Abstract
OBJECTIVE
Multifaceted care has been shown to reduce mortality and complications in type 2 diabetes. We hypothesized that structured care would reduce renal complications in type 2 diabetes.
RESEARCH DESIGN AND METHODS
A total of 205 Chinese type 2 diabetic patients from nine public hospitals who had plasma creatinine levels of 150–350 μmol/l were randomly assigned to receive structured care (n = 104) or usual care (n = 101) for 2 years. The structured care group was managed according to a prespecified protocol with the following treatment goals: blood pressure <130/80 mmHg, A1C <7%, LDL cholesterol <2.6 mmol/l, triglyceride <2 mmol/l, and persistent treatment with renin-angiotensin blockers. The primary end point was death and/or renal end point (creatinine >500 μmol/l or dialysis).
RESULTS
Of these 205 patients (mean ± SD age 65 ± 7.2 years; disease duration 14 ± 7.9 years), the structured care group achieved better control than the usual care group (diastolic blood pressure 68 ± 12 vs. 71 ± 12 mmHg, respectively, P = 0.02; A1C 7.3 ± 1.3 vs. 8.0 ± 1.6%, P < 0.01). After adjustment for age, sex, and study sites, the structured care (23.1%, n = 24) and usual care (23.8%, n = 24; NS) groups had similar end points, but more patients in the structured care group attained ≥3 treatment goals (61%, n = 63, vs. 28%, n = 28; P < 0.001). Patients who attained ≥3 treatment targets (n = 91) had reduced risk of the primary end point (14 vs. 34; relative risk 0.43 [95% CI 0.21–0.86] compared with that of those who attained ≤2 targets (n = 114).
CONCLUSIONS
Attainment of multiple treatment targets reduced the renal end point and death in type 2 diabetes. In addition to protocol, audits and feedback are needed to improve outcomes.
Athough the Steno-2 study reported that multifaceted care by a multidisciplinary team reduced all-cause mortality and cardiovascular complications in type 2 diabetes (1,2), these results need to be confirmed in a multicenter setting. In addition, renal insufficiency is a powerful predictor for cardiovascular diseases (3), and type 2 diabetes is the main cause of end-stage renal disease (ESRD) (4). The effects of multifaceted care on ESRD remain unexplored. We have reported 50–70% risk reduction in death and cardiorenal complications in type 2 diabetic patients managed by a diabetologist-led team (5,6) compared with usual care. In this randomized multicenter study, we compared the effects of structured care with predefined protocol and treatment targets, delivered by a diabetologist-led team, on death and ESRD compared with usual care.
RESEARCH DESIGN AND METHODS
In this 2-year randomized, multicenter study, which commenced in 2003 and completed in 2007, 205 type 2 diabetic patients with renal insufficiency were recruited from nine public hospitals. Each hospital runs a weekly session in which 10–15 diabetic patients undergo comprehensive assessment (7) and from which patients with plasma creatinine levels ≥150 μmol/l were invited for recruitment. All patients had ultrasound scanning to exclude reversible causes of renal insufficiency, e.g., renal artery stenosis.
With written informed consent, eligible patients were randomly assigned in a 1:1 manner to receive either structured care or usual care using a computer-generated allocation code with equal numbers in each group within the same hospital. The sealed envelope was opened sequentially by nonstudy personnel. The structured care group was followed up at the Diabetes Centre by the specialist team using a predefined protocol, whereas the usual care group was managed in their original clinic but returned for assessments at 2 years. The study was approved by the research ethics committee of each institution.
Inclusion criteria included a diagnosis of type 2 diabetes (8), age 35–75 years (inclusive), and plasma creatinine level 150–350 μmol/l (inclusive). Exclusioncriteria included malignancy or life-threatening diseases; nondiabetes renal disease (e.g., biopsy-proven glomerulonephritis orobstructive uropathy); unstable psychiatric illnesses, and renal function (≥20% difference in two consecutive plasma creatinine values within 3 months before recruitment).
The primary composite end point was death and/or ESRD, defined as the need for dialysis, or plasma creatinine level ≥500 μmol/l. The secondary end point was the percentage of patients attaining ≥3 treatment targets and their impacts on clinical outcomes.
Structured care
The structured care group was managed by a diabetes team including diabetologists, endocrine trainees, and diabetes nurses using a preprinted case report book containing predefined scheduled visits, assessment items, and treatment targets defined as follows: 1) blood pressure <130/80 mmHg, 2) A1C <7%, 3) calculated LDL cholesterol <2.6 mmol/l, 4) fasting plasma triglyceride <2 mmol/l, and 5) treatment with ACE inhibitors and/or angiotensin receptor blockers (ARBs) unless patients developed persistent hyperkalemia (≥5.5 mmol/l) or an acute rise in plasma creatinine (e.g., 30%) upon drug introduction or dose titration.
