigma-Aldrich (St. Louis, MO). PCB77 was a generous gift from Dr. Larry W. Robertson, University of Iowa, Iowa City, IA. 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) were purchased from AccuStandard, Inc. (New Haven, CT).
Cell culture
Primary porcine endothelial cells were isolated from pulmonary arteries as described previously (Hennig et al., 1984). Cells were cultured in M199 media (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Hyclone Laboratories, Logan, UT). Prior to treatments, cells were grown until confluent and then synchronized by maintaining them in 1% FBS for 16 h. All vehicle controls and treated cultures contained the same amount of DMSO (0.1% v/v). This concentration of DMSO did not have any effect on MCP-1 expression.
Animals
All animals were housed in the Association for Assessment and Accreditation of Laboratory Animal Care-certified animal facilities at the University of Kentucky. For our experiments mice were used with low density lipoprotein receptor (LDL-R)-deficient background. These mice are a preferred model for atherosclerosis studies because they mimic human lipoprotein levels and atherosclerosis development (Daugherty, 2002). We also have demonstrated previously that PCB77 increases aortic adhesion molecule expression in LDL-R-deficient mice (Hennig et al., 2005). LDL-R deficient (LDL-R−/−) mice on a C57BL/6 background and caveolin-1 (Cav-1−/−) deficient mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Cav-1 deficient mice were generated in the Sv129 strain and backcrossed onto a C57BL/6 background. Mice were bred at the University of Kentucky to generate LDL-R/caveolin-1 double null mice (LDL-R−/− Cav-1−/− mice). At 8 weeks of age, mice were placed on a standardized diet containing 20% calories from fat (Dyets Inc., Bethlehem, PA). After 2 weeks, mice were injected intraperitoneally with PCB77 (170 μmol/kg body weight) or vehicle (olive oil) and then 6 days later they were injected again. This dose of PCB77 was previously sufficient to induce aortic adhesion molecule expression in vivo (Hennig et al., 2002; Hennig et al., 2005), as well as the development of atherosclerotic lesions over the course of 6 weeks (Arsenescu et al., 2008). 24 h after the last treatment, mouse tissues were harvested.
Caveolin-1 small-interfering RNA (siRNA) and transfection
Caveolin-1 protein levels in endothelial cells were silenced using small inhibitory (si)RNA technique as described previously (Lim et al., 2007). Briefly, the cells were transfected with a mixture of two siRNAs targeted against caveolin-1 (40 nM each) or control siRNA (80 nM), synthetized by Dharmacon (Lafayette, CO) according to previously published sequences (Repetto et al., 2005), and by using GeneSilencer transfection reagent (Genlantis, San Diego, CA) in OptiMEM media (Invitrogen). Subsequently, cells were treated with vehicle (DMSO) or PCB77.
Real-time PCR
Total RNA was extracted from the cells using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. Mouse aortas were cleaned of periadventitial tissue and stored in RNAlater RNA stabilizing reagent (Qiagen, Valencia, CA) at −80°C. Total mRNA was purified using the RNeasy Fibrous Tissue Mini Kit (Qiagen) according to the manufacturer’s instructions. Reverse transcription was performed using the AMV reverse transcription system (Promega, Madison, WI). The levels of mRNAs expression were then assessed by real-time PCR using 7300 Real Time PCR System (Applied Biosystems, Foster City, CA) and SYBR Green (in endothelial cells) or TaqMan (in tissues) master mix (Applied Biosystems). MCP-1 or interleukin-6 (IL-6) mRNA levels were divided by β-actin (internal control). β-Actin and MCP-1 primer sequences for SYBR Green chemistry were designed using the Primer Express Software 3.0 for real-time PCR (Applied Biosystems) and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). β-actin sequences: sense, 5′-TCATCACCATCGGCAACG-3′; antisense, 5′-TTCCTGATGTCCACGTCG-3′; MCP-1 sequences:5′-CGGCTGATGAGCTACAGAAGAGT-3′; antisense, 5′-GCTTGGGTTCTGCACAGATCT-3′. For TaqMan reactions, TaqMan gene expression assays (Applied biosystems) were used.
