Abstract
Palmitoylation is a prevalent feature amongst G protein-coupled receptors. In this study we sought to establish whether the TPα and TPβ isoforms of the human prostanoid thromboxane (TX) A2 receptor (TP) are palmitoylated and to assess the functional consequences thereof. Consistent with the presence of three cysteines within its unique carboxyl-terminal domain, metabolic labelling and site-directed mutagenesis confirmed that TPβ is palmitoylated at Cys347 and, to a lesser extent, at Cys373,377 whereas TPα is not palmitoylated. Impairment of palmitoylation did not affect TPβ expression or its ligand affinity. Conversely, agonist-induced [Ca2+]i mobilization by TPβC347S and the non-palmitoylated TPβC347,373,377S, but not by TPβC373S or TPβC373,377S, was significantly reduced relative to the wild type TPβ suggesting that palmitoylation at Cys347 is specifically required for efficient Gq/phospholipase Cβ effector coupling. Furthermore, palmitoylation at Cys373,377 is critical for TPβ internalization with TPβC373S, TPβC373,377S and TPβC347,373,377S failing to undergo either agonist-induced or temperature-dependent tonic internalization. On the other hand, whilst TPβC347S underwent reduced agonist-induced internalization, it underwent tonic internalization to a similar extent as TPβ. The deficiency in agonist-induced internalization by TPβC347S, but not by TPβC373,377 nor TPβC347,373,377S, was overcome by over-expression of either β-arrestin1 or β-arrestin2. Taken together, data herein suggest that whilst palmitoylation of TPβ at Cys373,377 is critical for both agonist- and tonic-induced internalization, palmitoylation at Cys347 has a role in determining which pathway is followed, be it by the β-arrestin-dependent agonist-induced pathway or by the β-arrestin-independent tonic internalization pathway.
Abbreviations: AR, adrenergic receptor; C-tail, carboxyl-terminal tail; [Ca2+]i, intracellular calcium; eNOS, endothelial nitric oxide synthase; GFP, green fluorescent protein; GPCR, G protein-coupled receptor; HA, hemagglutinin; HEK, human embryonic kidney; 5-hydroxytryptamine (4a) receptor, 5-HT4(a); IP, prostacyclin receptor; IP3, inositol 1, 4, 5-trisphosphate; PAGE, polyacrylamide gel electrophoresis; PK, protein kinase; PL, phospholipase; TP, TXA2 receptor; TXA2, thromboxane A2
Keywords: Thromboxane A2 receptor, Receptor internalization, Palmitoylation
1. Introduction
The covalent addition of lipid moieties to proteins is widely recognized as an important regulatory mechanism in eukaryotic cells contributing to protein:membrane and/or protein:protein interactions [1,2]. Common lipidations include the co-translational attachment of myristate acid via a stable amide linkage to amino (N)-terminal glycine residues, post-translational attachment of farnesyl or geranylgeranyl isoprenoids to carboxy (C)-terminal cysteine(s) via thioether bond(s) and post-translational addition of palmitate or other fatty acyl group to cysteine(s) via labile thioester linkages [1–5]. The latter, generally referred to as palmitoylation but more accurately termed acyl thioesterification or S-acylation, is the least understood despite being the most common lipid modification [6]. Unlike myristoylation or isoprenylation, palmitoylation is a dynamic modification with proteins constantly undergoing enzymatic cycles of palmitoylation and depalmitoylation, events that are thought to regulate not only membrane/protein interactions but also protein function [1,6]. This has been supported with the recent identification of candidate mammalian protein:S-acyl-transferases (PATs) and S-acylprotein thioesterases (APTs) that catalyze such palmitoylations and depalmitoylations, respectively [5,6].
A diverse range of palmitoylated proteins have been identified, most notably amongst proteins participating in intracellular signalling including H- and N-Ras, endothelial nitric oxide synthase, growth cone-associated protein 43, SRC and several other protein tyrosine kinases ([4,5] and references therein). Palmitoylation is also a feature of heterotrimeric Gα subunits and of their downstream effectors, including Gαs, Gαq and adenylyl cyclase [1,4,5] and is a common modification of numerous G protein-coupled receptors (GPCRs). In fact, it is estimated that up to 80% of GPCRs contain at least one palmitoylable cysteine typically located some 10–14 amino acid residues downstream from transmembrane (TM) 7 within their carboxyl-terminal (C)-tail domain [7]. Rhodopsin, the first GPCR to be confirmed to be palmitoylated, undergoes palmitoylation at Cys322 and Cys323 within its C-tail domain and it was proposed that interaction of the hydrophobic palmitoyl moieties with the lipid bilayer of the plasma membrane may result in the formation of a fourth intracellular loop within its GPCR structure [8]. Numerous other GPCRs have since been shown to be palmitoylated within their C-tail domains including the α2A- and β2-adrenergic receptors [9,10], the dopamine D1 receptor [11,12], the 5-hydroxytryptamine (4a) receptor [13], the muscarinic acetylcholine receptor [14], the prostacyclin receptor [15] and the vasopressin V1A and V2 receptors [16,17]. Through these and many other specific examples, it is evident that while palmitoylation may potentially affect diverse aspects of GPCR function and regulation including processing and targeting, protein turnover, G protein coupling, phosphorylation and desensitization, sequestration and/or internalization [1–5], it is also apparent that such effects are generally receptor specific, requiring investigation at the individual GPCR level.
The prostanoid thromboxane (TX)A2 plays a critical role in vascular hemostasis regulating platelet activation status and vascular tone [18]. In humans, but not in non-primates, TXA2 signals through two distinct isoforms of the TXA2 receptor (TP) termed TPα and TPβ [18–21]. As members of the GPCR superfamily, TPα and TPβ are identical for their first N-terminal 328 amino acids and diverge exclusively within their carboxyl-terminal (C)-tail domains [18–22]. While TPα and TPβ arise by differential splicing [20], they display distinct patterns of mRNA and protein expression [23,24] and, more recently, it has been established that they are under the transcriptional regulation of distinct promoters within the single human TP gene [25–28]. Moreover, whilst both TPα and TPβ exhibit identical ligand binding and Gq-dependent activation of phospholipase (PL) Cβ, their primary effector [20,29–31], they oppositely regulate adenylyl cyclase through Gs and Gi, respectively [32] and TPα, but not TPβ, can couple to Gh/tissue transglutaminase activation [33].
Hence, it is evident that TPα and TPβ display differences in their patterns of expression and in their modes of intracellular signalling, suggesting that they may have distinct physiologic roles [22]. Consistent with this hypothesis, there is an abundance of emerging evidence suggesting that the greatest differences between TPα and TPβ lies in their modes of desensitization/regulation post-receptor signalling, through events that are largely regulated through sequences or functional determinants within their respective unique C-tail domains. For example, while both TPs undergo agonist-induced phosphorylation [24,29], TPβ, but not TPα, is subject to both agonist-induced and tonic internalization [34,35]. Agonist-induced TPβ internalization appears to be dynamin-, GRK2/3- and β-arrestin-dependent [34,35], requiring an active or dynamic actin cytoskeleton [36] and may also involve direct interaction between Rab 11 and the C-tail domain of TPβ [37]. On the other hand, tonic internalization of TPβ is both GRK- and β-arrestin-independent but dynamin-dependent and is also dependent on the presence of an intact internalization motif within its C-tail domain [34,35]. In studies investigating cross-talk between TXA2 and other prostanoids, it was established that signalling by TPα, but not TPβ, is subject to prostacyclin- and prostaglandin (PG)D2-mediated desensitization involving direct PKA phosphorylation of TPα at Ser329 within its unique C-tail domain [30,38]. Consistent with this, TPα, but not the TPβ, is also a target for nitric oxide (NO)-induced desensitization that occurs through a mechanism involving its direct PKG phosphorylation at Ser331 within its unique C-tail domain [39]. These latter studies point to an essential role for TPα in prostacyclin- and NO-regulated vascular hemostasis and point to a redundant or an, as yet, unidentified role for TPβ in this essential physiologic process [22,30,38,39]. Moreover, collectively, these data also indicate that TPα and TPβ are subject to distinct modes of regulation, intracellular signalling and expression and that such differences are largely determined by sequences within their unique C-tail domains.
Another notable difference between the TP isoforms, the significance of which has yet to be investigated, is the presence of multiple cysteine (Cys) residues within the unique C-tail of TPβ at Cys347, Cys373 and Cys377, while the C-tail domain of the TPα is entirely devoid of such residues. The presence of Cys residues within the unique C-tail domain of TPβ raises the possibility that they may be palmitoylated and that such modification(s) may contribute to the functional differences between the two TP isoforms. Hence, the aim of the current study was to investigate whether TPβ is subject to palmitoylation and to examine the functional consequences thereof. Our studies confirm that TPβ, but not TPα, is palmitoylated at Cys347 and, to a lesser extent, at Cys373/377, a modification that not only regulates agonist-induced second messenger signalling but also differentially influences agonist- and tonic-induced internalization of TPβ.
2. Experimental procedures
2.1. Materials
U46619 and SQ29,548 were obtained from Cayman Chemical Company. G418 sulfate and FURA2/AM were from Calbiochem. [3H]SQ29,548 (50.4 Ci/mmol) was obtained from DuPont NEN. Rat monoclonal 3F10 anti-HA-horse radish peroxidase-conjugated antibody (25 μg/ml) was obtained from Roche. Mouse monoclonal anti-hemagglutinin (HA)-101R antibody (1 mg/ml) was obtained from Eurogentec; goat anti-β-arrestin1 (K-16), goat anti-β-arrestin2 (N-16), horse radish peroxidase (HRP)-conjugated goat anti-mouse (400 μg/ml), HRP-conjugated mouse anti-goat (400 μg/ml) and HRP-conjugated goat anti-rabbit (400 μg/ml) secondary antibodies were from Santa Cruz; goat anti-mouse FITC-conjugated antibody, propidium iodide, protein G sepharose 4B Fast Flow (∼ 50% v/v slurry) were obtained from Sigma. AlexaFluor594 goat anti-mouse antibody (2 mg/ml) was from Molecular Probes. [9, 10-3H] Palmitic acid (60 Ci/mmol) was purchased from TOCRIS.
