Abstract
Successful organ cryopreservation will significantly benefit human health and biomedical research. One of the major challenges to this accomplishment is the need for optimization of cryoprotectant agent (CPA) perfusion procedures that involve highly complicated mass transfer processes in organs. The diffusivity of CPA is of critical importance for designing perfusion procedures to minimize the associated toxicity and osmotic damage. However, to date there have been no attempts to measure the CPA diffusivity in organs. In this study, we established a simple CPA diffusion model for relatively small organs, e.g. mouse ovaries, defined the apparent diffusivity (D̄) of CPA for these organs (please see Table 1 for symbol definitions), and established a practical approach to measure the value of D̄ through magnetic resonant imaging (MRI). Using rapid MRI techniques and water saturation analyses, the distribution of ethylene glycol (EG) concentration in the centric cross-section of mouse ovaries was measured at a series of time points during perfusion, and these data were fit to the integral form of the mass transfer equation in the established model. These fits resulted in a value of D̄ for EG in mouse ovaries of 6.1 ± 1.4×10-7cm2/s (mean ± SD). Based on these results, we proposed a modified perfusion procedure that may improve the survival of small organs or thin tissues during equilibrium cooling processes and assessed its efficiency through theoretical analyses.
Keywords: diffusivity, CPA, MRI, perfusion, organ cryopreservation
Introduction
Organ transplantation is one of the spectacular achievements of human biomedical research in the last century [12,20]. Storage of organs before transplantation is of critical importance for medical and commercial applications. The commonly used hypothermic methods with 4°C as the storage temperature, however, generally are limited by storage time (48-72 hrs for kidney, 6-12 hrs for heart, etc.) due to the related hypothermic injuries [11,19]. Cryopreservation of organs at the liquid nitrogen saturation temperature (-196°C) or dry ice sublimation temperature (-80°C) is potentially an approach to prevent hypothermic injury and may significantly prolong the storage time from hours to years [11,12,13]. Before cryopreservation, permeating cryoprotectant agents (CPA, e.g. ethylene glycol (EG), Me2SO, and glycerol) are commonly perfused into tissues and organs to prevent the damaging intra- and intercellular ice formation (IIF) induced by low temperatures [7,17], as well as attenuate cell damage due to the so-called “solution effects” [8,16]. However, these processes generate both toxic effects and osmotic damage to cells [4,17]. Therefore, proper CPA addition and removal procedures are critical to cryopreservation protocols. To optimize these CPA perfusion procedures, it is essential to measure the dynamic CPA distribution inside the organs to obtain CPA mass transfer properties and predict the efficiency of different procedures.
Magnetic resonance imaging (MRI) is a widely used technique to detect spatial distribution of chemical components inside 3D structures without invasive methods [5,10]. Different MRI techniques have been developed to investigate CPA perfusion processes in tissues. For example, Fuller et al. [6] and Walcerz et al. [24] used an MR spectroscopic technique to obtain averaged CPA concentration over the entire volume of the tissues, and their procedure was improved by the chemical shift-specific slice selection (C4S) technique [23] to obtain the distribution of CPA concentration. Combining both the chemical shift selection (CHESS) and fast low angel shot (FLASH), the image quality was further improved with an enhanced signal-to-noise ratio [1,2,9]. However, to date there has been no attempts to apply these MRI techniques to organs, due to the complicated mass transfer process inside organs and the lack of simple physical models.
Even for relatively simple organs such as mouse ovaries, there exist different cell and tissue types at different locations. During a typical CPA diffusion process, CPA and water molecules diffuse through both cell membranes and intercellular space. This results in complicated physical models and involve parameters that are technically difficult to measure [14,19]. To overcome these theoretical and technical difficulties, a simple model and MRI analysis process is required. In this study we proposed the use of a phenomenological mass transfer property of CPA: the apparent CPA diffusivity, D̄. Instead of measuring the CPA gradient inside tissues or MR spectrum frequency shift as previously described [10,14], we calculated the average CPA concentration in the centric cross-section (the cross-section with maximum area) of mouse ovaries from the MRI images and fit these data to the integral form of the mass transfer equations to obtain D̄. With this phenomenological parameterization the requirement of image quality is lowered, and the characteristics of the EG perfusion processes in mouse ovaries can be investigated and used to optimize CPA perfusion procedures.
