Abstract
The vascular smooth muscle cell (SMC)-specific isoform of α-actin (ACTA2) is a major component of the contractile apparatus in SMCs located throughout the arterial system. Heterozygous ACTA2 mutations cause familial thoracic aortic aneurysms and dissections (TAAD), but only half of mutation carriers have aortic disease. Linkage analysis and association studies of individuals in 20 families with ACTA2 mutations indicate that mutation carriers can have a diversity of vascular diseases, including premature onset of coronary artery disease (CAD) and premature ischemic strokes (including Moyamoya disease [MMD]), as well as previously defined TAAD. Sequencing of DNA from patients with nonfamilial TAAD and from premature-onset CAD patients independently identified ACTA2 mutations in these patients and premature onset strokes in family members with ACTA2 mutations. Vascular pathology and analysis of explanted SMCs and myofibroblasts from patients harboring ACTA2 suggested that increased proliferation of SMCs contributed to occlusive diseases. These results indicate that heterozygous ACTA2 mutations predispose patients to a variety of diffuse and diverse vascular diseases, including TAAD, premature CAD, ischemic strokes, and MMD. These data demonstrate that diffuse vascular diseases resulting from either occluded or enlarged arteries can be caused by mutations in a single gene and have direct implications for clinical management and research on familial vascular diseases.
Introduction
Mutations in genes inherited in a Mendelian manner and leading to adult-onset disorders have been identified for many diseases, including breast and colon cancer, as well as hypertrophic and dilated cardiomyopathy. In contrast, the identification of single genes that lead to premature onset of coronary artery disease (CAD) in the absence of abnormal lipoprotein levels has been limited to only a few genes.1,2 Furthermore, no single-gene disorders have been shown to cause large-vessel ischemic strokes. The slow progress made in identifying vascular disease genes is felt to be due to significant genetic heterogeneity for these diseases, along with the complex interactions between genes and environmental factors that contribute to the disease pathogenesis, including smoking, diabetes mellitus, obesity, and systolic blood pressure.3 Another factor potentially delaying the progress of gene identification for vascular diseases is that genes encoding proteins required for vascular SMC function and arterial-wall structure are expressed in arteries throughout the body, and mutations in these genes could lead to diffuse vascular diseases involving many arteries. Therefore, mapping strategies focused on a restricted vascular phenotype, such as ischemic stroke or aortic aneurysm, may delay the identification of disease genes. Supporting this hypothesis are the diffuse vasculopathies associated with many genetic syndromes caused by single-gene mutations, including vascular Ehlers-Danlos syndrome (EDS VI [MIM 225400]) and neurofibromatosis 1 (NF1 [MIM 162200]).4,5 The NF1 vasculopathy not only affects many different arteries but can present as either enlargement or occlusion of arteries. These facts raise the question of whether vascular-disease genes could be identified more rapidly if mapping and identification focused on premature onset of vascular disease in families irrespective of the vascular bed affected.
The major function of vascular smooth muscle cells (SMCs) is to contract in response to the stretch resulting from pulsatile blood flow, a process that is dependent on the cyclic interaction between thin filaments, composed of the SMC-specific isoform of α-actin (SM α-actin, encoded by ACTA2), and thick filaments, composed of SMC-specific β-myosin. The importance of SM α-actin for SMC function is supported by several lines of evidence. First, SM α-actin expression characterizes SMC differentiation and is the single most abundant protein in differentiated SMCs, accounting for ∼40% of the total cellular protein and ∼70% of the total actin.6,7 Second, Acta2 null mice exhibited normal cardiovascular development but compromised vascular contractility, tone, and blood flow.8,9 Finally, heterozygous mutations in ACTA2 in humans lead to an inherited predisposition for thoracic aortic aneurysms and dissections (TAAD). ACTA2 missense mutations are the major genetic cause of familial TAAD identified to date and are responsible for disease in 15% of families.10 Interestingly, in these families, only half of ACTA2 mutation carriers have aortic disease, a penetrance lower than that typically observed for genes leading to familial TAAD.
We noted that all ACTA2-mutation carriers in a large family had marked and persistent livedo reticularis (LR), a purplish reticular rash caused by occlusion of the dermal arteries.10 In ACTA2-mutant aortas, occlusion of the arteries in the vasa vasorum as a result of increased SMCs in the vessel wall was also noted. These observations raised the possibility that ACTA2 mutations could also result in vascular occlusion. To test this hypothesis, we assessed our families with ACTA2 mutations for the premature onset of vascular diseases, including coronary artery disease, ischemic strokes, and transient ischemic attacks (TIAs), before the age of 55 years in men and before age 60 years in women. Both linkage and association data from these families support the conclusion that ACTA2 mutations lead to vascular occlusive diseases. Furthermore, we found that explanted SMCs and myofibroblasts harboring ACTA2 mutations proliferate more rapidly than control cells, suggesting that ACTA2 mutations lead to occlusive disease through increased proliferation of SMCs. These data support the conclusion that a single-gene mutation can cause a diffuse and diverse vasculopathy.
Subjects and Methods
Family Characterization and Sample Collection
The institutional review board at the University of Texas Health Science Center at Houston (UTHSCH) approved this study. Families with multiple members with TAAD and no known genetic syndrome were recruited and characterized as previously described.11 Family members at risk for TAAD were imaged for the presence of asymptomatic ascending aortic aneurysms. Individuals were considered affected if they had dissection of the aorta, surgical repair of an ascending aortic aneurysm, or dilatation of the ascending aorta greater than two standard deviations as compared to the normal diameter of the aortic segment.12 These measurements were based on echocardiography images of the aortic diameter at the sinuses of Valsalva, the supra-aortic ridge, and the ascending aorta compared in healthy and affected individuals. Premature CAD and stroke are designated in this study as onset of the disease at the age of 55 years or younger in men and 60 years or younger in women. Medical records pertaining to vascular diseases and risk factors were collected of all family members for verification of diagnoses regardless of mutation-carrier status. The diagnosis of CAD is based on documentation of a myocardial infarct or 70% narrowing of one or more coronary arteries noted during either cardiac catheterization or cardiac pathology performed at the time of autopsy. Ischemic strokes were diagnosed on the basis of the presence of cerebral infarct in cerebral imaging or diagnosis of a transient ischemic attack (TIA). Diagnosis of Moyamoya disease (MMD) is based on cerebrovascular imaging demonstrating stenosis or occlusion of the terminal portion of the internal carotid artery, the formation of an abnormal vascular network in the vicinity of the arterial occlusion, and review of the patient's history and images for exclusion of other causes of stenosis, such as atherosclerosis.