2.2. Site-directed mutagenesis of TPβ to generate TPβC347S, TPβC373S, TPβC373,377S and TPβC347,373,377S
The plasmids pHM6:TPα, pHM6:TPβ and pHM6:hIP encoding HA epitope-tagged forms of TPα, TPβ and human prostacyclin receptor (IP), respectively, were previously described [30,40]. pRK5:β-arrestin1, pcDNA3:β-arrestin2, pEGFPN3:β-arrestin1 and pEGFPN1:β-arrestin2 encoding wild type and green fluorescent protein (GFP)-tagged forms of β-arrestin1 and β-arrestin2 have been described [41–43]. pCMV:Gαq, encoding Gαq, has been previously described [40,44].
Conversion of Cys347 to Ser347 of TPβ to generate pHM6:TPβC347S was achieved using pHM6:TPβ as template and the sense/antisense primer pair (5′ G ATC TCG GCT CAC TCC AAC CTC CGC CTC). Conversion of Cys373 to Ser373 to generate pHM6:TPβC373S was achieved using pHM6:TPβ as template and the sense/antisense primer pair (5′ GTA AGC CAC TCC GCC CGG CCT TGC). Conversion of Cys373,377 to Ser373,377 to generate pHM6:TPβC373,377S was performed using pHM6:TPβ as template and the sense/antisense primer pair (5′ GTA AGC CAC TCC GCC CGG CCT TCC ATG CTC TTT G). The plasmid pHM6:TPβC347,373,377S was generated using pHM6:TPβC347S as template and sense/antisense primer pair (5′ GTA AGC CAC TCC GCC CGG CCT TCC ATG CTC TTT G). For each primer pair, sequences shown correspond to the sense primer and the identity of the mutator codon is in boldface italics. All site-directed mutagenesis was performed using QuikChange™ (Stratagene) site-directed mutagenesis and all mutations were validated by DNA sequence analysis.
2.3. Cell culture and transfections
Human embryonic kidney (HEK) 293 cells were obtained from the American Type Culture Collection and were grown in minimal essential medium (MEM) containing 10% foetal bovine serum (FBS).
Routinely, approximately 48 h prior to transfection cells were plated at a density of 2 × 106 cells/10 cm culture dish in 8 ml media. Thereafter, cells were transiently transfected with 10 μg of pADVA [45] and 25 μg of pCMV- or pHM-based vectors using the calcium phosphate/DNA co-precipitation procedure, as previously described [44]. For transient transfections, cells were harvested 48 h after transfection. To create stable cell lines, HEK 293 cells were transfected with 10 μg Sca1 linearized pADVA plus 25 μg Sca1 linearized pHM-based vectors. Forty-eight hours post-transfection, G418 (0.8 mg/ml) was added; individual G418 resistant colonies were selected after approximately 21 days and clonal cell lines were expanded and were assessed for TP expression by radioligand binding assay using the radioligand [3H]SQ29,548. In this way, HEK.TPβC347S, HEK.TPβC373S, HEK.TPβC373,377S and HEK.TPβC347,373,377S cell lines stably over-expressing HA-tagged forms of TPβC347S, TPβC373S, TPβC373,377S and TPβC347,373,377S, respectively, were established. HEK.TPα, HEK.TPβ and HEK.hIP cells stably over-expressing HA-tagged form of TPα, TPβ and of the human IP, respectively, have been previously described [30,40].
2.4. Radioligand binding studies
TP radioligand binding assays were carried out at 30 °C for 30 min in the presence of 0–40 nM [3H]SQ29,548 for Scatchard analyses or in the presence of 20 nM [3H]SQ29,548 for saturation radioligand binding experiments as previously described [44]. Protein determinations were carried out using the Bradford assay.
2.5. Palmitoylation of the thromboxane receptor
Palmitoylation was carried out essentially as previously described [15]. Briefly, cells were plated 48 h in advance to achieve a density of approx. 3 × 105 cells/10-cm dish (∼ 80% confluency) on the day of metabolic labelling. Cells were washed once in PBS and then metabolically labelled in serum-free MEM (1.5 ml) containing 1.5 mCi of [3H] palmitic acid (60 Ci/mmol). Following incubation for 2 h at 37 °C, the labelling reaction was stopped by washing the cells in ice-cold PBS. Thereafter, cells were lysed by the addition of 600 μl RIPA buffer (20 mM Tris–Cl, pH 8.0, 0.15 M NaCl, 1% Triton X-100, 1% SDS, 1% deoxycholate, 10 mM EDTA, pH 8.0, 1 mM PMSF, 2 mM 1,10-phenanthroline, 10 μg/ml aprotinin, 10 μg/ml antipain, 1 μg/ml leupeptin, 10 μg/ml benzamidine) and were disrupted by passing through needles of decreasing bore size. The lysate was centrifuged at 13,000 rpm for 5 min and the supernatant (600–700 μg) was subjected to immunoprecipitation using the anti-HA 101R antibody (1:300 dilution) followed by the addition of 10 μl protein G Sepharose 4B (50% v/v slurry) and further incubation at room temperature for 1 h, as described [15]. Immunoprecipitates were then resuspended in SDS-PAGE sample buffer (25 mM Tris–Cl, pH 8.0, 1% SDS, 5% glycerol, 0.0013% (w/v) Bromophenol Blue, 1% β-Mercaptoethanol), incubated at room temperature for 15 min, and then resolved by 8% SDS-PAGE. After electroblotting onto PVDF membrane, the blots were soaked in Amplify (Amersham) for 30 min followed by fluorography using Kodak X-Omat XAR film for 60 days at − 70 °C. Thereafter, following fluorographic exposure, PVDF membranes were screened by immunoblot analysis using the anti-HA 3F10 peroxidase-conjugated antibody followed by chemiluminescence detection.
2.6. Calcium measurements
Measurements of agonist-induced intracellular calcium ([Ca2+]i) mobilization in response to the TXA2 mimetic U46619 (1 μM) were carried out in FURA2/AM preloaded HEK.TPβ, HEK.TPβC347S, HEK.TPβC373S, HEK.TPβC373,377S and HEK.TPβC347,373,377S cells that had been transiently co-transfected with pCMV:Gαq and pADVA some 48 h previously, essentially as described [44]. The agonist U46619, in ethanol, was diluted 1:1000 in the vehicle HBSSHB (modified Ca2+/Mg2+-free Hank's buffered salt solution, containing 10 mM HEPES, pH 7.67, 0.1% bovine serum albumin) and the agonist in vehicle (20 μl) or, as controls, the vehicle alone (20 μl) were added to the 2 ml cells (0.8 × 106 cells/ml) to achieve the desired working concentration. It was established that the vehicle had no effect on [Ca2+]i mobilization by either TP isoform and had no effect on experimental data. The ratio of the fluorescence at 340 nm to 380 nm is a measure of [Ca2+]i [46], assuming a Kd of 225 nM Ca2+ for FURA2/AM. The results presented in the figures are representative data from at least three/four independent experiments and are plotted as changes in intracellular Ca2+ mobilized (Δ[Ca2+]i (nM)) as a function of time (seconds, s) following agonist stimulation.
2.7. Internalization of TP receptors
Cells were seeded at a density of 5 × 104 cells/ml per well in 1 ml of MEM, 10% FBS media into 24-well plates, which had been pre-coated with 0.001% poly-l-lysine. The cells were then incubated for 48 h at 37 °C prior to experiments. To assess agonist-induced internalization of TPβ or its mutated derivatives, the media was changed to serum-free Dulbecco's modified essential medium and thereafter, cells were treated with 1 μM U46619 for 0, 1, 2 and 4 h at 37 °C. Cells were washed twice in ice-cold PBS prior to fixation in 3.7% paraformaldehyde, 1× PBS, pH 7.4, for 15 min at room temperature. After washing the cells in 1× TBS (20 mM Tris–HCl, pH 7.4, 0.1 M NaCl), non-specific sites were blocked with Blocking Buffer (5% dried skimmed milk powder in TBS) and then cells were incubated with anti-HA 101R antibody (1:1000 in Blocking Buffer) for 1 h at room temperature. The antibody solution was removed and the cells were washed twice in TBS, prior to incubation with goat anti-mouse HRP (1:1000) for 45 min at room temperature. Thereafter, the cells were washed trice in TBS and HA-tagged receptor expression was detected colorimetrically at 650 nm using the K-Blue substrate [47].
To assess tonic internalization, 48 h post-seeding, cells were washed in MEM, 10% FBS, prior to incubation in media containing mouse anti-HA 101R (1 in 1000 dilution), at 4 °C for 1 h. Thereafter, the cells were rinsed briefly in MEM to remove unbound antibody prior to incubating the cells at 4 °C and 37 °C for 2 h. Cells were washed twice in ice-cold PBS prior to fixation in 3.7% paraformaldehyde, 1× PBS, pH 7.4, for 15 min at room temperature. After washing the cells twice in 1× TBS (20 mM Tris–HCl, pH 7.4, 0.1 M NaCl), non-specific sites were blocked with Blocking Buffer followed by incubation with the secondary goat anti-mouse HRP (1:1000) for 45 min at room temperature. Detection of the HA-tagged receptors was via colorimetric K-Blue substrate (Neogen Corp), as previously described [47].
2.8. Immunofluorescence and confocal microscopy
Cells were seeded onto poly-l-lysine pre-treated coverslips in 6-well plates to achieve 60–70% confluency following 48 h incubation at 37 °C. Thereafter, the media was changed to MEM, 10% FBS and cells were incubated with anti-HA 101R (1 in 1000 dilution) at 4 °C for 1 h. Thereafter, unbound antibody was removed by washing twice with media. To investigate agonist-induced internalization, cells were washed once with serum-free MEM prior to incubation with 1 μM U46619 for 0, 2 and 4 h at 37 °C. To examine tonic internalization, following labelling with anti-HA 101R (1 in 1000 dilution) at 4 °C for 1 h, cells were washed briefly in MEM to remove unbound antibody prior to incubation at 4 °C and 37 °C for 2 h in MEM, 10% FBS.
After the appropriate incubation, cells were washed twice in ice-cold PBS prior to fixation in 3.7% paraformaldehyde, 1× PBS, pH 7.4, for 15 min at room temperature and thereafter washed three times with PBS. Cells to be permeabilized were then incubated with 0.2% Triton X-100 in PBS for 10 min on ice followed by washing in 1× TBS. Non-specific sites were blocked by incubating cells with Blocking Buffer. Thereafter, receptors that had been previously labelled with the anti-HA 101R antibody were labelled with the secondary goat anti-mouse FITC-conjugated antibody solution. Cells were washed 3 times in 1× TBS, prior to counterstaining with propidium iodide (1 μg/ml, in H2O). Excess propidium iodide was washed away with H2O prior to mounting coverslips in Vectashield (Vector Laboratories) mounting medium. Slides were then imaged using Carl Zeiss Lazer Scanning System LSM510 using Zeiss LSM Imaging software.