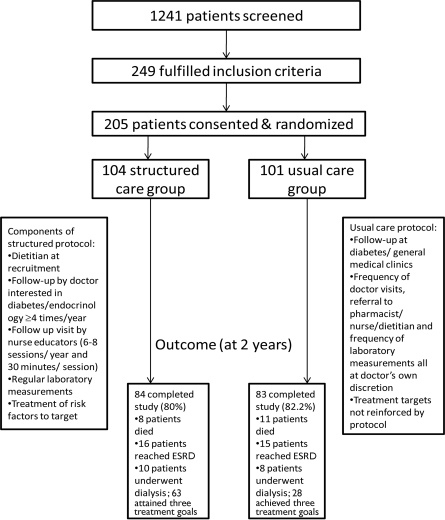
The structured care group was seen by a dietitian upon randomization to reinforce a low-protein and low-potassium diet. ACE inhibitor or ARB therapy was started in treatment-naive patients with monitoring of renal function at week 2, then every 4 weeks for 12 weeks and subsequently every 8–12 weeks, throughout the study period. All patients were seen by a doctor-nurse team every 3 months and more often if indicated. At each visit, blood pressure, body weight, and laboratory measurements were performed according to the protocol. Treatment compliance and self-care including drug use, insulin injection, self-monitoring of blood glucose, and lifestyle modification were reinforced by nurses either during clinic visits or using telephone calls. Patients also returned between clinic visits for measurement of blood pressure and body weight and blood taking by nurses (to test plasma glucose, A1C, and renal function). All laboratory results were available for review by a doctor at the scheduled visit for decision making. Complete blood results, plasma lipid levels (total cholesterol, HDL cholesterol, triglyceride, and calculated LDL cholesterol), and a bone profile were measured every 6 months.Figure 1 shows the overall study design and clinical outcomes for recruited patients.
Figure 1.
Overall study design and clinical outcomes of recruited patients.
Usual care
To ensure applicability of the results, patients randomly assigned to the usual care group were managed according to the usual clinic practice as defined by the respective hospital with no modification. Thus, patients might attend a diabetes clinic (managed by a diabetes team) or a general medical clinic (managed by a nondiabetes specialist or an internist), usually at 3- to 4-month intervals. All had access to laboratory tests, investigations, and counseling from a pharmacist, educator, or dietitian, but these were ordered at the doctor's discretion. Because different hospitals adopted different systems to manage diabetic patients, in some study sites, diabetic patients randomly assigned to usual care were followed up regularly at diabetes specialist clinics with a care standard comparable to that of the structured care group.
Statistical analysis
In a pilot study, the 2-year incidence of ESRD was 30% in 80 type 2 diabetic patients with plasma creatinine levels of 150–350 μmol/l who received structured care by a specialist team compared with 50% in a matched cohort receiving usual care in the same hospital. By using β = 0.8 and α = 0.05, the sample size was 96 for each group with a recruitment target number of 200–220, assuming a 10–20% default rate.
All data were analyzed by SPSS (Windows version 15.0; SPSS, Chicago, IL) using intention-to-treat analysis followed by per-protocol analysis, as defined by attainment of ≥3 treatment goals. All results are expressed as mean ± SD, n(percent), or median (range) where appropriate. Student's t test, χ2 test, and ANCOVA after adjustment for age, sex, and study centers were used for between-group comparisons. Relative risk (RR) reduction (95% CIs) was reported using Kaplan-Meier analysis.P < 0.05 (two-sided) was significant.
RESULTS
Of 205 patients recruited (mean ± SD age of 65 ± 7.2 years and disease duration of 14 ± 7.9 years), 104 were randomly assigned to structured care and 101 to usual care. The groups had similar clinical profiles except that the structured care group had a higher frequency of sensory neuropathy, coronary heart disease (CHD), and usage of ACE inhibitors or ARBs than the usual care group. Of these patients, 167 completed the 2-year study (84 structured care and 83 usual care). Reasons for withdrawal included death (n = 19), consent withdrawal (n = 3), loss to follow-up (n = 2), and referral to a nephrologist for dialysis (n = 14) (Table 1;Fig. 1).
Table 1.