Blood or buccal cells were collected for DNA. Mutation status of family members was determined by sequencing of their DNA or inferred by their location in the pedigree (e.g., their parent and offspring were documented to have the mutation). Individuals who were at 50% risk for inheriting a mutation and died of an aortic dissection were assumed to have the mutant gene because dissection is an uncommon vascular event, whereas at-risk individuals who died of stroke or CAD were not assumed to carry the mutation because of the frequency of these conditions in the population. Control DNA was obtained from whites, African Americans, Filipinos, and Hispanics who did not have cardiovascular disease.
TexGen Patient Sample Collection
Patients admitted to Texas Medical Center (TMC) hospitals were recruited for the study. With the patients' consent, samples and clinical data were collected, de-identified, and banked as part of TexGen Research. TexGen is a collaborative genetic-discovery research project within the TMC and includes The UTHSCH, The University of Texas M.D. Anderson Cancer Center, Baylor College of Medicine and their affiliated hospitals, and the Texas Heart Institute. Patients with strokes and TIAs were recruited at Memorial Hermann Hospital. Patients admitted for chest pain due to coronary artery disease were recruited for TexGen at the Texas Heart Institute at St. Luke's Episcopal Hospital and the Methodist Hospital. General health information, cardiovascular risk factors, clinical diagnosis, and treatment of patients were obtained from the patient or extracted from the medical records and recorded in the TexGen database.
Sequencing Protocol
Bidirectional sequencing of ACTA2 exons was done at the Human Genome Sequencing Center at Baylor College of Medicine with the use of intron-based, exon-specific primers. PCR amplifications were carried out with HotStar Taq DNA polymerase (QIAGEN, Valencia, CA). PCR products were treated with Exo_SAP (USB, Cleveland, OH) for digestion of primers, followed by sequencing PCR with the BigDye sequencing reaction mix (Applied Biosystems, Foster City, CA). The sequencing PCR products were purified via the BigDye XTerminator kit (Applied Biosystems) and then loaded on an ABI3730xl sequencing instrument with the Rapid36 run module. The DNA sequencing results were analyzed with SNP Detector software.13 Identified mutations were verified by bidirectional resequencing of the original DNA sample in another lab (D.M.M.'s lab).
Statistical Analysis
Two-point linkage analysis with ACTA2-mutation status was performed with TAAD and the combined phenotype of TAAD, CAD, and stroke or combinations of these phenotypes. The disease-allele frequency and penetrance of CAD and stroke were the same as those defined for TAAD, and 0.001 was the minor allele frequency of the ACTA2 mutation.14 Association of ACTA2 mutations with CAD and stroke was performed via Fisher's exact test as implemented in the software StatXact (Cytel Statistical Software and Services). Pairwise LOD scores were calculated with the MLINK program of the computer software FASTLINK, version 3.P.15
Structural Analyses
The atomic coordinates of actin (Protein Data Bank [PDB] ID: 1J6Z) were obtained from the PDB. Structure superposition and visualization were performed with O.16 Nonbonded and hydrogen-bonded contacts were determined with HBPLUS.17 Figures were generated with PyMOL.18
Pathology and Cell-Proliferation Assays
Formalin-fixed, paraffin-embedded tissue sections from patient and control aortas and coronary arteries and myocardium from two deceased patients were stained with H&E and Movat. Additional tissue sections were immunostained with monoclonal antibody for SM α-actin obtained from Sigma.10
SMCs were explanted from the ascending thoracic aorta above the sinuses of Valsalva, from two patients with ACTA2 mutations and two controls, and subcultured in SmBm containing 15% FBS (Table S1, available online).19 Cells at passage 2 were plated in triplicate at 10,000 cells per well in a 96-well plate. After 24 hr, the cells were placed in media containing 0.2% FBS and incubated with BrdU reagent for 24 hr for quantification of cell proliferation. Cells were fixed, and an ELISA for BrdU was carried out according to the manufacturer's instructions (Millipore). A Student's t test was used in determining whether the increase in cell proliferation was statistically significant. Data are represented with the standard error of the mean indicated.
Dermal fibroblasts were explanted from patients heterozygous for an ACTA2 mutation and from controls without vascular disease and were subcultured in DMEM containing 10% FBS (Table S1). Cells from passages 2 or 3 were plated in triplicate at a density of 20,000 cells per well and placed in DMEM containing 0.2% FBS. After 24 hr, fresh DMEM containing 0.2% FBS with and without 10 ng/mL TGF-β1 was added to the cells. Cells were incubated for 72 hr after TGF-β1 treatment, followed by 24 hr of incubation with BrdU reagent. Cells were fixed, and an ELISA for BrdU was done. Statistical analysis was performed as described for the SMCs. Data were tested for normal distribution via a D'Agostino-Pearson test. Data are represented with the standard error of the mean indicated and are a composite of three independent experiments. For confirmation that fibroblasts transformed to myofibroblasts with TGF-β1 exposure, cells were treated with and without TGF-β1, harvested, and lysed in RIPA buffer. Equal amounts of total protein were separated through gel electrophoresis, transferred to a PVDF membrane, and immunoblotted with antibodies directed against SM α-actin and GAPDH (Abcam) for confirmation of increased expression of SM α-actin 72 hr after exposure to TGF-β1.20,21
Results
Assessment of Vascular Diseases in Families with ACTA2 Mutations
Twenty families, with 127 members harboring heterozygous ACTA2 mutations, were phenotyped for premature vascular diseases, defined as an age at onset less than 55 years in men and less than 60 years in women (Table S2). Family members age 21 years and older were included, along with members who presented with vascular diseases at younger ages. The thoracic aortic disease was previously reported in 14 of these families.10 Six additional families with ACTA2 mutations are included in the present study (c.215C→A; p.P72Q in TAA018, c.479G→A; p.G160D in TAA200, c.116G→A; p.R39H in TAA252 and TAA331, c.353G→GA; R118Q in TAA441, and c.554G→A; p.R185Q in TAA455 [Figure 1 and Figure S1]). Mutations were confirmed by exclusion of the alteration in 192 ethnically matched controls and segregation of the alterations with aortic disease in the families. Note that bidirectional sequencing of the exons and flanking introns of ACTA2 in 192 white controls identified no variants.