2.9. β-arrestin1 and β-arrestin2 co-localization with TPβ receptors
HEK 293 cells (10-cm dishes, 60–70% confluent) were transiently co-transfected with pHM6-based plasmid encoding HA-tagged TPβ (365 ng), or its mutated variants, along with either pEGFPN3:β-arrestin1 or pEGFPN1:β-arrestin2, encoding green fluorescent protein (GFP)-tagged forms of the β-arrestin1/2 (365 ng), in the presence of pADVA (270 ng) using the Effectene (Qiagen) transfection reagent, as per manufacturer's instructions. Some 24 h post-transfection, cells were replated onto fibronectin (50 μg/ml, 2 μg/cm2) pre-coated glass coverslips (22 mm diameter) in 6-well plates and incubated for a further 48 h. Thereafter, cells were incubated with anti-HA 101R (1:1000 dilution in MEM, 10% FBS) at 4 °C for 1 h. Unbound antibody was removed by washing twice with media prior to incubating cells with 1 μM U46619 (prepared in media) for 30 min at 37 °C. Thereafter, cells were fixed in 3.7% paraformaldehyde, 1× PBS, pH 7.4, for 15 min at room temperature. Following washing (3 times with 1× PBS), cells were permeabilized by incubation on ice for 10 min in 0.2% Triton X-100 in PBS. Following washing twice in 1× TBS (20 mM Tris–HCl, pH 7.4, 0.1 M NaCl) and further incubation for 30 min with Blocking Buffer (5% dried skimmed milk powder in 1× TBS), HA-tagged receptors, previously labelled with the anti-HA 101R antibody, were visualized using the AlexaFluor594 goat anti-mouse IgG (1:1500) as secondary antibody. Cells were washed trice in TBS, prior to counterstaining with DAPI (1 μg/ml, in H2O). Excess DAPI was washed away with H2O prior to mounting coverslips in Vectashield mounting medium. A Zeiss fluorescence microscope coupled with AxioVision software (version 4.4) was used for acquiring multichannel, Z-stack images (19 slices, 0.125 μM apart) with filters appropriate for enhanced GFP and AlexaFluor 594 fluorescence. Subsequently, the multichannel, Z-stack images were processed by AxioVision Deconvolution Analysis, with enabled multichannel and Z-stack correction.
2.10. Data analyses
Radioligand binding data was analysed using GraphPad Prism V3.0 to determine the Kd and Bmax values. Statistical analyses were carried out using the unpaired Student's t test using the Statworks Analysis Package. p-Values of less than or equal to 0.05 were considered to indicate a statistically significant difference.
3. Results
3.1. Palmitoylation of the human TXA2 receptor, TP
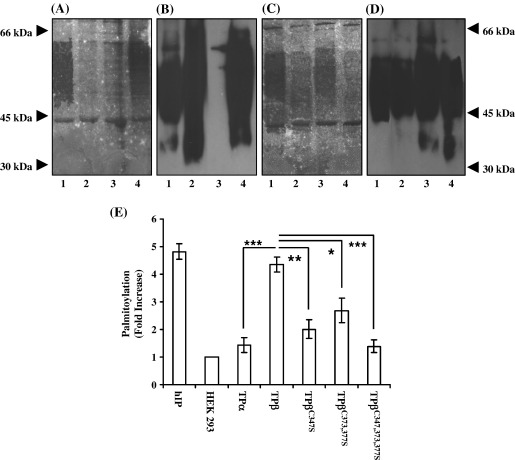
To investigate whether the human thromboxane (TX) A2 receptor (TP) isoforms are palmitoylated, human embryonic kidney (HEK) 293 cells stably over-expressing hemagglutinin (HA) epitope-tagged forms of TPα and TPβ were metabolically labelled with [3H] palmitic acid. As a control, metabolic labelling was also investigated in HEK.hIP cells stably over-expressing HA-tagged human (h) prostacyclin receptor (hIP), a GPCR previously confirmed to be palmitoylated [15]. Metabolic labelling and palmitoylation of both TPβ and the hIP was readily detected as evidenced by the presence of broad radiolabelled bands between 45 and 66 kDa markers in the immunoprecipitates from HEK.TPβ and HEK.hIP cells (Fig. 1A, lanes 1 and 4, respectively). In contrast, no metabolic labelling was associated with the immunoprecipitates from HEK.TPα cells or from control HEK 293 cells (Fig. 1A, lanes 2 and 3, respectively) confirming that, unlike that of TPβ, the TPα isoform does not undergo palmitoylation. Confirmation of equivalent expression and efficient recovery of the hIP, TPα and TPβ receptors in their respective immunoprecipitates were obtained by subsequent screening of the PVDF membrane by immunoblot analysis (Fig. 1B, lanes 1, 2 and 4, respectively) which also clearly indicated that failure to detect palmitoylation of TPα was not due to failure of its immunoprecipitation.
Fig. 1.
Analysis of palmitoylation in HEK.TPα and HEK.TPβ cells. Panels A and B, HEK.TPβ (lane 1), HEK.TPα (lane 2), HEK 293 (lane 3, negative control) and HEK.hIP (lane 4, positive control) cells were metabolically labelled for 2 h at 37 °C (Panel A). Panels C and D, HEK.TPβ (lane 1), HEK.TPβC347S (lane 2), HEK.TPβC373,377S cells (lane 3) and HEK.TPβC347,373,377S (lane 4) cells were metabolically labelled for 2 h at 37 °C (Panel C). Thereafter, the HA-tagged receptors were immunoprecipitated with anti-HA 101R and resolved by SDS-PAGE followed by electroblotting onto PVDF membrane. The blots (A and C) were soaked in Amplify prior to fluorography for 60–90 days at − 70 °C. Following fluorographic exposure, PDVF membranes in A and C were screened by immunoblot analysis using anti-HA 3F10 peroxidase-conjugated antibody followed by chemiluminescent detection to obtain Panels B and D, respectively. The positions of the molecular weight markers (kDa) are indicated to the left and right of Panels A and D, respectively. Panel E, the level of palmitoylation in HEK.hIP, HEK.TPα, HEK.TPβ, HEKHA.TPβC347S, HEK.TPβC373,377S and HEK.TPβC347,373,377S cells relative to basal levels, in HEK 293 cells, was determined by Phosphorimage analysis. Data is presented as mean fold increase in palmitoylation over basal levels ± S.E.M. and are expressed in arbitrary units. The data are representative of three independent experiments. The asterisks indicate that palmitoylation levels of TPα, TPβC347S, TPβC373,377S and TPβC347,373,377S cells were significantly lower than that of TPβ where ⁎, p < 0.05; ⁎⁎, p < 0.01; ⁎⁎⁎p < 0.005.
From Phosphorimage analysis, it was evident that both TPβ and the hIP were efficiently palmitoylated showing near equivalent 4.36- and 4.82-fold increases in palmitoylation relative to basal levels in HEK 293 cells, respectively (Fig. 1E). The efficient metabolic labelling of the hIP is consistent with the fact that this receptor undergoes palmitoylation at multiple Cys residues within its C-tail domain [15]. In the case of TPβ, it is notable that there are three cysteine residues within its unique C-terminal tail domain which may potentially be palmitoylated, possibly accounting for its near equivalent metabolic labelling relative to that of the hIP (Fig. 1A and E). Thus, to investigate the possible involvement of one or more of those Cys residues in palmitoylation and the implications thereof for TPβ function, site-directed mutagenesis was used to mutate Cys347, Cys373 and Cys377 to Ser residues, either individually and collectively, to generate TPβC347S, TPβC373S, TPβC373,377S and TPβC347,373,377S. HEK 293 cell stably over-expressing TPβC347S, TPβC373S, TPβC373,377S and TPβC347,373,377S were generated and initially characterised by saturation radioligand binding analysis using the selective TP antagonist SQ29,548. HEK.TPβC347S, HEK.TPβC373S, HEK.TPβC373,377S and HEK.TPβC347,373,377S cells were confirmed to display similar ligand binding characteristics (Kd and Bmax) relative to HEK.TPβ cells and the mutations per se had no affect on the receptor affinity (Table 1). It should be noted that despite repeated attempts, it was not possible to generate stable cell lines over-expressing TPβC377S.
Table 1.
Radioligand binding assays
| Cell lines |
Kd |
Bmax |
|---|---|---|
| nM ± S.E.M. | pmol/mg protein ± S.E.M. | |
| HEK.TPβ | 8.44 ± 1.44 | 3.24 ± 0.33 |
| HEK.TPβC347S | 7.81 ± 3.22 | 4.16 ± 0.86 |
| HEK.TPβC373S | 8.21 ± 3.10 | 4.93 ± 1.05 |
| HEK.TPβC373,377S | 8.57 ± 2.44 | 4.16 ± 2.06 |
| HEK.TPβC347,373,377S | 8.00 ± 1.41 | 4.60 ± 0.30 |
Saturation radioligand binding isotherms were carried out on HEK 293 cells stably over-expressing HA epitope-tagged forms of TPβ and its variants using the TP antagonist [3H] SQ29,548 (50.4 Ci/mmol, 0–40 nM) and 75 μg of whole cell protein/assay. Radioligand binding data were analysed using GraphPrism 3 (GraphPad Software Inc.) to determine the Kd and Bmax values. Data presented are the mean ± S.E.M. of 3 independent experiments.
To investigate whether Cys347 and/or Cys373,377 are actual targets for TPβ palmitoylation, HEK.TPβC347S, HEK.TPβC373,377S and HEK.TPβC347,373,377S cells were metabolically labelled with [3H] palmitic acid. Palmitoylation of TPβ and, to a lesser extent, of TPβC347S and TPβC373,377S was observed as evidenced by the broad radiolabelled band between 45 and 66 kDa in their respective immunoprecipitates (Fig. 1C, lanes 1–3, respectively). Phosphorimage analysis confirmed that while the levels of metabolic labelling of both TPβC347S and TPβC373,377S were significantly reduced relative to that of TPβ, palmitoylation of either mutated receptor was not actually abolished (compare 4.36-, 2.01- and 2.68-fold increase in palmitoylation for TPβ, TPβC347S and TPβC373,377S, respectively; Fig. 1E). In contrast, the levels of metabolic labelling of TPβC347,373,377S (1.39-fold increase) were significantly reduced relative to TPβ (Fig. 1C, lanes 2 and 4, respectively; Fig. 1E) and, in fact, were found not to be significantly greater than basal levels in immunoprecipitates from HEK 293 cells. Confirmation of equivalent expression and recovery of the TPβ, TPβC347S, TPβC373,377S and TPβC347,373,377S receptors in their respective immunoprecipitates was obtained by subsequent screening of the PVDF membrane by Western blot analysis (Fig. 1D, lanes 1–4, respectively) and confirmed that failure, or any reductions, in detecting palmitoylation was not due to failure of the immunoprecipitation itself.