Due to their relatively small size, mouse and rat ovaries may be cryopreserved using equilibrium cooling methods with relatively low CPA concentrations (~1.5 M) and slow cooling rates (~0.3 K/min) [3,15,25]. Investigators using the current perfusion methods generally attempt to achieve a uniform CPA concentration distribution inside ovaries before cooling [3,15,25]. However, this may not be the optimal approach. During a typical equilibrium cooling process, as temperature decreases and the solute concentration of freezing media increases, the cells near the ovary surface are able to efficiently lose intracellular water due to their direct contact with the extracellular solutions. In contrast, influenced by the slow diffusion process of water and CPA in tissues at subzero temperatures, the cells near the ovary center will be unable to lose enough intracellular water, resulting in a high possibility of IIF during cooling, which is generally believed to be lethal to cells [17,22]. Therefore, a relatively high concentration of CPA (~6-7 M) is required to prevent IIF in the center of ovaries. However, when the ovaries perfused with 6-7 M CPA are transferred into the solutions with relatively low CPA concentrations (~1.5 M) for the following equilibrium cooling procedures, the cells near the surface will encounter a large amount of water influx due to the significant solute concentration difference (~5 M) between intra- and extracellular solutions. This process may severely damage the cells by osmotic effects. Based on the analyses above, for relatively small organs that can be cryopreserved using equilibrium cooling methods, we hypothesize that a perfusion procedure that results in a relatively high CPA concentration near the organ center and a relatively low CPA concentration on the surface will be superior to currently used approaches. Obviously, traditional perfusion procedures are incapable of achieving such an “inverse” CPA distribution. This study was conducted to investigate an improved perfusion method to attenuate cell damage due to IIF near the center of ovaries and assess its efficiency through theoretical analyses.
Methods and Materials
Mouse Ovaries
Fresh ovaries were obtained from CD-1 female mice. They were first transferred into Flushing and Holding Medium (FHM; [15]) at 37°C. All procedures using animals were approved by our institutional animal care committee and conducted in accordance with standards as described in the Guide for the care and use of laboratory animals (National Research Council, Washington DC). Both ovaries were cleaned with tissue paper and mounted on a plastic sample holder as shown in Fig. 1. The sample holder was inserted into a perfusion tube, which was loaded with 20 ml 40% (w/w) EG and 0.9% saline solution. The time duration for the insertion process and the first image acquisition was recorded and appended in the intensity curve with a zero concentration value as the curve origin.
Fig. 1.

The sample holder for the MRI experiments.
Rapid MRI
Magnetic resonance imaging of mouse ovaries was carried out on a 7 T/ 210 mm horizontal bore Varian Unity Inova MRI system (Varian Inc., Palo Alto, CA) equipped with a quadrature driven birdcage RF coil (38 mm I.D). After the perfusion tube with ovaries was inserted into the RF coil, a fast gradient echo multi-slice (GEMS) pulse sequence was applied to collect ovary images at a series of time points. The proton magnetic resonance (MR) spectrum was demonstrated in Fig.2 when the perfusion tube was loaded with the EG solution, where the chemical shift between the protons of water and EG is 320 Hz at 7 T. The excitation frequency was centered on the resonant frequency of the –CH2 group in EG molecules as shown in Fig.2, so that the signal of the resonance of water protons was suppressed to prevent the influence of water molecules on MR images. Consequently, the images obtained were only the signals generated by the excitation of the protons in the -CH2 group of EG molecules, and thus the distribution of EG could be detected. The images were acquired with a flip angle of 10°, repetition time of 108 ms, echo time of 10 ms, three slices with 0.5 mm thickness, 20 repetitions, 128 × 128 data matrix, 20 × 20 mm field of view, and Fourier transformed with one zero filling in each dimension, yielding a 78 × 78 μm in-plane resolution. The time interval between images was chosen to be 5 min, to balance the image quality and sampling rate to achieve optimal curve-fitting results. For each image, the average image intensity of the centric cross-section of each ovary was calculated by VNMRJ software (Varian Inc., Palo Alto, CA) with the boundary of the ovaries manually determined.
Fig.2.