Figure 1.

Vascular Diseases in Families with ACTA2 Mutations
(A) Pedigrees of 12 families with ACTA2 mutations in which the mutation segregates with a number of vascular diseases, including TAAD, premature onset of CAD, and stroke. The disease and mutation status of individuals are as indicated in the figure legend.
(B) Three families with ACTA2 R258 mutations with early age at onset of stroke and MMD.
(C) Pedigrees of patients with ACTA2 mutations identified from Texgen cohort. Pedigrees of two patients with nonfamilial TAAD and de novo ACTA2 mutations and of one patient with nonfamilial TAAD, a sister with MMD, and a mother with premature CAD due to an ACTA2 mutation. Numbers and letters under each circle or square in the pedigree represent the following: the first line shows the individual ID number; the second line shows age at onset of the vascular disease(s), which is color coded based on the vascular disease (see the figure legend); and an asterisk next to a symbol indicates that individuals had MMD. Individuals in (C) are labeled with their sample ID number. Circles represent females, and squares represent males. A slash through a circle or square indicates a deceased person. A pound sign (#) indicates an individual who may have carried the ACTA2 mutation, on the basis of their location in the pedigree, but was not assumed to harbor a mutation in the genetic and clinical analysis of ACTA2-mutation carriers.
TAAD was the primary vascular disease in ACTA2-mutation carriers (76 individuals), but carriers also had premature onset of CAD (26 individuals) and ischemic strokes (15 individuals); 15 individuals had more than one vascular disease (Figure 1A). In contrast, none of the family members without an ACTA2 mutation had TAAD, premature CAD, or stroke in these families. Five families had multiple ACTA2-mutation carriers with CAD (TAA252, 327, 370, 441, and 445), including two families with the recurrent ACTA2 c.445C→T; p.R149C mutation previously shown not to be related.10 One family (TAA455) had more ACTA2-mutation carriers with premature CAD than with aortic disease. It is important to note that neither coronary occlusion nor stroke was caused by aortic dissection or aortic surgical repair in these family members.
Although premature ischemic strokes also occurred in ACTA2-mutation carriers in these families, very early onset strokes occurring under the age of 20 were noted in four families (TAA105, 252, 377, and 390 [Figures 1A and 1B]). Three of these families had an ACTA2 mutation altering arginine 258 (c.772C→T; p.R258C and c.773G→A; p.R258H), and in these families, 10 out of 14 mutation carriers had aortic disease and seven had onset of strokes at ages ranging from 5 to 46 years of age. Five of seven acute strokes were classified as MMD, a rare cerebral arteriopathy caused by occlusion of the supraclinoid portion of the internal carotid arteries as a result of fibrocellular proliferation in the arteries (Figure S2A). The other two individuals, TAA377:II:1 and III:2, had strokes at ages 32 and 17 years, respectively, but imaging records could not be obtained. Recent imaging studies identified fusiform cerebral aneurysms in both individuals (current ages 53 and 27 years [Figure S2B]).
Linkage analysis and penetrance of the various vascular diseases in all of the ACTA2-mutation families were analyzed. The LOD score for TAAD in the 20 ACTA2-mutation families was 6.7, with a calculated penetrance for TAAD between the ages of 40 and 60 years of 0.48 (Table S3). Evidence that ACTA2 mutations also caused premature occlusive vascular disease in these families was supported by positive LOD scores for premature CAD, stroke, and combined CAD and stroke vascular phenotypes: 2.49, 1.65, and 3.93, respectively. Because we specifically tested the role of ACTA2 mutation in premature CAD, stroke, and combined CAD and stroke vascular phenotypes, the p values associated with these LOD scores were highly significant (0.0004, 0.0029, and 1.06 × 10−5, respectively). The calculated penetrance of ACTA2 mutation for premature CAD and stroke between the ages of 40 and 60 years is 0.30 and 0.15, respectively. The LOD score for all vascular diseases combined—TAAD, premature CAD, and stroke—rose to 10.62 for the families, associated with a corresponding increase of the penetrance between 40 and 60 years to 0.80, further supporting our hypothesis that ACTA2 mutations lead to occlusive diseases.
As a control for other genetic and environmental factors predisposing family members to premature CAD and strokes, the number of family members with ACTA2 mutations and premature onset of CAD or strokes (40 individuals out of 127 carriers) was compared with the number of family members who had these premature vascular diseases but did not have an ACTA2 mutation (0 out of 104 family members) (p = 1.7 × 10−12). Thus, in these families, individuals with ACTA2 mutations are significantly more likely to have premature CAD and stroke compared with family members without ACTA2 mutations. These data support the hypothesis that ACTA2 mutations lead to premature stroke and CAD, in addition to the previously established predisposition to TAAD.