Thus, in summary, it is evident that TPβ is palmitoylated at multiple Cys residues within its C-tail domain and that mutation of Cys347 and Cys373,377 leads to a significant reduction in metabolic labelling confirming that palmitoylation occurs at both Cys347 and Cys373,377. Moreover, we also observed a significant reduction in the level of metabolic labelling of TPβC373S confirming that Cys373 is specifically palmitoylated (data not shown). However, as it was not possible to generate stable cell lines over-expressing TPβC377S, we cannot conclude with certainty whether Cys377 along with Cys373 is palmitoylated.
3.2. Effect of TPβC347S, TPβC373S, TPβC373,377S and TPβC347,373,377S on intracellular signalling
Numerous studies have indicated that the covalent attachment of palmitate has been implicated in the regulation of receptor G protein coupling and subsequent downstream signalling events [15,48]. To investigate whether palmitoylation has a role in Gq-coupled phospholipase (PL)Cβ activation by TPβ, we examined agonist-induced intracellular calcium ([Ca2+]i) mobilization by TPβC347S, TPβC373S, TPβC373,377S and TPβC347,373,377S in response to the TXA2 mimetic U46619. Consistent with previous reports [30], HEK.TPβ cells, transiently transfected with Gαq, showed efficient [Ca2+]i mobilization in response to U46619 (Fig. 2A). Stimulation of TPβC373S or TPβC373,377S each showed efficient intracellular signalling and it was found that U46619-mediated [Ca2+]i mobilization by either TPβC373S or TPβC373,377S was not significantly different to that of TPβ (Fig. 2C and D; p = 0.94 and 0.61, respectively). On the other hand, while TPβC347S did show a substantial signalling response (Fig. 2B), the level of [Ca2+]i mobilization by TPβC347S was significantly reduced relative to that of TPβ, TPβC373S or TPβC373,377S (Fig. 2F; p = 0.02). Moreover, consistent with this, U46619-induced [Ca+]i mobilization by TPβC347,373,377S was also impaired (Fig. 2E), showing significant reductions relative to that of TPβ, TPβC373S or TPβC373,377S (Fig. 2F; p = 0.01) whereas there was no significant difference in signalling between TPβC347S and TPβC347,373,377S (Fig. 2F; p = 0.79). Taken together, these data confirm that palmitoylation of TPβ plays a significant role in mediating its efficient coupling to Gq/PLCβ activation and, moreover, established that palmitoylation of Cys347 is specifically required for this regulation.
Fig. 2.
Analysis of U46619-mediated [Ca2+]i mobilization. HEK.TPβ (Panel A), HEK.TPβC347S (Panel B), HEK.TPβC373S (Panel C), HEK.TPβC373,377S (Panel D) and HEK.TPβC347,373,377S (Panel E) cells, transiently co-transfected with pCMV5:Gαq and preloaded with FURA2/AM, were stimulated with 1 μM U46619 where the ligand was added at times indicated by the arrows. The results are representative profiles from 4 independent experiments and are plotted as changes in intracellular Ca2+ mobilized (Δ[Ca2+]i, nM) as a function of time(s) following ligand stimulation. Data presented in Panel F is plotted as mean changes in Δ[Ca2+]i mobilization (nM ± S.E.M.) for each cell line. The asterisk in Panel F indicates that U46619-mediated [Ca2+]i mobilization by TPβC347S and TPβC347,373,377S was significantly lower than that of TPβ where ⁎, p < 0.05.
3.3. Effect of palmitoylation on agonist-induced internalization of TPβ
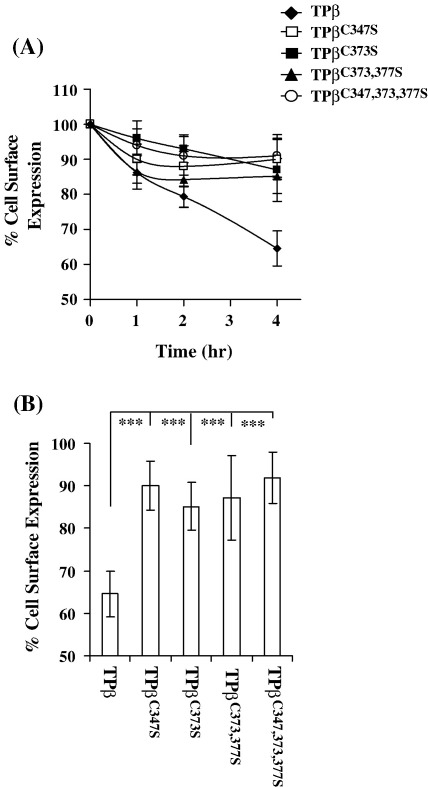
TPβ, but not TPα, is widely reported to undergo significant agonist-induced and tonic internalization, albeit through distinct mechanisms [34,35]. Hence, to further investigate the functional consequences of TPβ palmitoylation, we also assessed and compared agonist-induced internalization of TPβ, TPβC347S, TPβC373S, TPβC373,377S and TPβC347,373,377S, initially using an ELISA-based assay. Cells were treated with U46619 (1 μM) over a 4 h period, prior to fixation and immunolabelling with anti-HA 101R. Consistent with previous reports [34], TPβ underwent efficient agonist-induced internalization such that following 4 h exposure to U46619, its level of cell surface expression was reduced by approx. 40% (Fig. 3). On the other hand, mutation of any one of the Cys residues within the C-tail domain of TPβ, be it Cys347, Cys373 or Cys377 either singly or in combination, significantly impaired agonist-induced internalization (Fig. 3A). Specifically, following 4 h treatment with U46619, the level of cell surface expression of TPβC347S, TPβC347,373,377S, TPβC373S and TPβC373,377S had decreased by less than 10–15% (Fig. 3B).
Fig. 3.
Effect of palmitoylation on agonist-induced internalization of TPβ. Panel A, HEK.TPβ, HEK.TPβC347S, HEKHA.TPβC373S, HEK.TPβC373,377S and HEK.TPβC347,373,377S cells were incubated with 1 μM U46619 at 37 °C for 0–4 h prior to fixation and immunolabelling with anti-HA 101R. Results are expressed as mean cell surface expression at each time point as a percentage of that at 0 h (% cell surface expression ± S.E.M., n = 3) as a function time (h). Panel B, mean cell surface expression following stimulation of cells with 1 μM U46619, 37 °C for 4 h expressed as a percentage of that at 0 h (% cell surface expression ± S.E.M., n = 3). The asterisks indicate that at 4 h, U46619-mediated loss of cell surface expression of TPβC347S, TPβC373S, TPβC373,377S and TPβC347,373,377S was significantly reduced relative to that of TPβ where ⁎⁎⁎, p < 0.0001.
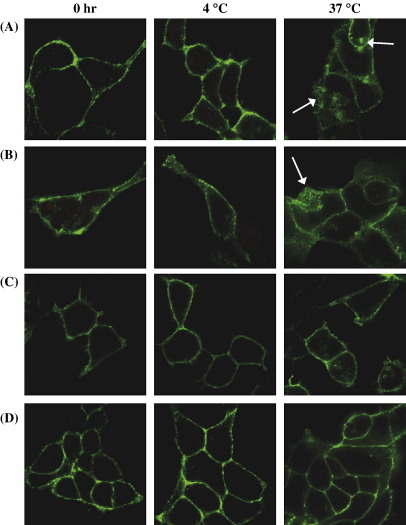
Agonist-induced internalization of TPβ, TPβC347S, TPβC373S, TPβC373,377S and TPβC347,373,377S was also examined by immunofluorescence and confocal microscopy (Fig. 4). Owing to the fact that at any one time, even in the absence of agonist stimulation, a significant proportion of TPβ expression is found intracellularly, due at least in part to its tonic internalization and/or retention within the endoplasmic reticulum [35,49], to facilitate our studies cell surface receptors were initially immunolabelled with anti-HA 101R prior to exposure of cells with the agonist U46619. Immunolocalizations were then captured under non-permeabilizing and permeabilizing conditions at 0, 2 and 4 h (2 h data not shown throughout). In the absence of agonist (0 h), there was a strong detection of HA-tagged TPβ, TPβC347S, TPβC373,377S and TPβC347,373,377S at their respective cell surfaces under both non-permeabilizing and permeabilizing conditions (Fig. 4, Panels A–D, 0 h). In the presence of U46619, cell surface expression of TPβ decreased with time, as indicated by reduced immunodetection in HEK.TPβ cells analysed in non-permeabilizing conditions, with a corresponding increase in TPβ internalization detected following permeabilization (Fig. 4A, 4 h). In permeabilized cells, TPβ expression was observed intracellularly at 4 h confirming that the HA-tagged TPβ had internalized from the cell surface into a cytoplasmic compartment. Indeed, there was a distinct punctuate staining within the perinuclear region, indicated by the arrow, appearing at the 4 h time point (Fig. 4A, permeabilized, 4 h). However, in contrast, the level of agonist-induced internalization of TPβC347S was significantly impaired relative to that of the wild type TPβ with no measurable decrease in surface expression and no increase in intracellular expression of TPβC347S detected under either non-permeabilizing or permeabilizing conditions (Fig. 4B). Moreover, U46619-stimulation did not result in a decrease in cell surface expression of TPβC373,377S and TPβC347,373,377S in cells analysed under non-pemeabilizing conditions, even following 4 h exposure to agonist (Fig. 4C and D, respectively) with no decrease in cell surface expression and no detection of internalized receptors when cells were analysed under permeabilizing conditions (Fig. 4C and D, 4 h). Similar results were observed for TPβC373S (data not shown).
Fig. 4.
Confocal microscopy of agonist-induced internalization. HEK.TPβ (Panel A), HEK.TPβC347S (Panel B), HEK.TPβC373,377S (Panel C) and HEK.TPβC347,373,377S (Panel D) cells were with pre-labelled with anti-HA 101R, at 4 °C for 1 h, prior to stimulation with 1 μM U46619 at 37 °C for the times indicated. Thereafter, following fixation, surface expression of the HA-tagged receptors was detected, under both non-permeabilizing and permeabilizing conditions, by immunolabelling anti-mouse FITC-conjugated antibody. Images were captured using a Carl Zeiss Lazer Scanning System LSM510 and Zeiss LSM Imaging software. The arrows show agonist-induced TPβ internalization as indicated by loss of cell surface HA-tagged TPβ detected in non-permeabilized cells and increased detection of intracellular TPβ in permeabilized cells. The colour version of this figure is available online at www.sciencedirect.com.