Baseline clinical and biochemical characteristics and end of study clinical events in 205 type 2 diabetic patients with renal insufficiency randomly assigned to receive either structured or usual care for 2 years
| Structured care | Usual care | |
|---|---|---|
| At baseline | ||
| n | 104 | 101 |
| Age (years) | 64.6 ± 7.5 | 65.4 ± 6.9 |
| Male sex (%) | 66 | 67 |
| Duration of diabetes (years) | 14.4 ± 7.9 | 13.8 ± 7.9 |
| BMI (kg/m2) | 25.4 ± 3.5 | 25.4 ± 3.8 |
| Systolic blood pressure (mmHg) | 145 ± 23.7 | 144 ± 26.7 |
| Diastolic blood pressure (mmHg) | 74 ± 11.7 | 74 ± 10 |
| Waist circumference (cm) | 90.1 ± 9.2 | 89.3 ± 9.0 |
| Waist-to-hip ratio | 0.95 ± 0.06 | 0.94 ± 0.06 |
| Coexisting diseases (%) | ||
| Hypertension | 96 | 96 |
| Coronary heart disease* | 19 | 12 |
| Congestive heart failure | 8 | 7 |
| Myocardial infarction | 1 | 3 |
| Revascularization | 2 | 3 |
| Peripheral vascular disease | 1 | 1 |
| History of cerebrovascular accident | 14 | 16 |
| Retinopathy | 50 | 38 |
| Sensory neuropathy | 20 | 34 |
| Medications (%) | ||
| ACE inhibitor or ARB | 77 | 56 |
| Aspirin | 37 | 30 |
| Statin | 48 | 42 |
| Fibrate | 9 | 8 |
| Insulin | 61 | 58 |
| No. of blood pressure–lowering drugs | 2.9 ± 1.2 | 2.4 ± 1.1 |
| At 2 years | ||
| No. of patients with composite renal end point | 24 | 24 |
| ESRD (Cr >500 μmol/l) | 16 | 15 |
| Dialysis | 10 | 8 |
| Death | 8 | 11 |
| No. of patients with composite cardiovascular end point | 21 | 19 |
| Hospitalization for heart failure | 13 | 15 |
| Hospitalization for angina | 1 | 0 |
| Hospitalization for arrhythmia | 5 | 1 |
| Myocardial infarction | 4 | 4 |
| Revascularization (PTCA/CABG) | 1 | 1 |
| Other revascularization | 1 | 0 |
| CVA or transient ischemic attack | 2 | 3 |
| Lower-limb amputation | 1 | 0 |
| Emergency room visits (n) | 1 (0–8) | 1 (0–18) |
| Hospitalizations (n) | 1 (0–15) | 1 (0–13) |
| Days of hospitalization | 2 (0–93) | 2 (0–116) |
Data are means ± SD, n, %, or median (range). P values comparing SC and UC: all NS.
*Coronary heart disease includes symptoms of angina or an abnormal electrocardiogram with confirmed stress test and/or coronary angiogram. CABG, coronary artery bypass graft; Cr, serum creatinine; CVA, cerebrovascular accident; PTCA, percutaneous transluminal coronary angioplasty.
In both groups, the composite end point occurred in 24 patients (RR 0.96 [95% CI 0.50–1.84]) with similar rates of clinical events, hospitalization, and emergency room visits (Table 1). Among the nine study sites, the structured care group had fewer event rates than the usual care group in five hospitals, higher rates in two hospitals, and similar rates in two hospitals.
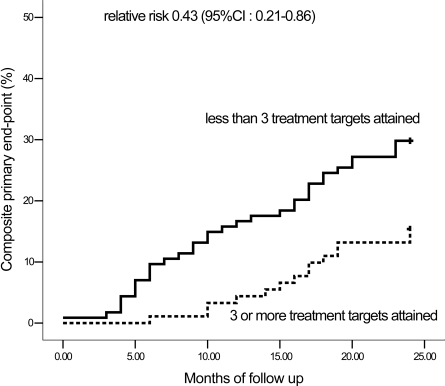
At 2 years and after adjustment for age, sex, and study sites, the structured care group had lower diastolic blood pressure and A1C levels and were more likely to attain ≥3 treatment goals (61% [ n = 63] vs. 28% [ n = 28]) (Table 2). With per protocol analysis, patients who attained ≥3 treatment goals (n = 91) had a 60% risk reduction in reaching the primary end point compared with those who did not attain ≥3 treatment goals (n = 114) (14 vs. 34; RR 0.43 [95% CI 0.21–0.86]) (Table 3; Fig. 2).
Table 2.