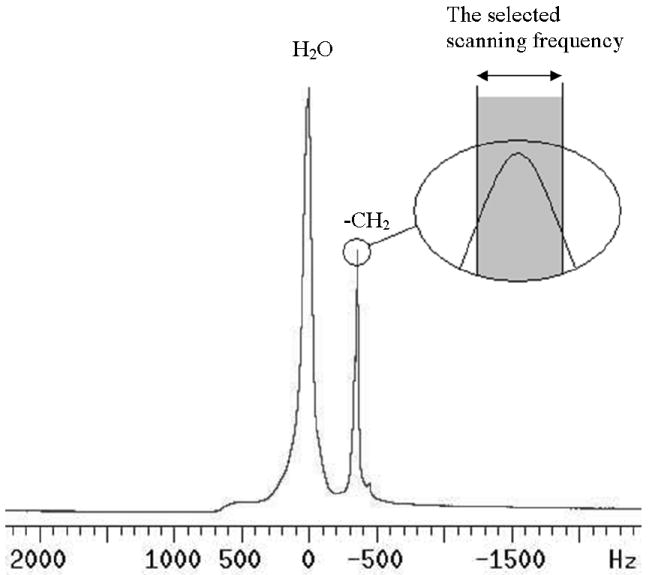
The proton MR spectrum at 7 T from the sample holder loaded with two ovaries and 40% (w/w) EG solution. The excitation frequency was centered at the resonance frequency for the –CH2 group in EG molecules.
Physical Model
To simplify the analytic procedures, the mouse ovaries were modeled as a sphere with the same cross-sectional area as ovaries. The apparent (i.e., volumetrically averaged) diffusivity (D̄) was used as the phenomenological parameter for the perfusion process in the whole ovaries. Therefore, the governing equation to describe the diffusion of CPA in the ovaries is
| Eq.1 |
or, in the spherical coordinates:
| Eq.2 |
Assuming the concentration of the EG solution in the perfusion tube is constant, the boundary condition is chosen as a constant EG concentration on the surface of ovaries:
| Eq.3 |
where R is the radius of the spherical ovaries, and was calculated by the square root of the maximum cross-sectional area of the ovaries divided by π, and Cext is the CPA concentration of the perfusion solution. Then the theoretical solution to this diffusion process is expressed as
| Eq.4 |
where C0(r) is the initial CPA distribution inside the ovaries. The spatially averaged concentration of the centric cross-section of the ovaries can be obtained through
| Eq.5 |
and in the case where C0(r) = 0, we can integrate explicitly and simplify to get
| Eq.6 |
With the value of C̄ obtained from the MRI experiments as a function of time, the value of D̄ can be calculated through curve-fitting Eq. 6 with a genetic algorithm [18]. Using this value of D̄ and Eq.4, we can predict the CPA distribution inside mouse ovaries with different perfusion procedures.
An “inverse” perfusion procedure
To achieve a relatively high concentration of CPA near the center of ovaries and a relatively low concentration near their surface, an “inverse” perfusion procedure was proposed. The ovary is first exposed to perfusion solutions with increasing CPA concentrations in a commonly used stepwise fashion, e.g. 1 M EG for 40 min, 4 M for 40 min and 7 M for 40 min. Then the ovary is perfused with decreasing CPA concentrations for relatively short times, e.g. 5 M for 5 min, 3 M for 5 min and 1.5 M for 5 min. The whole procedure will then result in a lower concentration on the surface and a higher concentration in the center. The reason for gradually lowering the EG concentration is to attenuate the osmotic damage to the cells near the ovary surface when they are exposed to these relatively low CPA concentrations. The change of CPA (EG) concentration in the perfusion solutions is demonstrated in Fig. 3. An analytic solution of the diffusion equation with time dependent boundary conditions exists, but in our case it is sufficient to use Eq. 4 at each constant concentration perfusion step, e.g. the step when EG concentration for perfusion remains as 4 M or 7 M for 40 min, with the initial concentration distribution C0(r) of the new perfusion step set to the ending concentration distribution of the last perfusion step. Therefore, the theoretical solution for the EG concentration distribution during the whole perfusion procedure can be still obtained through this stepwise strategy.
Fig.3.