Taken collectively, these data are consistent with previous studies confirming that TPβ is readily internalized in response to exposure to agonist [34]. However, in keeping with our ELISA-based assays, when palmitoylation of TPβ is disrupted, as in the case of either TPβC347S, TPβC373S, TPβC373,377S and/or TPβC347,373,377S, the ability of TPβ to undergo agonist-induced internalization is impaired, showing no significant reduction in cell surface expression by either mutated variant.
3.4. Effect of palmitoylation on tonic internalization of TPβ
As stated, TPβ, but not TPα, has also been reported to undergo tonic internalization through a temperature- and dynamin-dependent but GRK- and β-arrestin-independent manner [35]. Hence, due to the reduced ability of the palmitoylation deficient TPβ mutants to undergo agonist-induced internalization, we next sought to investigate the effect of palmitoylation on tonic internalization. Again, cell surface receptors in HEK 293 cells stably expressing TPβ, TPβC347S, TPβC373S, TPβC373,377S and TPβC347,373,377S were pre-labelled at 4 °C with anti-HA 101R prior to incubating the cells for 2 h at 4 °C and 37 °C, to evaluate tonic internalization initially using an ELISA-based assay. Consistent with previous reports [35], TPβ readily underwent tonic internalization as indicated by the 40% loss of cell surface expression of TPβ observed at 37 °C without any loss in such expression at 4 °C (Fig. 5). However, unlike that which occurred for its agonist-induced internalization, it was found that TPβC347S also underwent similar temperature-dependent tonic internalization to levels similar to that of the wild type TPβ. Specifically, it was found that cell surface expression of TPβC347S was reduced by approx. 40% at 37 °C and to a level that was not significantly different from that of the wild type TPβ (Fig. 5; p = 0.32). On the other hand, the level of tonic internalization of TPβC373S, TPβC373,377S or TPβC347,373,377S was substantially impaired relative to TPβ (Fig. 5) or TPβ C347S (p < 0.0005) showing, on average, only a 15% loss of cell surface receptor expression following 2 h at 37 °C.
Fig. 5.
Effect of palmitoylation on tonic internalization. HEK.TPβ, HEK.TPβC347S, HEK. TPβC373S, HEK.TPβC373,377S and HEK.TPβC347,373,377S cells were incubated in the presence of anti-HA 101R for 1 h at 4 °C. Thereafter, following washing, cells were incubated at 4 °C or 37 °C for 2 h. Following fixation, cell surface expression of the HA-tagged receptors was detected using anti-mouse HRP conjugate antibody followed by colorimetric detection. Results are expressed as cell surface expression at 2 h as percentage of that at 0 h at the respective temperatures (% cell surface expression ± S.E.M., n = 3). The asterisks indicates that at 37 °C, tonic internalization of TPβC373S, TPβC373,377S and TPβC347,373,377S was significantly reduced compared to that of TPβ where ⁎, p < 0.0001 and ⁎⁎, p < 0.0005.
To further investigate the influence of palmitoylation on internalization, we also monitored temperature-dependent tonic internalization of TPβ and its palmitoylation defective variants by confocal microscopy (Fig. 6). In brief, HEK 293 cell lines stably over-expressing HA-tagged TPβ, TPβC347S, TPβC373,377S or TPβC347,373,377S were pre-labelled with anti-HA 101R at 4 °C; thereafter images were captured either directly (0 h), or following incubation for 2 h at 4 °C or 37 °C under non-permeabilizing (data not shown) and permeabilizing conditions (Fig. 6). At time 0 and following 2 h incubation at 4 °C, there was no evidence of internalized receptor for TPβ, TPβC347S, TPβC373,377S or TPβC347,373,377S with all of the immunolabelled receptors showing cell surface expression. Following 2 h incubation at 37 °C, much of the expression of TPβ and TPβC347S was still found at the cell surface, albeit at a substantially reduced level, but in both cases a significant amount of the immunolabelled receptors was present intracellularly consistent with their temperature-dependent tonic internalization (Fig. 6A and B, 37 °C, indicated by arrows). As with the agonist-induced internalization of TPβ, the cytoplasmic staining of TPβ and TPβC347S exhibited a punctuate pattern in the perinuclear region. However, in the case of TPβC373,377S or TPβC347,373,377S, there was little evidence of tonic internalization at 37 °C with both receptors being predominantly located at the cell surface (Fig. 6A and B, 37 °C). These data, particularly when taken in consideration with our ELISA-derived data, clearly indicates that the ability of TPβC373,377S and TPβC347,373,377S to undergo tonic internalization is severely impaired. Moreover, consistent with the ELISA data herein, the level of tonic internalization of TPβC373S following 2 h, or even more prolonged, incubation at 37 °C was severely impaired (data not shown).
Fig. 6.
Confocal microscopy of tonic internalization. HEK.TPβ (Panel A), HEK.TPβC347S (Panel B), HEK.TPβC373,377S (Panel C) and HEK.TPβC347,373,377S (Panel D) cells were pre-labelled with anti-HA 101R at 4 °C for 1 h. Thereafter, following washing, cells were incubated for 2 h at 4 °C or 37 °C, followed by fixation in paraformaldehyde. Cell surface expression of the HA-tagged receptors was detected using anti-mouse FITC-conjugated antibody, under permeabilizing conditions. Images were captured using a Carl Zeiss Lazer Scanning System LSM510 and Zeiss LSM Imaging software. The arrows show temperature-induced tonic internalization of TPβ and TPβC347S. The colour version of this figure is available online at www.sciencedirect.com.
Hence, in summary, whilst TPβ undergoes agonist-induced and tonic internalization, disruption of palmitoylation by mutating Cys347, Cys373 or Cys377, either singly or in combination, abolishes agonist-induced internalization. However, like TPβ, TPβC347S actually retains the ability to undergo tonic internalization while that of TPβC373S, TPβC373,377S and TPβC347,373,377S is substantially impaired.
3.5. Effect of β-arrestin1 and -2 on TPβC347S, TPβC373,377S and TPβC347,373,377S internalization
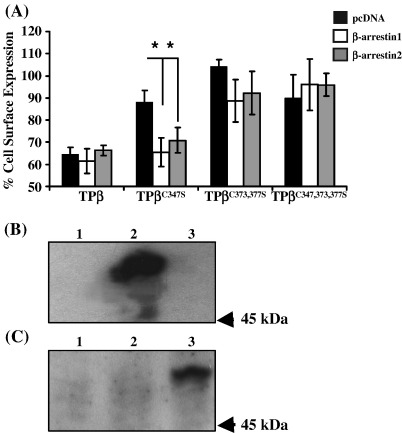
As stated, it is widely reported that agonist-induced internalization of TPβ shows a significant dependence on β-arrestin recruitment post-receptor activation and may, for example, be significantly reduced in the presence of dominant negative forms of either β-arrestin1 or β-arrestin2 [34]. Therefore, to further investigate the role of palmitoylation on TPβ internalization, we transiently transfected β-arrestin1 and β-arrestin2 into HEK 293 cells stably over-expressing TPβ and its variant TPβC347S, TPβC373,377S and TPβC347,373,377S with the view to assessing their effect on agonist-mediated internalization. In the case of TPβ, consistent with previous reports [34], over-expression of the wild type forms of either β-arrestin1 or β-arrestin2 had no significant effect on the overall level of U46619-induced internalization following 4 h, as assessed by the ELISA-based internalization assay (Figs. 3 and 7A). However, whilst TPβC347S does not undergo significant agonist-induced internalization (Fig. 3), over-expression of β-arrestin1 and β-arrestin2 appeared to compensate and resulted in a 30% loss of cell surface receptor following exposure of cells to U46619 for 4 h (Fig. 7A, compare the level of agonist-induced internalization in HEK.TPβC347S cells transiently co-transfected with cDNA encoding β-arrestin1 and β-arrestin2 relative to the empty vector; p = 0.017 and p = 0.042, respectively). On the other hand, consistent with previous data (Figs. 3 and 4), TPβC373,377S and TPβC347,373,377S did not undergo agonist-induced internalization and over-expression of either β-arrestin1 and β-arrestin2 had no significant effect on the overall level of cell surface expression or internalization by either receptor type (Fig. 7A, compare pcDNA to β-arrestin1 and -2, p > 0.2 and p > 0.3, respectively). Over-expression of β-arrestin1 and β-arrestin2 was confirmed in HEK.TPβ cells by Western blot analysis using anti-β-arrestin1 and anti-β-arrestin2 (Fig. 7B and C, respectively).
Fig. 7.
Effect of β-arrestin on agonist-induced internalization. Panel A, HEK.TPβ, HEK.TPβC347S, HEK.TPβC373S, HEK.TPβC373,377S and HEK.TPβC347,373,377S cells, transiently co-transfected with pRK5.β-arrestin1, pcDNA1.β-arrestin2 or, as a control, with the empty vector pcDNA were incubated with U46619 (1 μM) at 37 °C for 4 h, prior to fixation and immunolabelling with anti-HA 101R. Following fixation, cell surface expression of the HA-tagged receptors was detected using anti-mouse HRP-conjugated antibody followed by colorimetric detection. Results are expressed as mean cell surface expression at 4 h as a percentage of that at 0 h (% cell surface expression ± S.E.M., n = 3). The asterisk (⁎, p < 0.05) indicates that over-expression of β-arrestin1 and β-arrestin2 significantly reduced the level of cell surface expression of TPβC347S in the presence of U46619. Panels B and C, HEK.TPβ cells, transiently co-transfected with pRK5.β-arrestin1 (lane 2), pcDNA1.β-arrestin2 (lane 3) or, as a control, with the empty vector pcDNA (lane 1) were analysed by SDS-PAGE (50 μg whole cell protein analysed/lane) followed by Western blot analysis using anti-β-arrestin1 (K-16: Panel B) and anti-β-arrestin2 (N-16: Panel C). Data presented are representative immunoblots from three independent experiments. The relative position of the 45 kDa molecular size marker is indicated to the right of Panels B and C.