MCP-1 and IL-6 protein level
Cell culture media were harvested on ice and centrifuged at 2000 × g for 10 min at 4°C. The supernatants were collected and MCP-1 protein levels were measured using Quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Mouse blood was collected in tubes containing EDTA, centrifuged at 1500 g for 20 min at 4°C, and plasma was removed and stored at −80°C. Plasma levels of MCP-1 and IL-6 were measured using Mouse Adipokine LINCOplex kit (Millipore, St. Charles, MO) according to the manufacturer’s instructions. Luminex 100 (Luminex Corporation, Austin, TX) and Multiplex Data Analysis Software 1.0 (Upstate USA, Inc., Chicago, IL) were utilized for signal detection and data analysis, respectively.
Cell viability
Cell viability was evaluated using the commercially available MTS test (Sigma-Aldrich). Cells were plated into 96-well culture plates and treated with vehicle (DMSO) or increasing concentrations of PCB77 for 24 h. The MTS test was performed according to the manufacturer’s instructions. Absorbance at 490 nm was measured using a SpectraMax M2 (Molecular Devices, Sunnyvale, CA).
Statistical analysis
Values are reported as means ± SE of at least three independent groups. Comparisons between two treatments were made by t-test; comparisons among three or more groups by one-way or two-way analysis of variance (ANOVA) followed by post-hoc Fisher’s LSD test using SigmaStat 2.0 software (Systat Software, Point Richmond, CA). Statistical probability of p < 0.05 was considered significant.
Results
PCB77, as well as other AhR agonists, increase MCP-1 mRNA and protein levels in endothelial cells
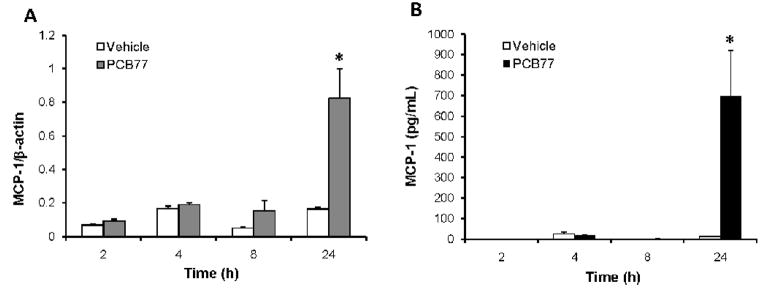
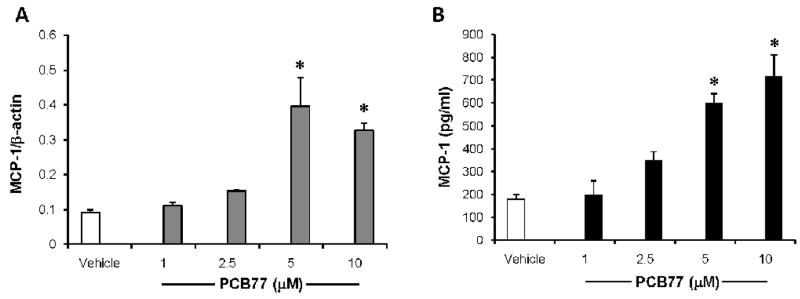
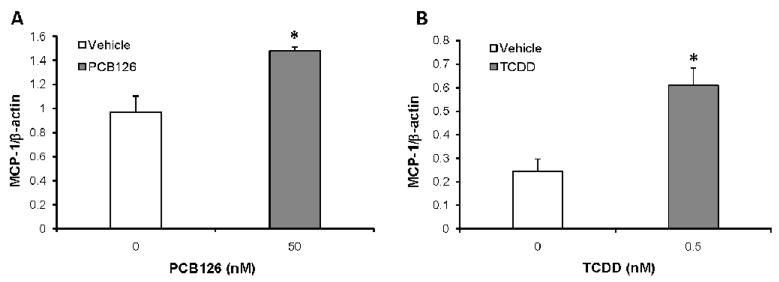
MCP-1 is a chemokine involved in recruitment of monocytes from blood stream to sub-endothelial space in early stages of atherosclerosis development. In order to test whether coplanar PCB77 can increase the expression of MCP-1 in endothelial cells, cells were treated with vehicle control or PCB77 (5 μM) for various time intervals ranging from 2–24 hours. Significant increases in both mRNA expression (measured by RT PCR) (Fig. 1A) and protein levels released into culture media (measured by ELISA) (Fig. 1B) were observed only after 24 hours. In order to find out the lowest concentration of PCB77 to achieve MCP-1 up-regulation, increasing concentrations of PCB77 ranging from 1–10 μM were used. A significant increase in MCP-1 mRNA a protein expression was observed at both 5 and 10 μM concentrations of PCB77 (Figs. 2A and 2B). Using the MTS test, cell viability was not affected at any of these concentrations. Since 5 μM was the lowest effective concentration of