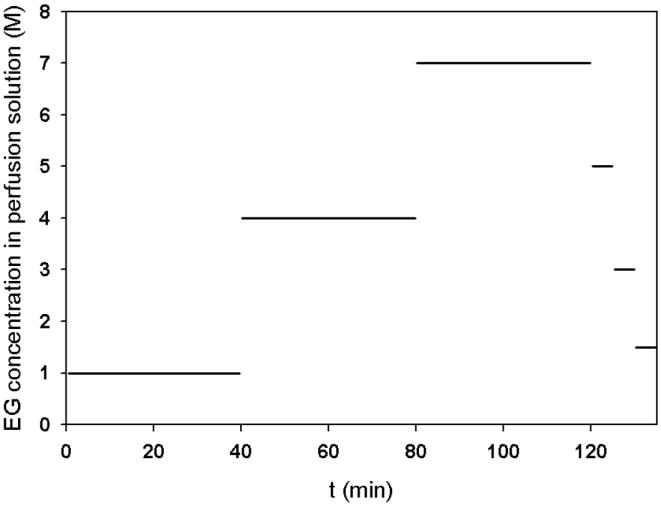
A schematic of the EG concentration change for the proposed perfusion procedures.
Results
To calibrate the relationship between the image intensity and EG concentration in ovaries, ovaries containing 0, 10, 20, 30, 40% (w/w) EG were scanned in five plastic straws using the same MRI procedure. The average image intensity of the centric cross-section of each ovary was calculated. Figure 4 demonstrates that the image intensity is a linear function (R2=0.99) of the EG concentration in these ovaries. Therefore, the value of C̄ can be calculated from the image intensity by fitting to this linear function. During the EG perfusion procedure in the tube as shown in Fig. 1, the image intensity of the centric cross-section of the ovaries continuously increased until it reached a plateau. Two sample images are as shown in Fig. 5. The average image intensity across the centric cross-section of each ovary was calculated and the values of C̄ were obtained through the linear relationship demonstrated in Fig. 4. Figure 6 shows the value of C̄ inside an ovary with a radius of 1.1 mm as a function of time, and the fitted curve using the theoretical model given by Eq. 6. The same procedures were repeated for 8 ovaries and the value of D̄ of EG was calculated as 6.1 ± 1.4×10-7 cm2/s (mean ± SD).
Fig.4.
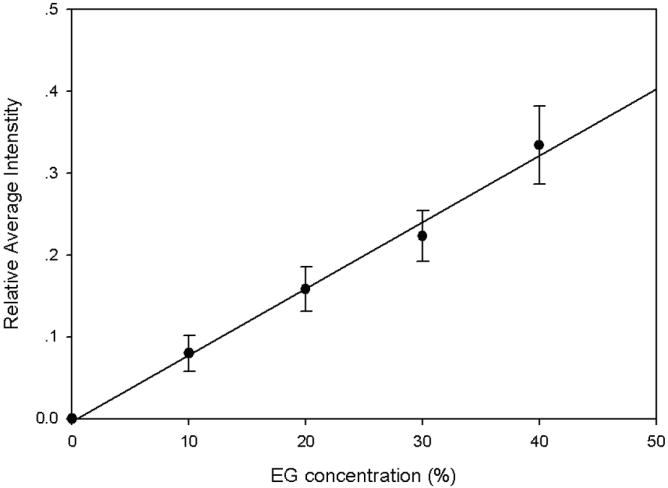
The linear relationship between the EG concentration in ovaries vs. the average image intensity.
Fig.5.
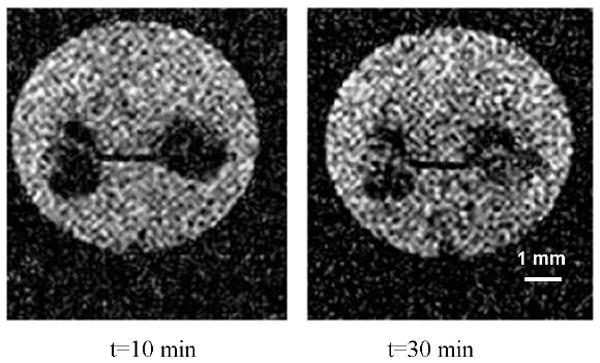
Two sample MR images, with water signal saturated, showing the increasing EG concentration in ovaries during perfusion.
Fig.6.