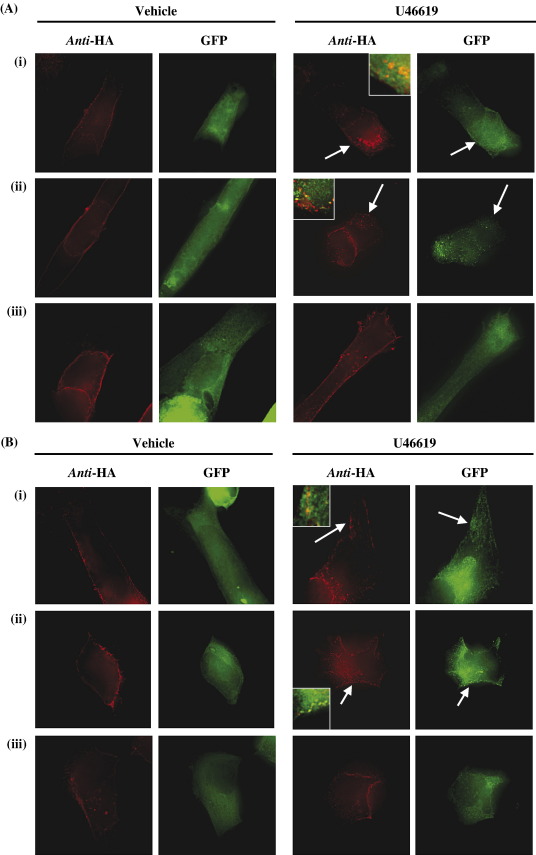
To further assess the role of the β-arrestins in agonist-induced internalization of TPβ and its palmitoylation defective variants, HEK 293 cells were transiently co-transfected with respective plasmids encoding HA-tagged TPβ or its variants TPβC347S, TPβC373S (data not shown), TPβC373,377S (data not shown) and TPβC347,373,377S along with GFP-tagged forms of either β-arrestin1 and β-arrestin2. HA-tagged cell surface receptors were initially immunolabelled prior to stimulation with U46619 for 30 min; thereafter, cells were permeabilized and HA-tagged receptor or GFP-tagged β-arrestin expression and co-localizations were captured by fluorescence microscopy (Fig. 8A and B). While TPβ, TPβC347S and TPβC347,373,377S were each clearly expressed on the cell surface in vehicle-treated cells (Fig. 8A and B), TPβ was significantly internalized even following exposure of cells to U46619 for 30 min in the presence of both GFP-tagged β-arrestin1 or β-arrestin2 (Fig. 8Ai and Bi). Moreover in the presence of either GFP-tagged β-arrestin1 or β-arrestin2, TPβC347S also underwent significant U46619-induced internalization (Fig. 8Aii and Bii). On the other hand, neither TPβC373S, TPβC373,377S (data not shown) nor TPβC347,373,377S underwent internalization even in the presence of over-expressed β-arrestins (Fig. 8Aiii and Biii). Consistent with previous reports [50], in vehicle-treated cells, both β-arrestins exhibit diffuse uniform staining in the cytoplasm whilst β-arrestin1, but not β-arrestin2, also showed significant nuclear staining (Fig. 8A and B). Upon U46619-stimulation and in the presence of TPβ, the GFP staining pattern associated with both β-arrestin1 and β-arrestin2 was significantly altered. Specifically, GFP fluorescence became punctuate with a translocation of both β-arrestin1 and β-arrestin2 from the cytoplasm to the periphery of the cell, consistent with their co-localization and interaction with agonist-activated TPβ on the cell surface (Fig. 8Ai and Bi, respectively). Similarly, in the presence of TPβC347S, both β-arrestin1 and β-arrestin2 showed translocation and enhanced co-localization with TPβC347S at the cell surface in response to U46619-stimulation (Fig. 8Aii and Bii). On the other hand, there was no evidence of β-arrestin1/2 translocation or receptor co-localization with TPβC373S, TPβC373,377S (data not shown) or TPβC347,373,377S (Fig. 8Aiii and Biii) in response to U46619-stimulation. Taken together, these data demonstrated that over-expression of β-arrestin1 or β-arrestin2 could compensate for the defect in agonist-induced internalization of TPβC347S and suggest that palmitoylation of TPβ, in particular at Cys347, is specifically required to mediate β-arrestin1/2 translocation and receptor interaction in response to agonist-stimulation.
Fig. 8.
Effect of palmitoylation on TPβ and β-arrestin co-localization. Panels A and B, HEK 293 cells transiently co-transfected with (i) pHM6.TPβ, (ii) TPβC347S and (iii) TPβC347,373,377S along with pEGFPN3:β-arrestin1 (Panel A) or pEGFPN1:β-arrestin2 (Panel B) were immunolabelled with anti-HA 101R for 1 h at 4 °C. Thereafter, cells incubated with vehicle or U46619 (1 μM) for 30 min at 37 °C. Following fixation and permeabilization, cell surface HA-tagged TPs were labelled with the AlexaFluor 594 anti-mouse IgG. Images were captured using the Zeiss Fluorescence microscope with AxioVision software (version 4.4) and processed by AxioVision Deconvolution analysis. The arrows show agonist-induced β-arrestin1 and β-arrestin2 translocation and TPβ internalization while the insets to the panels show the co-localization of the β-arrestins with the TPβ receptors. The colour version of this figure is available online at www.sciencedirect.com.
4. Discussion
Palmitoylation is a prevalent feature of GPCRs with up to 80% of the superfamily estimated to contain at least one cysteine in their C-tail domain, typically but not exclusively proximal to transmembrane (TM) 7, which may potentially be palmitoylated [5,7]. In this study, we sought to establish whether TPα and/or TPβ are palmitoylated and to investigate the functional consequences thereof. Whilst TPα does not contain any cysteines within its unique C-tail domain, TPβ possesses Cys347 close to the TM7/C-tail boundary and the couplet Cys373 and Cys377 located some 30 amino acids from its C-terminus. The results herein confirm that TPβ, but not TPα, is palmitoylated. Furthermore, site-directed mutagenesis and in vivo metabolic labelling determined that TPβ is palmitoylated at Cys347 and, to a lesser extent, at Cys373,377. Whilst palmitoylation was confirmed to occur at Cys373, attempts to further ascertain whether Cys377, along with Cys373, is specifically palmitoylated were not possible as stable cell lines over-expressing TPβC377S could not be generated despite repeated attempts.
A typical feature of palmitoylation is that it is viewed as a reversible modification, with the propensity to regulate protein:membrane or protein:protein association, protein localization and/or function in a dynamic manner [1,6]. Moreover, several GPCRs are known to undergo increased palmitoylation or depalmitoylation in an agonist-dependent manner, modulating receptor signalling potential in response to ligand engagement [13,51,52]. In the current study, we found no evidence of altered palmitoylation status of TPβ in response to agonist-stimulation under the conditions of metabolic labelling employed and, hence, data presented throughout are those generated in the absence of ligand activation. However, as the process of metabolic labelling and detection of palmitoylation is a relatively inefficient process, with poor-signal-to-noise ratio, we cannot exclude the possibility that TPβ palmitoylation is also subject to dynamic modulation, such as in response to agonist-engagement.
Numerous studies have indicated that the consequences of palmitoylation of GPCRs are wide-ranging with many aspects of receptor function being potentially implicated including receptor processing, G protein/effector coupling, phosphorylation and desensitization, sequestration and internalization, trafficking and/or protein turnover [1–5]. In examining the functional consequences herein it was established that palmitoylation of TPβ at Cys347 is required for its efficient Gq-mediated PLCβ activation, the primary effector of TP signalling. Specifically in the case in TPβC347S and TPβC347,373,377S, there was a 30% reduction in U46619-mediated [Ca2+]i mobilization compared to that of the wild type TPβ. In contrast, U46619-mediated [Ca2+]i mobilization by TPβC373S and TPβC373,377S was not significantly different from TPβ, indicating that palmitoylation at Cys373 and/or Cys377 does not influence Gq/PLCβ effector signalling. The reduction in [Ca2+]i mobilization by TPβC347S or TPβC347,373,377S was not due differences in their ligand binding properties as each of the palmitoylation deficient mutants displayed similar affinities for the selective TP radioligand [3H]SQ29,548 and their overall level of expression was not significantly different to that of the wild type TPβ. The specific requirement for palmitoylation at Cys347 for TPβ-mediated Gq/PLCβ activation is somewhat similar to that previously reported for the hIP where it was established that palmitoylation at Cys308, but not at Cys311, is specifically required for its Gq/PLCβ coupling [15].
A number of studies have demonstrated that impairment of receptor palmitoylation, as exemplified by the CCR5 receptor, can result in the accumulation of GPCRs in intracellular stores and in the lowering of cell surface expression [53]. In the case of TPβ and its variants, as stated, we have established that impairment of palmitoylation per se does not affect the overall level of surface expression and does not appear to affect the maturation or surface expression of TPβ. As TPβ is reported to undergo agonist-dependent and agonist-independent or tonic internalization, albeit through distinct mechanisms, we next sought to determine whether palmitoylation may affect TPβ internalization [34,35]. It was established that mutation of Cys347, Cys373 or Cys377, either alone or in combination, significantly impaired agonist-induced TPβ internalization reducing the movement of the respective cell surface receptors (TPβC347S, TPβC373S, TPβC373,377S or TPβC347,373,377S) into intracellular compartment(s) relative to the wild type TPβ. On the other hand, while tonic internalization by TPβC373S, TPβC373,377S and TPβC347,373,377S was also significantly impaired, it was striking that the variant TPβC347S retained the ability to undergo tonic internalization. Furthermore, over-expression of either β-arrestin1 or β-arrestin2 was able to overcome the inability of TPβC347S to undergo agonist-induced internalization, an effect not observed with the other palmitoylation deficient mutants including TPβC373S, TPβC373,377S and TPβC347,373,377S. These data indicated a critical role for palmitoylation of Cys373,377 in both agonist-dependent and tonic internalization of TPβ, while palmitoylation of TPβC347 is also required for agonist-induced, but not tonic internalization.