ACTA2 Mutations in Patients with Thoracic Aortic Disease and Premature Onset of Coronary Artery Disease and Stroke
We sought to determine the frequency of ACTA2 mutations in patients with nonfamilial TAAD. The nine exons and flanking intronic regions of ACTA2 were sequenced in 237 patients who were admitted for surgical repair of a thoracic aortic aneurysm or acute aortic dissection and did not have a family history of aortic disease (Table 1). Heterozygous ACTA2 mutations were identified in five white men and one woman (2.5%), and these alterations were not present in 192 white controls. Three of these men had ischemic strokes at ages 22, 26, and 41 years prior to presenting with an acute aortic dissection at ages 27, 26 and 42 years, respectively (2340, 6375, and 5875 [Table S4]). Two men were confirmed to have de novo mutations; the mutation was not present in the parental DNA, and paternity was confirmed through analysis of polymorphic microsatellite markers (Figure 1C). The sister and mother of patient 8622 were found to be heterozygous for the ACTA2 c.635G→A; p.R212Q mutation. His sister was diagnosed with MMD when she presented with an acute stroke at the age of 17 years, and his mother presented with CAD requiring stent placement at the age of 55 years (Figure 1C). Patient 9025 had a heterozygous ACTA2 alteration (c.977→A; p.T326N) and had an ascending aortic aneurysm and bicuspid aortic valve diagnosed at the age of 59 years. Her son carried the alteration and had a stroke at the age of 25 years. Her daughter, age 57 years, also harbored the mutation and has declined aortic imaging. The identification of premature strokes due to ACTA2 mutations in nonfamilial TAAD patients, along with the segregation of premature occlusive disease (stroke and CAD) in family members, further supports the conclusion that ACTA2 mutations lead to occlusive diseases.
Table 1.
Texgen Patients with Sporadic Thoracic Aortic Aneurysm and Dissection Patients
| Clinical Data | No. (%) |
|---|---|
| Ascending aneurysma | 182 (76) |
| Ascending dissection | 40 (17) |
| Descending aneurysm | 56 (24) |
| Descending dissection | 25 (11) |
| Mean Age (yrs) | 61 ± 14; range 16–86 |
| Male | 155 (65) |
| Female | 83 (35) |
| White | 200 (84) |
| African-American | 19 (8) |
| Hispanic | 9 (4) |
| Native American | 1 (< 1) |
| Other ethnicity | 9 (4) |
| BMI | 28 ± 6 (range, 10 - 52) |
| Hypertension | 170 (72) |
| Diabetes | 23 (10) |
| Current smoker | 46 (19) |
| Past smoker | 107 (45) |
| Never smoked | 84 (35) |
| BAV | 25 (10) |
| CAD: Male, age < 55 | 7 (3) |
| CAD: Female, age < 60 | 1 (<1) |
| Stroke | 11 (5) |
n = 237.
A subset of patients had more than one type of thoracic aortic disease.
For determination of the contribution of ACTA2 mutations to premature strokes and CAD, genomic DNA collected from TexGen patients with these vascular diseases was sequenced for ACTA2 mutations. DNA was sequenced from 216 patients with CAD and 271 patients with acute strokes or TIAs who were age 55 years or younger (men) or 60 years or younger (women) (Table 2). Although primarily ischemic strokes were observed in families with ACTA2 mutations, fusiform aneurysms were documented in two family members, and MMD can present with either ischemic or hemorrhagic strokes. Therefore, patients with both ischemic and hemorrhagic strokes were included for mutational analysis. A missense mutation in ACTA2 (p.T326N) was identified in a 53-year-old man of Northern European descent who presented with an anterior myocardial infarct due to left anterior descending stenosis (TS2088 [Table S4]). The patient's family declined to be studied. No ACTA2 mutations confirmed to disrupt protein structure were identified in patients with premature strokes. Two unique ACTA2 alterations leading to synonymous changes in the amino acid were noted in the coding region of a 45-year-old African American man with CAD and a 51-year-old of Northern European descent with an ischemic stroke (c.417G→A and c.936C→T, respectively) and were not present in matched controls. The biological and clinical significance of these alterations is not known.
Table 2.
Texgen Patients with Premature Coronary Artery Disease and Stroke
| Clinical Data | No.: CAD (%) | No.: Stroke (%) |
|---|---|---|
| TIA | 22 (8) | |
| Infarct | 158 (58) | |
| Intracranial hemorrhage | 78 (29) | |
| Unknown | 13 (5) | |
| Mean Age (yrs) | 48 ± 6; range 25–60 | 55 ± 8; range 17–60 |
| Male | 151 (70) | 165 (61) |
| Female | 65 (30) | 106 (39) |
| White | 138 (64) | 111 (41) |
| African-American | 34 (16) | 108 (40) |
| Hispanic | 35 (16) | 46 (17) |
| Asian | 9 (4) | 4 (1) |
| Other | 0 (0) | 2 (1) |
| BMI | 30.98 ± 7.5; range 15.64–49.81 | 25.98 ± 4.19; range 20.12–9.93 |
| Hypertension | 150 (69) | 171 (63) |
| Diabetes | 139 (64) | 61 (23) |
| Smoking history (present and past) | 64 (30) | 238 (88) |
| Family history: father | 107 (50) | 36 (13) |
| Family history: mother | 60 (28) | 54 (20) |
| Family history: siblings | 49 (23) | 29 (11) |
| Cholesterol (mg/dl) | 182.19 ± 80.00 | 187.90 ± 50.88 |
| HDL (mg/dl) | 38.15 ± 12.71 | 41.84 ± 16.30 |
| LDL (mg/dl) | 108.89 ± 76.89 | 118.77 ± 40.80 |
| Triglyceride (mg/dl) | 92.87 ± 195.02 | 147.66 ± 125.85 |
n = 216 CAD patients, 271 stroke patients.
Genotypes Associated with Vascular Phenotypes
Analysis of the mutated sites along the SM α-actin sequence suggests that different vascular diseases are associated with specific ACTA2 missense mutations (Figure 2). Assessment of mutations with greater than 15 carriers (p.R118Q, p.R149C, p.R258C, and p.R258H) indicates that p.R258C/H mutations are associated primarily with strokes, including those with an age at onset less than 20 years, and not with CAD, whereas p.R118Q and p.R149C lead primarily to CAD and are less frequently associated with strokes (Figure 2A). Of the individuals with either the p.R118Q or the p.R149C mutation, 18 (30%) had CAD and 42 (70%) did not. In comparison, in individuals with other ACTA2 mutations, 8 (11.9%) had CAD and 59 (88.1%) did not. On the basis of a Fisher's exact test, these two mutations predispose one to CAD at a 3.1-fold increased risk (confidence interval [CI]:1.16–9.15, p value = 0.01) compared with other ACTA2 mutations. A similar analysis of the p.R258C/H mutations indicates that these mutations result in a 6.51-fold increased risk of stroke (CI:1.71–24.72, p value = 0.02).