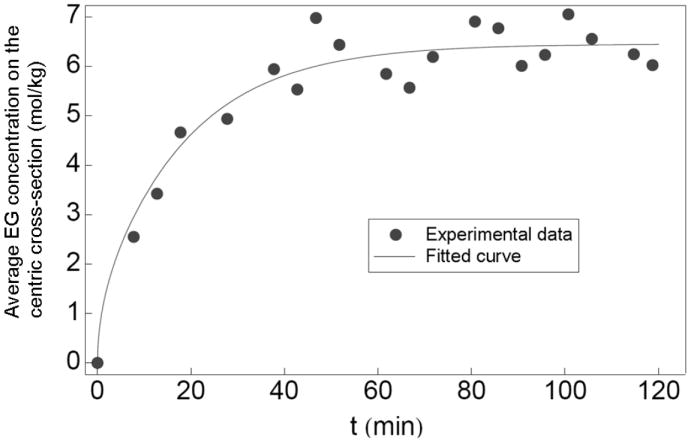
The experimental data with their fitted curve for the average EG concentration change on the centric cross-section of an ovary with 1.1 mm as its identical radius.
With this value of D̄ and the stepwise strategy to obtain the theoretical solutions, when the inverse perfusion procedure is followed, the final distribution of EG concentration in a spherical mouse ovary with a radius of 1 mm is shown in Fig. 7. During the whole perfusion process, the EG concentration as a function of time at different locations in the ovary is shown in Fig. 8.
Fig.7.
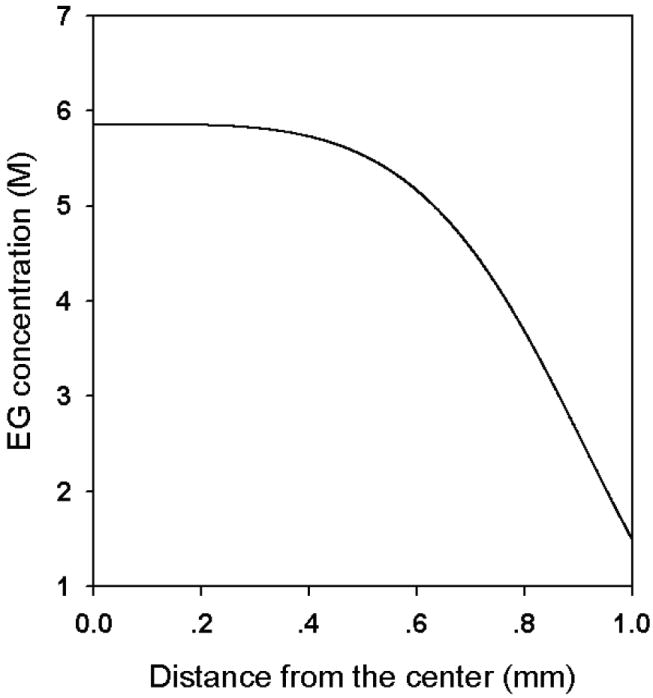
The theoretical results of the “inverse” EG distribution in an ovary of 1mm in radius after the proposed perfusion procedure is used.
Fig.8.
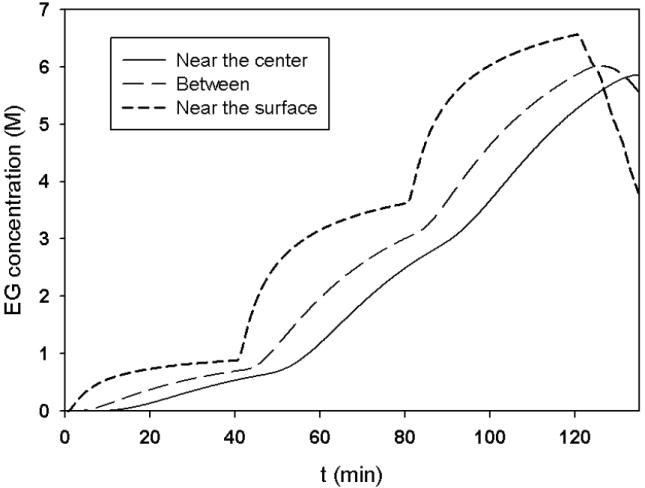
The change of EG concentration in the ovary using the proposed perfusion procedure.
Discussion
During the CPA perfusion processes in tissues or organs, water diffusion accompanies with CPA diffusion, and calculating the MR image intensity from the excitation of the protons in both water and CPA molecules will result in complicated physical analyses. On the other hand, to design a perfusion procedure, the primary concern is the CPA concentration distribution. Considering these two facts, water saturation techniques were applied in this study to eliminate the water signal and reveal the movement of EG molecules into the different locations of ovaries.