Taken together, our results indicate that TPβ is palmitoylated at 2 independent sites Cys347 and Cys373,377 separated by some 30 amino acid residues within its unique C-tail domain, to regulate G protein coupling and receptor internalization. One of the structural consequences of GPCR palmitoylation is that, by increasing local hydrophobicity, the palmitoyl moiety(s) is thought to become inserted into the membrane lipid bilayer resulting in the formation of a putative fourth intracellular loop within its C-tail domain, such as originally demonstrated for rhodopsin [8,54,55]. Moreover, in certain cases, palmitoylation at multiple independent sites, either alone or in combination with other post-translational lipid modifications, has been proposed to result in the formation of loop structures within the C-tail domain of the given GPCR. For example, in the case of the 5-hydroxytryptamine (5-HT)4(a) receptor, Ponimaskin et al. [13,48] established that it undergoes palmitoylation at two adjacent Cys residues (Cys328/Cys329) located proximal to TM7 but is also palmitoylated at Cys386, located one amino acid residue from the C-terminus. They proposed that membrane insertion of the palmitates at the proximal Cys328/Cys329 and distal Cys386 residues resulted in the formation of a double loop structure within the C-tail of the 5-HT4(a) receptor. While palmitoylation deficient mutants of 5-HT4(a) receptor were indistinguishable from the wild type receptor in mediating G protein/effector signalling, mutation of the proximal and distal residues was shown not only to differentially modulate constitutive activation but also to modulate agonist-dependent phosphorylation, desensitization and β-arrestin internalization of the 5-HT4(a) receptor [13,48,56]. Similarly, it has been proposed that the C-tail domain of the hIP also contains a double loop structure anchored by dynamically regulated proximal palmitoyl groups, at Cys308/Cys311, and by a distal farnesyl isoprenoid permanently attached to its C-terminus and that collectively these dual lipidations modulate G protein coupling and effector signalling [15].
In order to rationalise the functional consequences of palmitoylation of TPβ we present a model, as outlined in Fig. 9, whereby it is proposed that palmitoylation of TPβ at Cys347 and at Cys373,377 also leads to the formation of a double loop structure within its C-tail domain. In our model, palmitoylation at Cys347 and membrane insertion leads to the formation of Loop A which, though not obligatory for Gq coupling, leads to more efficient Gq/PLCβ activation and [Ca2+]i mobilization. On the other hand, palmitoylation at the couplet Cys373,377, leads to the formation of Loop B or C, depending on whether Cys347 is also palmitoylated or not, respectively (Fig. 9). Whilst palmitoylation at Cys373,377 is essential for tonic internalization, palmitoylation at both Cys347 and Cys373,377, leading to the formation of Loops A and B, is required for agonist-induced internalization. In investigating the mechanism of agonist-induced internalization of TPβ, Parent et al., established that amino acid residues between 355 and 366 within its unique C-tail are essential for such β-arrestin-dependent internalization [34]. Moreover, it has since been established that Ser357 within the latter internalization domain is a specific target for agonist-dependent G protein-coupled receptor kinase (GRK)2/3 phosphorylation leading to β-arrestin recruitment and TPβ desensitization and internalization [57]. It is indeed noteworthy that both the latter agonist-dependent internalization motif and the GRK phospho-target are located between the palmitoylation sites at Cys347 and Cys373,377 within the C-tail of TPβ. Hence in our model (Fig. 9) and as supported by our experimental findings, we propose that palmitoylation of TPβ at both Cys347 and Cys373,377, leading to the formation of Loops A and B, would expose the agonist-induced internalization motif and GRK phosphorylation site at Ser357 thereby enabling β-arrestin recruitment. Whilst formation of Loop C by palmitoylation at Cys373,377 alone, as observed in TPβC347S, may also expose the internalization motif and GRK site at Ser357, we propose that the C-tail is in an altered, less favorable conformation for β-arrestin interaction and with reduced affinity for β-arrestin binding. Hence, palmitoylation of Cys373,377 alone, as in the case of TPβC347S, yields a receptor with impaired agonist-induced internalization owing to its reduced affinity for β-arrestin1/2. Consistent with this, through our experimental observations, over-expression of either β-arrestin1/2 overcame this reduced affinity by TPβC347S and facilitated its internalization in response to U46619-stimulation to levels that were indistinguishable from those of the wild type TPβ. Moreover, our immunolocalization data supported the agonist-dependent recruitment of GFP-tagged forms of β-arrestin1 and β-arrestin2 to both the agonist-activated TPβ and TPβC347S but not to TPβC347,373,377S, for example.
Fig. 9.
Model of palmitoylation of TPβ. Palmitoylation of TPβ occurs at 2 distinct sites through the attachment of palmitates at Cys347 and Cys373/377. Following palmitoylation, it is proposed that membrane insertion of the palmitate moieties at Cys347 and Cys373/377 leads to the formation of a double loop structure within the C-tail domain of TPβ referred to as Loop A and Loop B, respectively. A third conformation of the C-tail of TPβ occurs when Cys373,377, but not Cys347, is palmitoylated resulting in the formation of Loop C, such as is found in TPβC347S. TPβC373,377S undergoes palmitoylation at Cys347 resulting in the formation of Loop A only while TPβC347,373,377S does not contain Loops A, B or C. It was found that Loop A is required for efficient Gαq/PLCβ coupling as evidenced by the reduced agonist-induced [Ca2+]i mobilization by TPβC347S and TPβC347,373,377S. It is proposed that Loop B is required for agonist-induced GRK phosphorylation at Ser357, followed by β-arrestin recruitment and TPβ internalization, as evidenced by the impaired U46619-induced β-arrestin1/2 recruitment and internalization by TPβC347S, TPβC373,377S and TPβC347,373,377S. Like the wild type TPβ, TPβC347S (which contains Loop C only) retains the ability to undergo tonic internalization while TPβC373,377S (which contains Loop A only) or TPβC347,373,377S fails to undergo tonic or agonist-induced internalization. From these latter data, it is proposed that palmitoylation at Cys373 and/or Cys377, resulting in the formation of Loop C only (in TPβC347S) or Loop A plus Loop B (in wild type TPβ) is necessary to present a tonic internalization motif, such as the proposed Y339X3Φ343[35], in the correct orientation for interaction with the internalization machinery.
Our proposed model accounting for the role of palmitoylation in the regulation of β-arrestin-dependent agonist-induced internalization of TPβ is consistent with a number of other studies. In the case of the V2 vasopressin receptor, for example, Charest and Bouvier established that palmitoylation enhances the β-arrestin recruitment to the activated V2R, facilitating internalization and other processes requiring the scaffolding action of β-arrestin including activation of the mitogen-activated protein kinase cascades [16]. In contrast, other studies demonstrated that palmitoylation may actually inhibit β-arrestin interaction [58]. For example, palmitoylation of the luteinizing hormone/human chorionic gonadotropin receptor physically hinders receptor:β-arrestin interaction such that the palmitoylation status of the receptor determines whether internalization actually proceeds through a β-arrestin-dependent or independent mechanism [58].
Whilst TPβ undergoes agonist-induced internalization via a dynamin-, GRK2/3- and β-arrestin-dependent mechanism, requiring an active or dynamic actin cytoskeleton [34–36], tonic internalization is reported to occur, at least in part, through a dynamin-dependent mechanism with no requirement for either GRK phosphorylation or the β-arrestins [35]. In studies investigating the determinants of tonic internalization, Parent et al., identified the presence of a tonic internalization motif Y339X3Φ343, where X is any residue and Φ is a bulky hydrophobic isoleucine, within the C-tail domain of TPβ. Hence, the critical residues required for tonic internalization lie between residues 339 and 343 in close proximity to the Cys347 within the proposed Loop A structure associated with TPβ (Fig. 9). Hence, it is tempting to speculate that palmitoylation at Cys347 may modulate tonic internalization of TPβ. Our experimental finding that TPβC347S, in addition to the wild type TPβ, undergoes tonic internalization suggests that the conformation of Loop C is sufficient to present or orientate the latter motif for internalization to occur. However, the fact that TPβC373,377S which contains Loop A, but not Loop B, does not undergo tonic internalization leads us to propose that other unidentified sequence determinants within Loop B, associated with TPβ, may also be required for efficient tonic internalization and that the conformation of Loop C may also orientate those sequences to facilitate that internalization. The identities of those additional sequences required for tonic internalization remain to be established.
In summary, various studies have indicated that many GPCRs are palmitoylated and that the functional consequences are diverse between receptors [3–5]. In this study we have established that TPβ, but not TPα, is palmitoylated and that palmitoylation occurs at distinct 2 sites, Cys347 and Cys373,377, within its unique C-tail domain. Whilst palmitoylation at Cys347 is required for efficient Gq/PLCβ effector coupling, palmitoylation at Cys373,377 is essential for receptor internalization, with palmitoylation at Cys347 acting as a structural determinant of the route by which the receptor is internalized, be it β-arrestin-dependent or independent internalization. Due to the importance of TXA2 in vascular hemostasis, a clearer understanding of how the TP isoforms are differentially regulated is critical. Palmitoylation provides a dynamic and reversible post-translational modification that can regulate TPβ, in terms of its signalling, receptor desensitization and internalization and, as such, acts as a major determinant of TPβ function. Moreover, data presented herein provide a further rational explanation for the clear functional and mechanistic differences between the TPα and TPβ isoforms.
Acknowledgements
This research was supported by grants to BTK from the Wellcome Trust and The Health Research Board (Ireland). We are very grateful to Dr Stephen Ferguson, University of Western Ontaria, Canada for the gift of pRK5:β-arrestin1 and pEGFPN3:β-arrestin1; to Dr Jeffrey Benovic, Thomas Jefferson University, Philadelphia, USA for pcDNA3:β-arrestin2 and to Dr Robert Lefkowitz, Howard Hughes Medical Institute, Duke University, North Carolina for the gift of pEGFPN1:β-arrestin2.