Figure 2.
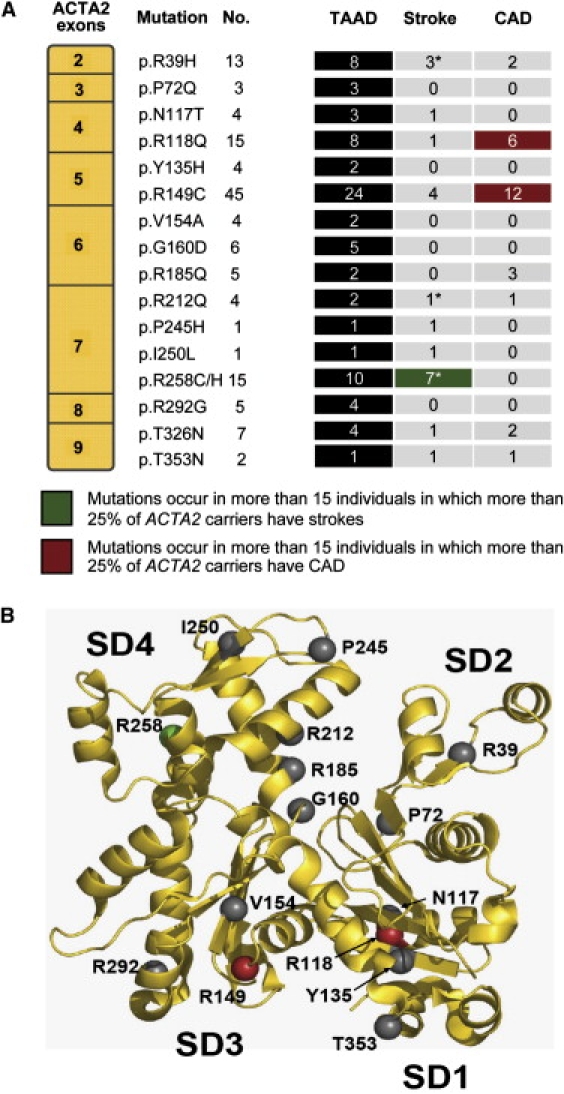
Genotype-Phenotype Correlation between Location of Mutation and Vascular Diseases
(A) Location of all ACTA2 mutations and the specific phenotypes observed in patients heterozygous for those mutations. The mutations are listed in sequential order and align with the exon in which they are located. “No.” indicates the number of identified individuals who carry each specific mutation. The headings TAAD, Stroke, and CAD denote the number of individuals affected by each of these conditions. An asterisk indicates a mutation leading to the onset of ischemic strokes prior to the age of 20 years.
(B) The ribbon diagram of SM α-actin, identifying the location of the different mutations at the protein-structure level. The recurrent ACTA2 mutation leading to ischemic strokes at a young age (p.R258C/H, shown in green) lies within subdomain 4, whereas mutations occurring in multiple individuals and leading primarily to premature CAD (p.R149C and p.R118Q, shown in red) all lie within the hydrophobic cleft.
The p.R258C/H mutations lie within the SM α-actin SD4 domain (Figure 2B). This domain participates in the opening and closing of the nucleotide binding cleft. Notably, p.R212Q, which also causes MMD, also resides in this domain. Furthermore, an additional mutation causing stroke before the age of 20 years (p.R39H) is found on the SD2 domain, whose dynamics are essential for ATP hydrolysis. Conversely, the mutations leading primarily to premature CAD (p.R149C and p.R118Q, along with p.T353N) lie within the hydrophobic cleft of α-actin, which serves as both the binding determinant of several regulatory proteins and the target of diverse marine macrolide toxins that permanently cap the barbed end and block promoter addition or removal. It is important to note the ACTA2 p.R185Q is found in a region that directly contacts the nucleotide and, therefore, is more than likely to perturb ATP hydrolysis. On the basis of the five individuals harboring this mutation, this alteration causes TAAD and CAD and not stroke, so the perturbation of ATP hydrolysis may not completely predict a predisposition to stroke.
Vascular Pathology and Increased Proliferation of ACTA2-Mutant Cells
Available vascular tissues from patients harboring heterozygous ACTA2 were obtained for a determination of whether the vascular pathology would provide insight into the etiology of the occlusive vascular diseases in affected individuals. Aortic samples from two patients with ACTA2 mutations demonstrated increased diameter and thickening of the walls of the vasa vasorum vessels as compared to control aortas, a finding that had been previously noted (data not shown).10 This thickening was due to increased SMCs in the medial layer, ascertained via immunostaining for SM α-actin. Despite the increased outer diameter of the arteries, the lumen was stenotic or occluded in some arteries.
We sought to determine whether the cardiac arteries in ACTA2-mutation patients demonstrated similar proliferation of SMCs. Cardiac tissue was available from autopsies of two ACTA2-mutation-positive members of family TAA441. The coronary artery of a healthy 28-year-old member (IV:3) who died of an acute aortic dissection demonstrated significant stenosis of the artery due to intimal and medial fibrocellular accumulation associated with an atherosclerotic plaque (Figure 3A). The atherosclerotic lesion was more cellular and had fewer lipids than typical atherosclerotic plaques. The majority of the cells in the lesion were SMCs, ascertained via SM α-actin staining (Figure 3A). In family member III:7, who died after surgical repair of chronic descending aortic dissection, the intracardiac arteries also had thickened walls due to medial thickening (Figure 3B). Similar thickening of the intracardiac arteries was noted at autopsy of TAA015:III:1.
Figure 3.

ACTA2 Mutations Lead to Increased SMC and Myofibroblast Proliferation in Culture
(A) Coronary artery from 28-year-old TAA441:IV:4, stained with Movat's stain and SM α-actin antibody. The coronary artery showed 70% narrowing of the vessel due to a fibrocellular atherosclerotic plaque (top two panels; magnification 40×; scale bar represents 1.0 mm). Higher magnification (200×; scale bar represents 200 μm) revealed that these cells contain SM α-actin.