Different methods have been developed to measure CPA diffusivity in tissues [1,2,6,10,24]. In organs, even as simple as mouse ovaries, there exist different types of tissues. As a result, the CPA perfusion rate in ovaries is non-uniform as shown in Fig. 5 and previous methods cannot be applied in this situation. Meanwhile, for the application of any perfusion technique, it is important to know the overall perfusion time to design the perfusion procedures. Therefore, an average value of CPA diffusivity in organs is needed to simplify the analyses and technical designs. We defined the phenomenological parameter D̄ to solve this problem and the curve-fitting result as shown in Fig.6 demonstrates that the use of D̄ satisfies the need to determine the time duration required for EG perfusion procedures. With the measured value of D̄, we can treat the ovaries as porous media with a uniform EG diffusivity the same as D̄, and the osmotic behavior of cells at different locations can be simulated with the extracellular solute concentrations determined by the simple diffusion process in such hypothetical porous media. Through this approach, we can optimize the perfusion procedures to minimize the osmotic damage to cells. Compared to the EG diffusivity in water [21], our result for the value of D̄ in the mouse ovaries is approximately one order lower.
Commonly used cryopreservation protocols achieve uniform CPA concentrations (~1.5 M) distributions prior to cooling. Then, during cooling the cells near the center of ovaries can lose little water during equilibrium cooling procedures, due to relatively low water diffusivity in tissues at subzero temperatures (on the order of 10-7 cm2/s). As a result, the possibility of IIF in these center cells is significantly increased. When the perfusion method we proposed is used, the concentration of the EG in the center of the ovaries can still maintain ~6M at the end of the perfusion procedure, which should significantly lower the possibility of IIF by decreasing the degree of supercooling and increasing the solution viscosity [22].
Conclusion
In this study, a simple physical model was proposed to investigate CPA perfusion processes in organs. The apparent EG diffusivity in mouse ovaries was defined and measured using rapid MRI with water saturation techniques to overcome the technical difficulties generated by the multiple tissue and cell types. To achieve a relatively high concentration of CPA in the center of organs to prevent IIF when equilibrium cooling procedures are used, an “inverse” perfusion method was proposed. The efficiency of the method was assessed by theoretical analyses, and it was demonstrated that this method significantly increases the CPA concentration in the ovary center without influencing the concentration near the surface.
Table 1.
Symbol definitions and units
| Symbol | Definition | Units |
|---|---|---|
| D̄ | Diffusivity | cm2/s |
| C | Concentration | mol/kg |
| t | Time | s |
| r | Radial coordinate | m |
| R | Radius of spherical ovary | m |
| Subscript 0 | Initial value | |
| Subscript ext | External to ovaries |
Acknowledgments
The work was supported by a grant from the National Institutes of Health (R01 5RL1HD058293), the University of Missouri Comparative Medicine Center, and the Cryobiology Research Institute. We also acknowledge the MRI facility support provided by the VA Biomolecular Imaging Center at the Harry S Truman VA Hospital and the University of Missouri.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bidault NP, Hammer BE, Hubel A. Use of acombined C4S-Keyhole imaging technique to study the dynamics of cryoprotective agents in an engineered tissue. Proc ISMRM. 1998:1455. [Google Scholar]
- 2.Bidault NP, Hammer BE, Hubel A. Rapid MR imaging of cryoprotectant permeation in an engineered dermal replacement. Cryobiology. 2000;40:13–26. doi: 10.1006/cryo.1999.2216. [DOI] [PubMed] [Google Scholar]