References
- 1.Milligan G., Parenti M., Magee A.I. Trends Biochem. Sci. 1995;20(5):181. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- 2.Dunphy J.T., Linder M.E. Biochim. Biophys. Acta. 1998;1436(1–2):245. doi: 10.1016/s0005-2760(98)00130-1. [DOI] [PubMed] [Google Scholar]
- 3.Bijlmakers M.J., Marsh M. Trends Cell Biol. 2003;13(1):32. doi: 10.1016/s0962-8924(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 4.Qanbar R., Bouvier M. Pharmacol. Ther. 2003;97(1):1. doi: 10.1016/s0163-7258(02)00300-5. [DOI] [PubMed] [Google Scholar]
- 5.Torrecilla I., Tobin A.B. Curr. Pharm. Des. 2006;12(14):1797. doi: 10.2174/138161206776873716. [DOI] [PubMed] [Google Scholar]
- 6.Magee T., Seabra M.C. Curr. Opin. Cell Biol. 2005;17(2):190. doi: 10.1016/j.ceb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Probst W.C., Snyder L.A., Schuster D.I., Brosius J., Sealfon S.C. DNA Cell Biol. 1992;11(1):1. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- 8.Ovchinnikov Y.A., Abdulaev N.G., Bogachuk A.S. FEBS Lett. 1988;230(1–2):1. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy M.E., Limbird L.E. J. Biol. Chem. 1993;268(11):8003. [PubMed] [Google Scholar]
- 10.Kennedy M.E., Limbird L.E. J. Biol. Chem. 1994;269(50):31915. [PubMed] [Google Scholar]
- 11.Jin H., Zastawny R., George S.R., O'Dowd B.F. Eur. J. Pharmacol. 1997;324(1):109. doi: 10.1016/s0014-2999(97)00059-9. [DOI] [PubMed] [Google Scholar]
- 12.Ng G.Y., Mouillac B., George S.R., Caron M., Dennis M., Bouvier M., O'Dowd B.F. Eur. J. Pharmacol. 1994;267(1):7. doi: 10.1016/0922-4106(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 13.Ponimaskin E.G., Schmidt M.F., Heine M., Bickmeyer U., Richter D.W. Biochem. J. 2001;353(Pt 3):627. doi: 10.1042/0264-6021:3530627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi M.K., Haga T. Arch. Biochem. Biophys. 1997;340(2):376. doi: 10.1006/abbi.1997.9906. [DOI] [PubMed] [Google Scholar]
- 15.Miggin S.M., Lawler O.A., Kinsella B.T. J. Biol. Chem. 2003;278(9):6947. doi: 10.1074/jbc.M210637200. [DOI] [PubMed] [Google Scholar]
- 16.Charest P.G., Bouvier M. J. Biol. Chem. 2003;278(42):41541. doi: 10.1074/jbc.M306589200. [DOI] [PubMed] [Google Scholar]
- 17.Schulein R., Liebenhoff U., Muller H., Birnbaumer M., Rosenthal W. Biochem. J. 1996;313(Pt 2):611. doi: 10.1042/bj3130611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narumiya S., Sugimoto Y., Ushikubi F. Physiol. Rev. 1999;79(4):1193. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 19.Hirata M., Hayashi Y., Ushikubi F., Yokota Y., Kageyama R., Nakanishi S., Narumiya S. Nature. 1991;349(6310):617. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- 20.Raychowdhury M.K., Yukawa M., Collins L.J., McGrail S.H., Kent K.C., Ware J.A. J. Biol. Chem. 1994;269(30):19256. [PubMed] [Google Scholar]
- 21.Raychowdhury M.K., Yukawa M., Collins L.J., McGrail S.H., Kent K.C., Ware J.A. J. Biol. Chem. 1995;270(12):7011. doi: 10.1074/jbc.270.12.7011. (Published erratum) [DOI] [PubMed] [Google Scholar]
- 22.Kinsella B.T. Biochem. Soc. Trans. 2001;29(Pt 6):641. doi: 10.1042/0300-5127:0290641. [DOI] [PubMed] [Google Scholar]
- 23.Miggin S.M., Kinsella B.T. Biochim. Biophys. Acta. 1998;1425(3):543. doi: 10.1016/s0304-4165(98)00109-3. [DOI] [PubMed] [Google Scholar]
- 24.Habib A., FitzGerald G.A., Maclouf J. J. Biol. Chem. 1999;274(5):2645. doi: 10.1074/jbc.274.5.2645. [DOI] [PubMed] [Google Scholar]
- 25.Coyle A.T., Kinsella B.T. FEBS J. 2005;272(4):1036. doi: 10.1111/j.1742-4658.2004.04538.x. [DOI] [PubMed] [Google Scholar]
- 26.Coyle A.T., Kinsella B.T. Biochem. Pharmacol. 2006;71(9):1308. doi: 10.1016/j.bcp.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Coyle A.T., Miggin S.M., Kinsella B.T. Eur. J. Biochem. 2002;269(16):4058. doi: 10.1046/j.1432-1033.2002.03098.x. [DOI] [PubMed] [Google Scholar]
- 28.Coyle A.T., O'Keeffe M.B., Kinsella B.T. FEBS J. 2005;272(18):4754. doi: 10.1111/j.1742-4658.2005.04890.x. [DOI] [PubMed] [Google Scholar]
- 29.Habib A., Vezza R., Creminon C., Maclouf J., FitzGerald G.A. J. Biol. Chem. 1997;272(11):7191. doi: 10.1074/jbc.272.11.7191. [DOI] [PubMed] [Google Scholar]
- 30.Walsh M.T., Foley J.F., Kinsella B.T. J. Biol. Chem. 2000;275(27):20412. doi: 10.1074/jbc.M907881199. [DOI] [PubMed] [Google Scholar]
- 31.Walsh M., Foley J.F., Kinsella B.T. Biochim. Biophys. Acta. 2000;1496(2–3):164. doi: 10.1016/s0167-4889(00)00031-8. [DOI] [PubMed] [Google Scholar]
- 32.Hirata T., Ushikubi F., Kakizuka A., Okuma M., Narumiya S. J. Clin. Invest. 1996;97(4):949. doi: 10.1172/JCI118518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vezza R., Habib A., FitzGerald G.A. J. Biol. Chem. 1999;274(18):12774. doi: 10.1074/jbc.274.18.12774. [DOI] [PubMed] [Google Scholar]
- 34.Parent J.L., Labrecque P., Orsini M.J., Benovic J.L. J. Biol. Chem. 1999;274(13):8941. doi: 10.1074/jbc.274.13.8941. [DOI] [PubMed] [Google Scholar]
- 35.Parent J.L., Labrecque P., Driss Rochdi M., Benovic J.L. J. Biol. Chem. 2001;276(10):7079. doi: 10.1074/jbc.M009375200. [DOI] [PubMed] [Google Scholar]
- 36.Laroche G., Rochdi M.D., Laporte S.A., Parent J.L. J. Biol. Chem. 2005;280(24):23215. doi: 10.1074/jbc.M414071200. [DOI] [PubMed] [Google Scholar]
- 37.Hamelin E., Theriault C., Laroche G., Parent J.L. J. Biol. Chem. 2005;280(43):36195. doi: 10.1074/jbc.M503438200. [DOI] [PubMed] [Google Scholar]
- 38.Foley J.F., Kelley L.P., Kinsella B.T. Biochem. Pharmacol. 2001;62(2):229. doi: 10.1016/s0006-2952(01)00661-x. [DOI] [PubMed] [Google Scholar]
- 39.Reid H.M., Kinsella B.T. J. Biol. Chem. 2003;278(51):51190. doi: 10.1074/jbc.M309314200. [DOI] [PubMed] [Google Scholar]
- 40.Hayes J.S., Lawler O.A., Walsh M.T., Kinsella B.T. J. Biol. Chem. 1999;274(34):23707. doi: 10.1074/jbc.274.34.23707. [DOI] [PubMed] [Google Scholar]
- 41.Wei H., Ahn S., Shenoy S.K., Karnik S.S., Hunyady L., Luttrell L.M., Lefkowitz R.J. Proc. Natl. Acad. Sci. U. S. A. 2003;100(19):10782. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menard L., Ferguson S.S., Zhang J., Lin F.T., Lefkowitz R.J., Caron M.G., Barak L.S. Mol. Pharmacol. 1997;51(5):800. [PubMed] [Google Scholar]
- 43.Zhang J., Barak L.S., Anborgh P.H., Laporte S.A., Caron M.G., Ferguson S.S. J. Biol. Chem. 1999;274(16):10999. doi: 10.1074/jbc.274.16.10999. [DOI] [PubMed] [Google Scholar]
- 44.Kinsella B.T., O'Mahony D.J., Fitzgerald G.A. J. Pharmacol. Exp. Ther. 1997;281(2):957. [PubMed] [Google Scholar]
- 45.Gorman C.M., Gies D.R., McCray G. DNA Protein Eng. Tech. 1990;2:3. [Google Scholar]
- 46.Grynkiewicz G., Poenie M., Tsien R.Y. J. Biol. Chem. 1985;260(6):3440. [PubMed] [Google Scholar]
- 47.Miggin S.M., Lawler O.A., Kinsella B.T. Eur. J. Biochem. 2002;269(6):1714. doi: 10.1046/j.1432-1327.2002.02817.x. [DOI] [PubMed] [Google Scholar]
- 48.Ponimaskin E.G., Heine M., Joubert L., Sebben M., Bickmeyer U., Richter D.W., Dumuis A. J. Biol. Chem. 2002;277(4):2534. doi: 10.1074/jbc.M106529200. [DOI] [PubMed] [Google Scholar]
- 49.Valentin F., Field M.C., Tippins J.R. J. Biol. Chem. 2004;279(9):8316. doi: 10.1074/jbc.M306761200. [DOI] [PubMed] [Google Scholar]
- 50.Oakley R.H., Laporte S.A., Holt J.A., Barak L.S., Caron M.G. J. Biol. Chem. 2001;276(22):19452. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 51.Chen C.A., Manning D.R. J. Biol. Chem. 2000;275(31):23516. doi: 10.1074/jbc.M003439200. [DOI] [PubMed] [Google Scholar]
- 52.Hawtin S.R., Tobin A.B., Patel S., Wheatley M. J. Biol. Chem. 2001;276(41):38139. doi: 10.1074/jbc.M106142200. [DOI] [PubMed] [Google Scholar]
- 53.Percherancier Y., Planchenault T., Valenzuela-Fernandez A., Virelizier J.L., Arenzana-Seisdedos F., Bachelerie F. J. Biol. Chem. 2001;276(34):31936. doi: 10.1074/jbc.M104013200. [DOI] [PubMed] [Google Scholar]
- 54.Moench S.J., Moreland J., Stewart D.H., Dewey T.G. Biochemistry. 1994;33(19):5791. doi: 10.1021/bi00185a017. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z., Wen X.H., Ablonczy Z., Crouch R.K., Makino C.L., Lem J. J. Biol. Chem. 2005;280(26):24293. doi: 10.1074/jbc.M502588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ponimaskin E., Dumuis A., Gaven F., Barthet G., Heine M., Glebov K., Richter D.W., Oppermann M. Mol. Pharmacol. 2005;67(5):1434. doi: 10.1124/mol.104.008748. [DOI] [PubMed] [Google Scholar]
- 57.Kelley-Hickie L.P., Kinsella B.T. Biochim. Biophys. Acta. 2006;1761(9):1114. doi: 10.1016/j.bbalip.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 58.Munshi U.M., Peegel H., Menon K.M. Eur. J. Biochem. 2001;268(6):1631. doi: 10.1046/j.1432-1327.2001.02032.x. [DOI] [PubMed] [Google Scholar]