(B) The epicardial arteries from a 50-year-old man, TAA441:III:7, and a 53-year-old woman, TAA015:III:1. H&E, Movat, and α-actin immunostaining of the tissue showed thickened vessels within the myocardium (magnification 200×; scale bar represents 200 μm).
(C) Cell-proliferation assays (BrdU incorporation) illustrate that SMCs explanted from patients heterozygous for an ACTA2 mutation (n = 2) proliferate more rapidly than matched control SMCs (n = 2).
(D) Myofibroblasts (fibroblasts exposed to TGF-β1 for 72 hr) from patients heterozygous for ACTA2 mutations (n = 9) proliferate more rapidly than matched controls (n = 10). Data are expressed as means ± SEM, and p values are indicated. Immunoblotting for SM α-actin from the cell lysates confirms that TGF-β1 exposure increased cellular SM α-actin.
The increased number of SMCs in the medial layers of arteries suggested that the presence of an ACTA2 missense mutation leads to increased proliferation of SMCs. To test this hypothesis, proliferation rates of SMCs and myofibroblasts explanted from affected patients were compared with age-, sex-, and passage-matched control cells (Table S1). SMCs explanted from ACTA2-mutation patients proliferated significantly more rapidly in culture as compared with control cells (p < 0.05 [Figure 3C]). Explanted fibroblasts from dermal biopsies were available from nine patients with ACTA2 mutations. Dermal fibroblasts plated in confluent cultures express low levels of SM α-actin and, with exposure to TGF-β1, are transformed into myofibroblasts expressing high levels of SM α-actin (Figure 3D).20,21 Proliferation assays confirmed that the patients' myofibroblasts proliferated significantly more rapidly than control myofibroblasts. The increased proliferation observed in SMCs with heterozygous ACTA2 mutations and confirmed through mutant myofibroblasts expressing SM α-actin may contribute to the increased numbers of SMCs observed in vascular lesions in affected individuals.
Discussion
Accumulating data from families harboring heterozygous ACTA2 mutations support the conclusion that ACTA2 mutations predispose individuals to occlusive vascular diseases, specifically premature CAD and strokes, in addition to the already established predisposition to TAAD. The first suggestion that ACTA2 mutations cause occlusive vascular lesions was the presence of livedo reticularis in affected patients. Further evidence was provided by linkage analysis of families with ACTA2 mutations; the LOD score and penetrance of the disease rose from 6.7 and 0.48 to 10.62 and 0.8 when TAAD was combined with premature CAD and stroke. In addition, positive LOD scores were obtained for premature CAD, stroke, and the combined CAD and stroke phenotype, with a combined phenotype LOD score of 3.93 (p = 1.06 × 10−5). None of the 104 family members without a mutation had premature CAD or stroke, whereas 40 out of 127 ACTA2 carriers had premature onset of these vascular diseases, indicating a highly significant association of familial occlusive vascular diseases with the mutation. Additional support was based on the fact that none of the ACTA2-mutation-positive members with premature occlusive vascular disease had a major risk factor for the early onset of these vascular occlusive diseases, such as plasma cholesterol levels consistent with monogenic hypercholesterolemia (>280 mg/dl) or uncontrolled and severe hypertension, and only one individual had diabetes mellitus. A nonfamilial TAAD patient with an ACTA2 mutation had family members with the ACTA2 mutations and premature occlusive disease, and an ACTA2 mutation was also identified independent of TAAD in a man with premature CAD. Finally, very early onset strokes and MMD (between the ages of 5 and 44 years) occurred in members of three unrelated families who all harbored mutations altering R258 in SM α-actin, a strong association supporting the idea that ACTA2 mutations also lead to ischemic strokes.
The contribution of ACTA2 mutations to nonfamilial vascular diseases was also assessed in this study. Only 2.5% of patients with sporadic TAAD had an ACTA2 mutation. The contribution of ACTA2 mutations to premature CAD and stroke in the general population was even less, with only one patient with CAD identified with an ACTA2 mutation in the Texgen premature CAD and stroke cohorts that were studied. Therefore, ACTA2 mutations contribute to premature CAD and strokes in families harboring ACTA2 mutations but appear to be a rare cause of CAD and stroke in the general population.
Atherosclerosis, the underlying cause of CAD and ischemic strokes, is associated with a number of risk factors, including hypercholesterolemia, hypertension, diabetes mellitus, and family history. The identification of genes predisposing one to occlusive arterial disease, both private mutations linked to the disease and disease-associated polymorphic variants, has identified four distinct pathogenetic pathways: (1) hypercholesterolemia leading to increased cholesterol deposition in the artery, (2) loss of endothelial integrity, (3) factors promoting arterial inflammation, and (4) factors increasing thrombosis in vessels.22 Our data implicate a fifth pathway for genetic predisposition for occlusive arterial disease, in which a mutant gene triggers inappropriate proliferation of vascular SMCs, leading to stenosis of vessels. The fact that SMCs and myofibroblasts from affected individuals proliferate more rapidly in culture than control cells supports this pathway. Similar results were obtained in the Acta2-deficient mouse, in which increased proliferation of myofibroblasts was noted both in vivo and in vitro when compared with wild-type myofibroblasts.23 Pathologic examination of affected aortic and cardiac tissue showing enlarged and stenotic arteries with increased SMCs primarily in the medial layer supports the hypothesis that SMC proliferation leads to occlusive lesions.
ACTA2 mutations associated with MMD provide further evidence that early-onset strokes may occur via a similar pathway of excessive SMC proliferation leading to arterial occlusion. MMD is a specific cerebrovascular disease characterized by stenosis or occlusion of the terminal portions of the internal carotid arteries and the formation of an abnormal vascular network in the vicinity of the arterial occlusion. The etiology of MMD is not understood, but it is established that genetic factors play a role, given that 6%–12% of MMD cases are familial.24,25 Histopathologic studies of the involved internal carotid arteries show fibrocellular proliferation of intimal SMCs as the cause of the arterial occlusion.26 Therefore, the MMD vascular lesions demonstrate the same increased SMC proliferation as observed in the ACTA2 vascular lesions.