PCB77, this dose was used in the subsequent inhibitor studies. This concentration also was used in our previous work on endothelial dysfunction (Slim et al., 1999), and is similar to PCB levels reported in acutely exposed populations (3.4 μM, or 1 ppm). PCB77 is an example of a coplanar PCB with dioxin-like activity; thus, we also tested other AhR ligands, such as PCB126 (3,3′,4,4′,5-pentachlorobiphenyl) and TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin), on induction of MCP-1 expression. Both coplanar PCB126 (Fig. 3A) and TCDD (Fig. 3B) significantly induced MCP-1 mRNA levels after 24 h. Toxicant concentrations used in these experiments were normalized relative to their toxic equivalency factor (TEF). In contrast to the AhR ligands, non-coplanar PCB153 (2,2′,4,4′,5,5′-hexachlorobiphenyl) did not affect MCP-1 expression (data not shown).
Figure 1. PCB77 up-regulates MCP-1 in a time-dependent manner.
Cells were treated with vehicle (DMSO) or PCB77 (5 μM) for indicated time periods. MCP-1 mRNA expression levels (A) were measure using Real-time PCR, and MCP-1 protein levels in culture media (B) were measured using a human MCP-1 OptiEIA ELISA Kit. Results represent mean ± SEM of 4 experiments. *Significantly different compared to vehicle control (p<0.05).
Figure 2. PCB77 up-regulates MCP-1 in a concentration-dependent manner.
Cells were treated with vehicle (DMSO) or increasing concentrations of PCB77 for 24 h. MCP-1 mRNA expression levels (A) were measure using Real-time PCR, and MCP-1 protein levels in culture media (B) were measured using the MCP-1 OptiEIA ELISA Kit. Results represent mean ± SEM of 4 experiments.
*Significantly different compared to vehicle control (p<0.05).
Figure 3. PCB126 and TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) induce MCP-1 mRNA expression.
Cells were treated with vehicle (DMSO) or PCB126 (50 nM) (A), or TCDD (0.5 nM) (B) for 24 h. MCP-1 mRNA expression levels were measured using Real-time PCR. Results represent mean ± SEM of 3 experiments. *Significantly different compared to vehicle control (p<0.05).
MCP-1 up-regulation is regulated through the aryl hydrocarbon receptor (AhR)
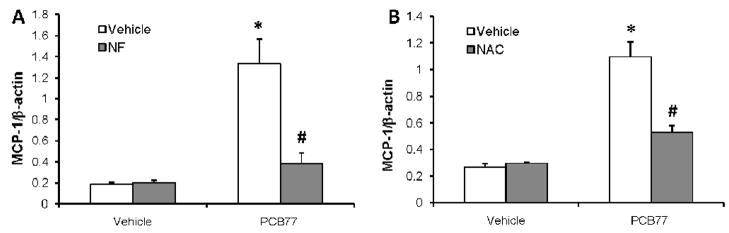
Many endothelial responses to coplanar PCBs are mediated through their binding to AhR (Hennig et al., 2002). In order to test the role of AhR in MCP-1 up-regulation by PCB77, the AhR antagonist α-naphthoflavone (α-NF) was used. Cells were pre-treated with α-NF (0.01 μM), followed by vehicle (DMSO) or PCB77 treatment. MCP-1 mRNA expression was significantly increased by PCB77, but this was prevented by α-NF pre-treatment (Fig. 4A).
Figure 4. Endothelial MCP-1 up-regulation by PCB77 is AhR- and reactive oxygen species-dependent.