- 3.Deanesley R. Immature rat ovaries grafted after freezing and thawing. J Endocrinol. 1954;11:197–200. doi: 10.1677/joe.0.0110197. [DOI] [PubMed] [Google Scholar]
- 4.Fahy GM, Lilley T, Lindsell H, Douglas M, Meryman H. Cryoprotectant toxicity: In search of molecular mechanisms. Cryobiology. 1990;27:257–268. doi: 10.1016/0011-2240(90)90025-y. [DOI] [PubMed] [Google Scholar]
- 5.Frahm J, Haase A, Matthaei D. Rapid NMR imaging of dynamic processes using the FLASH technique. Magn Reson Med. 1986;3:321–327. doi: 10.1002/mrm.1910030217. [DOI] [PubMed] [Google Scholar]
- 6.Fuller BJ, Busza AL. Proton NMR studies on the permeation of tissue tragments by dimethyl sulphoxide: Liver as a model for compact tissues. Cryo-Letters. 1994;15:131–134. [Google Scholar]
- 7.Gao DY, Critser JK. Encyclopedia of biomaterials and biomedical engineering. Marcel Dekker. Inc; New York: 2003. Cryopreservation of living cells. [Google Scholar]
- 8.Gao DY, Critser JK. Mechanisms of cryoinjury in living cells. ILAR J. 2000;41:187–196. doi: 10.1093/ilar.41.4.187. [DOI] [PubMed] [Google Scholar]
- 9.Haase A, Frahm J, Hanicke W, Matthaei DH. NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985;30:341–344. doi: 10.1088/0031-9155/30/4/008. [DOI] [PubMed] [Google Scholar]
- 10.Isbell SA. Development of protocol for quantitative evaluation of contrast in NMR images of cryoprotective solvents in intact tissues. Cryobiology. 1997;34:165–175. doi: 10.1006/cryo.1996.1995. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen IA, Pegg DE. Cryopreservation of organs: A review. Cryobiology. 1984;21:377–384. doi: 10.1016/0011-2240(84)90076-2. [DOI] [PubMed] [Google Scholar]
- 12.Karrow AM, Critser JK. Reproductive Tissue Banking: Scientific Principles. San Diego, USA: Academic Press; 1997. [Google Scholar]
- 13.Khirabadi B, Fahy GM. Permanent life support by kidneys perfused with a vitrifiable (7.5 molar) cryoprotectant solution. Transplantation. 2000;70:51–57. [PubMed] [Google Scholar]
- 14.Le Bihan D. Applications to functional MRI. Raven Press; New York: 1995. Diffusion and perfusion magnetic resonance imaging. [Google Scholar]
- 15.Liu L, Wood GA, Morikawa L, et al. Restoration of fertility by orthotopic transplantation of frozen adult mouse ovaries. Human reproduction. 2008;23:122–128. doi: 10.1093/humrep/dem348. [DOI] [PubMed] [Google Scholar]
- 16.Mazur P. Freezing of living cells: mechanisms and implication. American Journal of physiology. 1984;143:125–142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 17.Mazur P, Leibo SP, Chu EHY. A two factor hypothesis of freezing injury: Evidence from Chinese hamster tissue-culture cells. Exp Cell Res. 1972;71:345–355. doi: 10.1016/0014-4827(72)90303-5. [DOI] [PubMed] [Google Scholar]
- 18.Michalewicz Z, Fogel DB. How to solve it: modern heuristics. Springer-Verlag; Berlin: 2000. [Google Scholar]
- 19.Pegg DE. The biophysics of organ preservation. Plenum; NY: 1984. [Google Scholar]
- 20.Petechuk D. Organ transplantation (health and medical issues today) Greenwood press; 2006. [Google Scholar]
- 21.Ternstrom G, Sjostrand A, Aly G, Jernqvist A. Mutual diffusion coefficients of water + ethylene glycol and water + glycerol mixtures. J Chem Eng Data. 1996;41:876–879. [Google Scholar]
- 22.Toner M, Cravalho EG, Karel M. Thermodynamics and kinetics of intracellular ice formation during freezing of biological cells. J Appl Phys. 1990;67:1582–1593. [Google Scholar]
- 23.Volk A, Tiffon B, Mispelter J, Lhoste JM. Chemical shift-specific slice selection. A new method for chemical shift imaging at high magnetic field. J Magn Reson. 1987;71:168–174. [Google Scholar]
- 24.Walcerz DB, Taylor MJ, Busza AL. Determination of the kinetics of permeation of dimethyl sulfoxide in isolated corneas. Cell Biophys. 1995;26:79–102. doi: 10.1007/BF02796236. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Chen H, Yin H, et al. Fertility after intact ovary transplantation, frozen banking of whole organs for transplantation is starting to look feasible. Nature. 2002;415:385. doi: 10.1038/415385a. [DOI] [PubMed] [Google Scholar]