The increased proliferation observed in ACTA2-mutant SMCs may be due to the role that α-actin polymerization plays in modulating the SMC phenotype between a quiescent cell expressing high levels of contractile and cytoskeletal proteins and a proliferating cell not expressing contractile proteins.7,27 We have previously demonstrated that the ACTA2 missense mutations lead to decreased SM α-actin polymerization into filaments in SMCs, suggesting a dominant-negative effect of the mutant α-actin on actin-filament formation by disruption of polymerization of monomeric α-actin (G-actin) into polymerized (F-actin) actin.10 It is already established that when F-actin polymerization is inhibited, leading to an increased concentration of free G-actin, a signal is transduced to the nucleus and MRTF-A and MRTF-B are translocated from the nucleus into the cytosol. In the nucleus, these proteins form a complex with serum response factor (SRF) and myocardin and drive expression of SMC contractile genes. Thus, the nuclear export of MRTF-A/B leads to downregulation of contractile protein expression. Furthermore, reduction of nuclear MRTF-A/B allows ternary complex factors (TCFs, members of the Ets family of transcription factors) to bind to SRF because MRTFs and TCFs compete for binding to a common surface of SRF.28,29 The TCF-SRF complex activates a subset of SRF-regulated growth-responsive genes, leading to cellular proliferation.
Despite the presence of mutant SM α-actin in all vascular SMCs, pathology and clinical imaging in patients suggest that SMC proliferation occurs in localized regions of the arteries. Histologic examination of ACTA2-mutant aortas documents that a subset of the vasa vasorum arteries are occluded as a result of SMC proliferation. In addition, the imaging from the patients with Moyamoya disease and ACTA2 p.R258C/H mutations demonstrates occlusion of a discrete region of the internal carotid artery. Therefore, we hypothesize that the SMC proliferation leading to arterial occlusion is dependent on the presence of the mutation and an additional factor, most likely vascular injury or increased biomechanical stress.
ACTA2 mutations lead to dilatation of the aorta but occlusion of smaller arteries, and this differential response to an underlying defect in SM α-actin may be due to a number of factors. First, the aorta and many of its large arterial branches are elastic arteries, in which the media is composed of SMCs lying between layers of elastic fibers. In contrast, medium and small arteries are muscular arteries, in which the SMC are attached to each other and not layers of elastic fibers.30 Elastin has an established role in increasing SMC differentiation and decreasing SMC proliferation, and the lack of SMC and elastic-fiber interaction may increase proliferation in muscular arteries.31 Second, the ascending aorta bears the majority of the force of the pulsatile blood as it is ejected from the contracting heart, whereas other arteries experience significantly less force from blood flow. The physiological forces may activate different pathways in the mutant SMCs, leading to different vascular disease presentation. In addition, different lineage origins of vascular SMCs may contribute to the differing vascular diseases. The ascending aortic- and cerebral-vessel SMCs are neural crest derived, whereas other vascular SMCs are mesoderm derived.32
The identification of ACTA2 mutations as causes of TAAD, premature CAD, and stroke put forth the concept that a mutation in a single gene can cause a variety of vascular diseases, as opposed to a single type of vascular disease, within a family. Recent genome-wide association studies also support this concept through the identification of single-nucleotide polymorphisms on chromosome 9p21 associated with a variety of vascular diseases, including CAD, abdominal aortic aneurysm, and cerebral aneurysms.33 Current studies for the identification of genes leading to vascular diseases focus on families with multiple members with a particular vascular disease, such as CAD or ischemic stroke, and the data presented here should modify these studies. Our data also has clinical implications for determining the risk for vascular disease on the basis of family history. Additionally, the clinical management of patients harboring ACTA2 mutations should be modified on the basis of these data. In addition to the currently recommended routine imaging of the aorta for aneurysms, ACTA2-mutation carriers should be monitored for CAD and occlusive cerebrovascular disease. Finally, inappropriate proliferation of SMCs as a result of an ACTA2 mutation may represent a novel pathogenetic pathway contributing to occlusive vascular disease.
Supplemental Data
Supplemental Data include two figures and four tables and can be found with this article online at http://www.ajhg.org/.
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
Ensembl Genome Browser, http://www.ensembl.org/index.html
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
PyMol Molecular Viewer, http://www.pymol.org/
Acknowledgments
The authors are extremely grateful to the families and their physicians involved in this study. The authors also wish to acknowledge Richard Gibbs (Director) and Chris Akers for the excellent graphic assistance. The following sources provided funding for these studies: P50HL083794-01 (D.M.M.), RO1 HL62594 (D.M.M.), UL1 RR024148 (CTSA), P50NS044227 (J.C.G.), INSERM and Programme National de Recherche Cardiovasculaire (X.J.), the Vivian L. Smith Foundation, and the TexGen Foundation. D.M.M. is a Doris Duke Distinguished Clinical Scientist, C.S.R. is a Pew Scholar, and C.L.P. is a Schissler Fellow.