Cells were pre-treated with the AhR antagonist α-naphthoflavone (NF, 0.01 μM) (A) or the glutathione precursor N-acetyl cysteine (NAC, 0.1 mM) (B) for 1 h followed by vehicle (DMSO) or PCB77 (5 μM) treatment for 24 h. MCP-1 mRNA expression levels were measured using Real-time PCR. Results represent mean ± SEM of 4 experiments. *Significantly different compared to vehicle control (p<0.05). #Significantly different compared to PCB77-treated control without NF (A) or without NAC (B) (p<0.05).
MCP-1 up-regulation is oxidative stress-dependent
PCB77 is known to cause AhR-mediated up-regulation and subsequent uncoupling of cytochrome P450 (CYP) 1A1 (Schlezinger et al., 2006). Oxidative stress can cause MCP-1 up-regulation in vascular endothelium (Woo Lee et al., 2001). Here, the glutathione precursor and antioxidant N-acetyl cysteine (NAC) was used to increase antioxidant potential of endothelial cells. Cells were pre-treated with 100 μM NAC for 1 h followed by vehicle (DMSO) or PCB77 (5 μM) treatment. PCB77-induced MCP-1 mRNA levels were blocked by NAC pre-treatment (Fig. 4B).
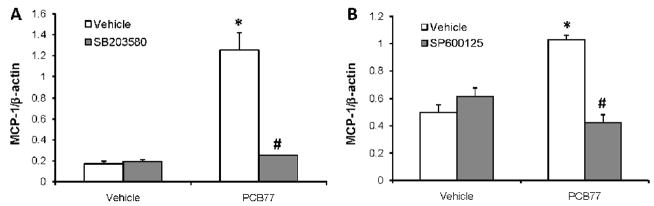
MCP-1 up-regulation is mediated by p38 and JNK MAPKs
Mitogen activated protein kinases (MAPKs) play a role in cellular responses to environmental stress, including endothelial activation. SB203580 (SB), a selective p38 kinase inhibitor, was used to examine the role of p38 kinase in MCP-1 expression. Pre-treatment for 1 h with 5 μM SB blocked PCB77-induced MCP-1 mRNA expression (Fig. 5A). Similarly, SP600125, a selective inhibitor of c-Jun K-terminal kinase (JNK) pretreatment (1 h, 20 μM) prevented PCB77-induced MCP-1 mRNA expression (Fig. 5B). In contrast, the ERK1/2 inhibitor, PD98029, at concentrations ranging from 1–20 μM had no effect on PCB77-induced over-expression of MCP-1 (data not shown).
Figure 5. MCP-1 up-regulation by PCB77 is regulated by p38 and JNK.
Cells were pre-treated with p38 (SB203580, 5 μM) (A) or JNK (SP600125, 20 μM) (B) inhibitors, respectively, for 30 min followed by vehicle (DMSO) or PCB77 (5 μM) treatment for 24 h. MCP-1 mRNA expression levels were measured using Real-time PCR. Results represent mean ± SEM of 4 experiments. *Significantly different compared to vehicle control (p<0.05). #Significantly different compared to PCB77-treated control without inhibitors (p<0.05).
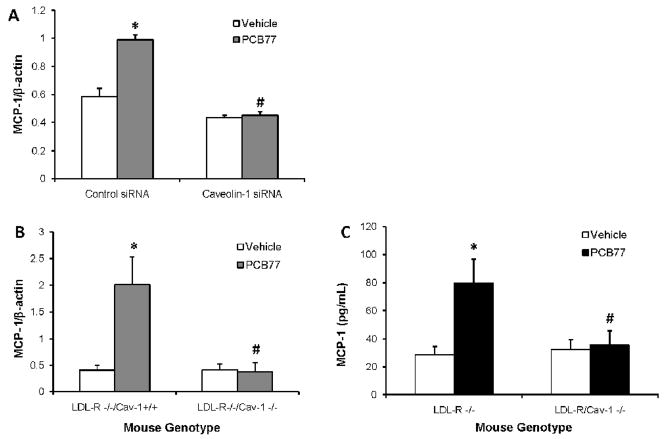
Caveolin-1 silencing prevents MCP-1 up-regulation by PCB77in vitro and in vivo
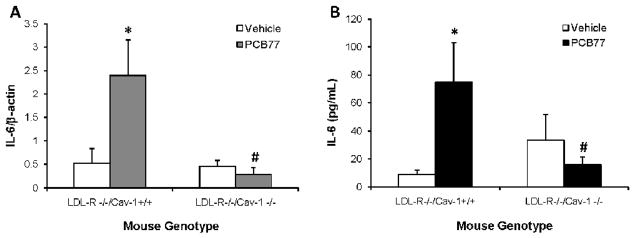
To investigate the role of caveolae in MCP-1 induction by PCB77, caveolin-1 was silenced using the siRNA technique. Cavelin-1 is the major structural protein of caveolae that is required for caveolae formation in endothelial cells (Drab et al., 2001). PCB77 induction of MCP-1 mRNA was abolished in the cells lacking caveolin-1, suggesting that intact caveolae are required for this response (Fig. 6A). To confirm these findings in vivo, atherosclerosis-prone LDL-R−/− mice were compared to LDL-R−/− Cav-1−/− mice. PCB77 treatment increased both aortic mRNA expression levels of MCP-1 in LDL-R−/− mice, as measured by real time PCR, and plasma protein levels of MCP-1 protein, assessed by LincoPLEX kit. However, no MCP-1 induction was detected in LDL-R−/− Cav-1−/− mice (Figs. 6B and 6C). Taken together, caveolin-1 is required for MCP-1 up-regulation by PCB77 both in vitro and in vivo. In addition to MCP-1, interleukin-6 (IL-6) also is an important mediator of acute phase response and risk factor for cardiovascular disease (Yudkin et al., 2000; Song and Schindler, 2004). Upregulation of aortic IL-6 mRNA (Fig. 7A) and plasma protein (Fig. 7B) levels by PCB77 followed a similar pattern as observed with MCP-1. These data suggest that caveolin-1 is a common regulator of PCB77-induced vascular inflammatory response.
Figure 6. Caveolin-1 deficiency prevents MCP-1 up-regulation by PCB77 in endothelial cells and mouse vasculature.
Cells were transfected with control or caveolin-1 siRNAs for 48 hours and treated with vehicle (DMSO) or PCB77 (5 μM) for 24 h. MCP-1 mRNA expression levels were measured using Real-time PCR with SYBR Green chemistry (A). LDL-R−/−/Cav-l+/+ (control) and LDL-R−/−/Cav-1−/− (caveolin-1 deficient) mice were treated with vehicle control (olive oil) or PCB77 (170 μmole/kg). Aortic mRNA was isolated and MCP-1 mRNA expression levels were measured using Real-time PCR with TaqMan chemistry (B). Plasma samples were analyzed for MCP-1 levels using mouse adipokine LINCOplex kit (C). Results represent the mean ± SEM of 6 experiments/animals. *Significantly different compared to vehicle control (p<0.05). #Significantly different compared to PCB77-treated control, i.e., siRNA cell or LDL-R−/−/Cav-l+/+ mice (p<0.05).
Figure 7. Caveolin-1 deficiency prevents IL-6 up-regulation by PCB77 in mouse vasculature.
LDL-R−/−/Cav-1+/+ (control) and LDL-R−/−/Cav-l−/− (caveolin-1 deficient) mice were treated with vehicle control (olive oil) or PCB77 (170 μmole/kg). Aortic mRNA was isolated and IL-6 mRNA expression levels were measured using Real-time PCR with TaqMan chemistry (A). Plasma samples were analyzed for IL-6 levels using mouse adipokine LINCOplex kit (B). Results represent the mean ± SEM of 6 animals. *Significantly different compared to vehicle control (p<0.05). #Significantly different compared to PCB77-treated LDL-R−/−/Cav-l+/+ mice (p<0.05).