References
- 1.Wang L., Fan C., Topol S.E., Topol E.J., Wang Q. Mutation of MEF2A in an inherited disorder with features of coronary artery disease. Science. 2003;302:1578–1581. doi: 10.1126/science.1088477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mani A., Radhakrishnan J., Wang H., Mani A., Mani M.A., Nelson-Williams C., Carew K.S., Mane S., Najmabadi H., Wu D. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q. Molecular genetics of coronary artery disease. Curr. Opin. Cardiol. 2005;20:182–188. doi: 10.1097/01.hco.0000160373.77190.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepin M., Schwarze U., Superti-Furga A., Byers P.H. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N. Engl. J. Med. 2000;342:673–680. doi: 10.1056/NEJM200003093421001. [DOI] [PubMed] [Google Scholar]
- 5.Friedman J.M., Arbiser J., Epstein J.A., Gutmann D.H., Huot S.J., Lin A.E., McManus B., Korf B.R. Cardiovascular disease in neurofibromatosis 1: Report of the NF1 Cardiovascular Task Force. Genet. Med. 2002;4:105–111. doi: 10.1097/00125817-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Fatigati V., Murphy R.A. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J. Biol. Chem. 1984;259:14383–14388. [PubMed] [Google Scholar]
- 7.Owens G.K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 8.Schildmeyer L.A., Braun R., Taffet G., Debiasi M., Burns A.E., Bradley A., Schwartz R.J. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle alpha-actin null mouse. FASEB J. 2000;14:2213–2220. doi: 10.1096/fj.99-0927com. [DOI] [PubMed] [Google Scholar]
- 9.Tomasek J.J., Haaksma C.J., Schwartz R.J., Vuong D.T., Zhang S.X., Ash J.D., Ma J., Al-Ubaidi M.R. Deletion of smooth muscle alpha-actin alters blood-retina barrier permeability and retinal function. Invest. Ophthalmol. Vis. Sci. 2006;47:2693–2700. doi: 10.1167/iovs.05-1297. [DOI] [PubMed] [Google Scholar]
- 10.Guo D.C., Pannu H., Papke C.L., Yu R.K., Avidan N., Bourgeois S., Estrera A.L., Safi H.J., Sparks E., Amor D. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat. Genet. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 11.Pannu H., Fadulu V., Chang J., Lafont A., Hasham S.N., Sparks E., Giampietro P.F., Zaleski C., Estrera A.L., Safi H.J. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005;112:513–520. doi: 10.1161/CIRCULATIONAHA.105.537340. [DOI] [PubMed] [Google Scholar]
- 12.Roman M.J., Devereux R.B., Kramer-Fox R., O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am. J. Cardiol. 1989;64:507–512. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., Wheeler D.A., Yakub I., Wei S., Sood R., Rowe W., Liu P.P., Gibbs R.A., Buetow K.H. SNPdetector: A software tool for sensitive and accurate SNP detection. PLoS. Comput. Biol. 2005;1:e53. doi: 10.1371/journal.pcbi.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo D., Hasham S., Kuang S.Q., Vaughan C.J., Boerwinkle E., Chen H., Abuelo D., Dietz H.C., Basson C.T., Shete S.S. Familial thoracic aortic aneurysms and dissections: Genetic heterogeneity with a major locus mapping to 5q13–14. Circulation. 2001;103:2461–2468. doi: 10.1161/01.cir.103.20.2461. [DOI] [PubMed] [Google Scholar]
- 15.Cottingham R.W., Idury R.M., Schaffer A.A. Faster sequential genetic linkage computations. Am. J. Hum. Genet. 1993;53:252–263. [PMC free article] [PubMed] [Google Scholar]
- 16.Jones T.A., Zou J.Y., Cowan S.W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 17.McDonald I.K., Thornton J.M. Satisfying hydrogen bonding potential in proteins. J. Mol. Biol. 1994;238:777–793. doi: 10.1006/jmbi.1994.1334. [DOI] [PubMed] [Google Scholar]
- 18.Delano W.L. DeLano Scientific; San Carlos, CA: 2002. The PyMOL User's Manual. [Google Scholar]
- 19.He R., Guo D.C., Estrera A.L., Safi H.J., Huynh T.T., Yin Z., Cao S.N., Lin J., Kurian T., Buja L.M. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J. Thorac. Cardiovasc. Surg. 2006;131:671–678. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Desmouliere A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora P.D., McCulloch C.A. The deletion of transforming growth factor-beta-induced myofibroblasts depends on growth conditions and actin organization. Am. J. Pathol. 1999;155:2087–2099. doi: 10.1016/s0002-9440(10)65527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topol E.J., Smith J., Plow E.F., Wang Q.K. Genetic susceptibility to myocardial infarction and coronary artery disease. Hum. Mol. Genet. 2006;15 doi: 10.1093/hmg/ddl183. Spec No 2, R117–R123. [DOI] [PubMed] [Google Scholar]
- 23.Takeji M., Moriyama T., Oseto S., Kawada N., Hori M., Imai E., Miwa T. Smooth muscle alpha-actin deficiency in myofibroblasts leads to enhanced renal tissue fibrosis. J. Biol. Chem. 2006;281:40193–40200. doi: 10.1074/jbc.M602182200. [DOI] [PubMed] [Google Scholar]
- 24.Kitahara T., Ariga N., Yamaura A., Makino H., Maki Y. Familial occurrence of moya-moya disease: Report of three Japanese families. J. Neurol. Neurosurg. Psychiatry. 1979;42:208–214. doi: 10.1136/jnnp.42.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott R.M., Smith J.L., Robertson R.L., Madsen J.R., Soriano S.G., Rockoff M.A. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J. Neurosurg. 2004;100:142–149. doi: 10.3171/ped.2004.100.2.0142. [DOI] [PubMed] [Google Scholar]
- 26.Fukui M., Kono S., Sueishi K., Ikezaki K. Moyamoya disease. Neuropathology. 2000;20(Suppl):S61–S64. doi: 10.1046/j.1440-1789.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 27.Parmacek M.S. Myocardin-related transcription factors: Critical coactivators regulating cardiovascular development and adaptation. Circ. Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 28.Posern G., Sotiropoulos A., Treisman R. Mutant actins demonstrate a role for unpolymerized actin in control of transcription by serum response factor. Mol. Biol. Cell. 2002;13:4167–4178. doi: 10.1091/mbc.02-05-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaromytidou A.I., Miralles F., Treisman R. MAL and ternary complex factor use different mechanisms to contact a common surface on the serum response factor DNA-binding domain. Mol. Cell. Biol. 2006;26:4134–4148. doi: 10.1128/MCB.01902-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphrey J.D. Springer; New York: 2002. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. pp. 249–264. [Google Scholar]
- 31.Brooke B.S., Bayes-Genis A., Li D.Y. New insights into elastin and vascular disease. Trends Cardiovasc. Med. 2003;13:176–181. doi: 10.1016/s1050-1738(03)00065-3. [DOI] [PubMed] [Google Scholar]
- 32.Topouzis S., Majesky M.W. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta 3. Dev. Biol. 1996;178:430–445. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- 33.Helgadottir A., Thorleifsson G., Magnusson K.P., Gretarsdottir S., Steinthorsdottir V., Manolescu A., Jones G.T., Rinkel G.J., Blankensteijn J.D., Ronkainen A. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat. Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