Discussion
Persistent organic pollutants (Ha et al., 2007) and in particular PCBs (Gustavsson and Hogstedt, 1997), were associated with an increased risk of cardiovascular disease. In this study, we have documented for the first time that coplanar PCBs, such as PCB77, can increase transcription and secretion of MCP-1 by endothelial cells. This is likely an important step in vascular inflammatory events involved in the promotion of atherosclerosis development by coplanar PCBs (Arsenescu et al., 2008). For example, MCP-1-mediated recruitment of monocytes is a critical event in atherosclerotic lesion formation (Boring et al., 1998; Gu et al., 1998).
There is evidence that the aryl hydrocarbon receptor (AhR) may play a critical regulatory role in the induction of proatherogenic inflammatory markers. For example, MCP-1 was previously reported to be induced in various tissues by other environmental toxicants, such benzo[a]pyrene (B[a]P) (Knaapen et al., 2007), or 2,3,7,8-tetrachlorodibenzo-para-dioxin (TCDD) (Vogel et al., 2007). AhR is a nuclear receptor that, after a ligand binding, translocates into the nucleus and initiates transcription through dioxin responsive elements (DREs) in regulatory regions of AhR-responsive genes. The majority of toxic effects of coplanar PCBs are initiated by their binding to AhR (Safe et al., 1985). Our data demonstrate that both coplanar PCB77 and PCB126, as well as TCDD, induce MCP-1 mRNA expression levels, suggesting AhR to be a common mediator of this pathway. In the present study, we used the AhR antagonist α-naphthoflavone (α-NF) (Merchant et al., 1993) to test the hypothesis that MCP-1 up-regulation by coplanar PCB77 is AhR-dependent, and we were able to abolish MCP-1 up-regulation by α-NF pre-treatment. We have previously demonstrated that coplanar PCBs trigger AhR-mediated up-regulation of cytochrome P450 (CYP1A1) levels and activity in endothelial cells (Ramadass et al., 2003). PCB-induced up-regulation of CYP1A1 can cause overproduction of reactive oxygen species and activation of oxidative stress-sensitive transcription factors such as nuclear factor-κB (NF-κB) (Hennig et al., 2002), a possible prerequisite for MCP-1 induction. Indeed, pre-treatment with the glutathione precursor and anti-oxidant N-acetyl cysteine (NAC) prevented MCP-1 induction, thus supporting a role of oxidative stress in this process.
Mitogen-activated protein kinases (MAPKs) mediate cellular responses to various environmental stimuli and are important signaling components upstream of transcription factors that regulate an inflammatory response. There are three best characterized MAPKs sub-families, extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 kinases (Johnson and Lapadat, 2002), and all of them can be regulated by oxidative stress (McCubrey et al., 2006).
JNK and p38 in particular seem to be important mediators of pro-atherogenic events (Hoefen and Berk, 2002; Zakkar et al., 2008) (Zakkar et al., 2008), and p38 activation can lead to endothelial MCP-1 up-regulation (Sung et al., 2001; Liu et al., 2008). Our data demonstrated that MCP-1 up-regulation by PCB77 could be diminished by both p38 and JNK inhibition.
We have evidence that caveolae may provide a critical signaling platform in the regulation of PCB-induced endothelial cell dysfunction and that caveolin-1 can be a biomarker of PCB toxicity (Lim 2008). Caveolae are membrane domains enriched in cholesterol and sphingolipids, and the major structural proteins caveolins (Thomas and Smart, 2008). Caveolae are highly abundant in endothelial cells (Frank et al., 2003) where they are proposed to serve as platforms to compartmentalize and selectively modulate cell signaling events. Caveolin-1, a 22 kDa protein (Rothberg et al., 1992) is required for caveolae formation in endothelial cells (Drab et al., 2001), and mice which lack caveolin-1 demonstrate increased severity of atherosclerosis (Frank et al., 2004). In the current study we used caveolin-1 siRNA to test the hypothesis that functional caveolae facilitate MCP-1 up-regulation by PCB77. Indeed, we found that in the absence of caveolin-1, MCP-1 up-regulation was diminished. These results were confirmed in vivo following PCB77 treatment of mice lacking the caveolin-1 gene. LDL-R deficient mice were used as a background control in this study as a model of atherosclerosis because their elevated LDL fraction resembles the lipoprotein profile of hypercholesterolemic humans (Daugherty, 2002). We found that PCB77 increased aortic mRNA expression of both MCP-1 and IL-6 in control LDL-R−/− mice, which was accompanied by increased plasma MCP-1 and IL-6 protein levels. In contrast, caveolin-1−/− LDL-R−/− mice were resistant to PCB77-induced MCP-1 and IL-6 up-regulation, suggesting the physiological importance of caveolae in PCB-induced inflammation and atherosclerosis.
Our recent study showed that AhR binds caveolin-1 in endothelial cells and that deletion of the caveolin-1 gene partially decreased CYP1A1 induction by coplanar PCBs (Lim et al., 2008). We also demonstrated that the production of reactive oxygen species induced by PCB77 was diminished in the absence of caveolin-1 (Lim 2008). Since AhR activation and oxidative stress were required for MCP-1 up-regulation, our data suggest that PCB77-mediated AhR binding and activation and subsequent up-regulation of MCB-1 requires functional caveolae. In a related study we also found that caveolin-1 was required for ICAM-1 up-regulation by B[a]P in endothelial cells and that this effect was also mediated by p38 activation (Oesterling et al., 2008). In addition, caveolin-1 was demonstrated to bind p38 in endothelial cells and to facilitate its phosphorylation and activation of downstream targets (Siddiqui et al., 2007). These data all suggest that regulation of inflammatory pathways through caveolae might be a mechanism shared by environmental toxicants that are AhR agonists.
In conclusion, we have demonstrated that coplanar PCB77 can up-regulate endothelial levels of MCP-1, a critical regulator of early stages of atherosclerosis. This process is regulated by caveolin-1 and caveolae-associated signaling pathways, including induction of AhR and reactive oxygen species, as well as p38 and JNK MAP kinases. It appears that functional caveolae are important for endothelial cell dysfunction, and caveolin-1 may emerge as a critical biomarker of cardiovascular toxicity by environmental contaminants.
Acknowledgments
We thank Jason Stevens at the University of Kentucky Center for Oral Health Research for the assistance in processing the LINCOplex data. This research was supported by grants from NIEHS/NIH (P42ES07380) and the University of Kentucky Agricultural Experiment Station.
Footnotes
Conflict of interest statement
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2 −/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- Daugherty A. Mouse models of atherosclerosis. Am J Med Sci. 2002;323:3–10. doi: 10.1097/00000441-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Haase RF, Santiago-Rivera A, Morse G, McCaffrey RJ, Rej R, Carpenter DO. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ Res. 2008;106:226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Am J Ind Med. 1997;32:234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999–2002. Environ Health Perspect. 2007;115:1204–1209. doi: 10.1289/ehp.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hennig B, Reiterer G, Toborek M, Matveev SV, Daugherty A, Smart E, Robertson LW. Dietary fat interacts with PCBs to induce changes in lipid metabolism in mice deficient in low-density lipoprotein receptor. Environ Health Perspect. 2005;113:83–87. doi: 10.1289/ehp.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Shasby DM, Fulton AB, Spector AA. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984;4:489–497. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- Hoefen RJ, Berk BC. The role of MAP kinases in endothelial activation. Vascul Pharmacol. 2002;38:271–273. doi: 10.1016/s1537-1891(02)00251-3. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Knaapen AM, Curfs DM, Pachen DM, Gottschalk RW, de Winther MP, Daemen MJ, Van Schooten FJ. The environmental carcinogen benzo[a]pyrene induces expression of monocyte-chemoattractant protein-1 in vascular tissue: a possible role in atherogenesis. Mutat Res. 2007;621:31–41. doi: 10.1016/j.mrfmmm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Lim EJ, Majkova Z, Xu S, Bachas L, Arzuaga X, Smart E, Tseng MT, Toborek M, Hennig B. Coplanar polychlorinated biphenyl-induced CYP1A1 is regulated through caveolae signaling in vascular endothelial cells. Chem Biol Interact. 2008;176:71–78. doi: 10.1016/j.cbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H3340–3347. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- Liu SW, Qiao SB, Yuan JS, Liu DQ. Visfatin Stimulates Production of Monocyte Chemotactic Protein-1 and lnterleukin-6 in Human Vein Umbilical Endothelial Cells. Horm Metab Res. 2008 doi: 10.1055/s-0028-1102914. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Merchant M, Krishnan V, Safe S. Mechanism of action of alpha-naphthoflavone as an Ah receptor antagonist in MCF-7 human breast cancer cells. Toxicol AppI Pharmacol. 1993;120:179–185. doi: 10.1006/taap.1993.1101. [DOI] [PubMed] [Google Scholar]
- Mineo C, Shaul PW. Circulating cardiovascular disease risk factors and signaling in endothelial cell caveolae. Cardiovasc Res. 2006;70:31–41. doi: 10.1016/j.cardiores.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Oesterling E, Toborek M, Hennig B. Benzo[a]pyrene induces intercellular adhesion molecule-1 through a caveolae and aryl hydrocarbon receptor mediated pathway. Toxicol Appl Pharmacol. 2008;232:309–316. doi: 10.1016/j.taap.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- Ramadass P, Meerarani P, Toborek M, Robertson LW, Hennig B. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicol Sci. 2003;76:212–219. doi: 10.1093/toxsci/kfg227. [DOI] [PubMed] [Google Scholar]
- Repetto S, Salani B, Maggi D, Cordera R. Insulin and IGF-I phosphorylate eNOS in HUVECs by a caveolin-1 dependent mechanism. Biochem Biophys Res Commun. 2005;337:849–852. doi: 10.1016/j.bbrc.2005.09.125. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Safe S, Bandiera S, Sawyer T, Robertson L, Safe L, Parkinson A, Thomas PE, Ryan DE, Reik LM, Levin W, et al. PCBs: structure-function relationships and mechanism of action. Environ Health Perspect. 1985;60:47–56. doi: 10.1289/ehp.856047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat Toxicol. 2006;77:422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Siddiqui SS, Siddiqui ZK, Uddin S, Minshall RD, Malik AB. p38 MAPK activation coupled to endocytosis is a determinant of endothelial monolayer integrity. Am J Physiol Lung Cell Mol Physiol. 2007;292:L114–124. doi: 10.1152/ajplung.00257.2005. [DOI] [PubMed] [Google Scholar]
- Slim R, Toborek M, Robertson LW, Hennig B. Antioxidant protection against PCB-mediated endothelial cell activation. Toxicol Sci. 1999;52:232–239. doi: 10.1093/toxsci/52.2.232. [DOI] [PubMed] [Google Scholar]
- Song L, Schindler C. IL-6 and the acute phase response in murine atherosclerosis. Atherosclerosis. 2004;177:43–51. doi: 10.1016/j.atherosclerosis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Sung FL, Slow YL, Wang G, Lynn EG, O K. Homocysteine stimulates the expression of monocyte chemoattractant protein-1 in endothelial cells leading to enhanced monocyte chemotaxis. Mol Cell Biochem. 2001;216:121–128. doi: 10.1023/a:1017383916068. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Smart EJ. Caveolae structure and function. J Cell Mol Med. 2008;12:796–809. doi: 10.1111/j.1582-4934.2008.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, Espandiari P, Robertson LW, Hennig B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. J Biochem Toxicol. 1995;10:219–226. doi: 10.1002/jbt.2570100406. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Nishimura N, Sciullo E, Wong P, Li W, Matsumura F. Modulation of the chemokines KC and MCP-1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice. Arch Biochem Biophys. 2007;461:169–175. doi: 10.1016/j.abb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Woo Lee Y, Joo Park H, Hennig B, Toborek M. Linoleic acid induces MCP-1 gene expression in human microvascular endothelial cells through an oxidative mechanism. J Nutr Biochem. 2001;12:648–654. doi: 10.1016/s0955-2863(01)00186-3. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Zakkar M, Chaudhury H, Sandvik G, Enesa K, Luong le A, Cuhlmann S, Mason JC, Krams R, Clark AR, Haskard DO, Evans PC. Increased endothelial mitogen-activated protein kinase phosphatase-1 expression suppresses proinflammatory activation at sites that are resistant to atherosclerosis. Circ Res. 2008;103:726–732. doi: 10.1161/CIRCRESAHA.108.183913. [DOI] [PubMed] [Google Scholar]