Metabolic control and attainment of treatment goals in type 2 diabetic patients with renal insufficiency randomly assigned to either usual or structured care for 2 years
| Structured care | Usual care | P* | |
|---|---|---|---|
| Completed 2 years of follow-up (%) | 81 | 82 | 0.55 |
| Systolic blood pressure (mmHg) | |||
| Baseline | 145 ± 24 | 144 ± 26 | 0.15 |
| Last available | 135 ± 25 | 137 ± 21 | 0.15 |
| Diastolic blood pressure (mmHg) | |||
| Baseline | 74 ± 12 | 74 ± 10 | 0.93 |
| Last available | 68 ± 12 | 71 ± 12 | 0.02 |
| A1C (%) | |||
| Baseline | 8.2 ± 1.9 | 8.4 ± 0.2 | 0.62 |
| Last available | 7.3 ± 1.3 | 8.0 ± 1.6 | <0.01 |
| Plasma triglycerides (mmol/l) | |||
| Baseline | 2.3 ± 1.7 | 2.5 ± 2.2 | 0.61 |
| Last available | 1.8 ± 1.3 | 1.9 ± 1.1 | 0.06 |
| HDL cholesterol (mmol/l) | |||
| Baseline | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.84 |
| Last available | 1.2 ± 0.4 | 1.2 ± 0.3 | 0.45 |
| LDL cholesterol (mmol/l) | |||
| Baseline | 3.1 ± 1.1 | 3.0 ± 1.0 | 0.60 |
| Last available | 2.49 ± 0.81 | 2.84 ± 1.1 | 0.14 |
| Serum creatinine (μmol/l) | |||
| Baseline | 196.3 ± 3.5 | 198.8 ± 48.7 | 0.68 |
| Last available | 281.9 ± 134.7 | 290.3 ± 28.7 | 0.37 |
| Estimated glomerular filtration rate (ml/min per 1.73m2) | |||
| Baseline | 31.4 ± 8.14 | 31.3 ± 8.2 | 0.96 |
| Last available | 24.0 ± 10.2 | 26.6 ± 12.4 | 0.11 |
| Use of ACE inhibitors or ARBs (%) | |||
| Baseline | 77 | 56 | <0.01 |
| Last visit | 69 | 49 | <0.01 |
| Use of insulin (%) | |||
| Baseline | 61 | 58 | 0.75 |
| Last visit | 65 | 71 | 0.29 |
| Patients attaining number of targets at last review visit (%) | |||
| 0 | 2 | 9 | |
| 1 | 13 | 19 | |
| 2 | 24 | 45 | |
| 3 | 29 | 16 | |
| 4 | 22 | 9 | |
| 5 | 10 | 3 | |
| Attained at least 3 treatment goals (%) | 61 | 28 | <0.01 |
| % of patients attaining treatment target at last review visit | |||
| Blood pressure <130/80 mmHg | 49 | 27 | <0.01 |
| LDL cholesterol <2.6 mmol/l | 56 | 41 | 0.02 |
| Triglycerides <2.0 mmol/l | 63 | 47 | 0.24 |
| A1C <7% | 39 | 26 | 0.19 |
Data are means ± SD or %.
*After adjustment for age, sex, and study centers.
Table 3.
Major clinical events in type 2 diabetic patients with renal insufficiency stratified by attainment of ≥3 prespecified treatment targets after 2 years of follow-up
| Attained ≥3 treatment goals* | Attained ≤2 treatment goals* | P† | |
|---|---|---|---|
| n | 91 | 114 | |
| Composite primary end point | 14 | 34 | 0.04 |
| Dialysis | 6 | 12 | 0.28 |
| ESRD (Cr >500 μmol/l) | 10 | 21 | 0.14 |
| Death | 4 | 15 | 0.11 |
| Composite cardiovascular end point | 19 | 21 | 0.22 |
| Hospitalization for heart failure | 12 | 16 | 0.77 |
| Hospitalization for arrhythmia | 4 | 2 | 0.41 |
| Acute myocardial infarction | 3 | 5 | 0.97 |
| Revascularization (PTCA/CABG) | 1 | 1 | 0.83 |
| Hospitalization for angina | 0 | 1 | 0.31 |
| Other revascularization | 0 | 1 | 0.99 |
| Lower-limb amputation | 1 | 0 | 0.14 |
Data are n.
*Treatment targets: 1) blood pressure <130/80 mmHg, 2) A1C <7%, 3) LDL cholesterol <2.6 mmol/l, 4) fasting plasma triglyceride <2 mmol/l, and 5) treatment with ACE inhibitors and/or ARBs.
†After adjustment for age, sex, and study centers. CABG, coronary artery bypass graft; Cr, serum creatinine; PTCA, percutaneous transluminal coronary angioplasty.
Figure 2.
Kaplan-Meier analysis showing the cumulative incidence of the primary composite end point of death or ESRD defined as dialysis or the need for dialysis or plasma creatinine level ≥500 μmol/l in type 2 diabetic patients with renal insufficiency stratified by attainment of ≥3 prespecified treatment targets. Treatment targets: 1) blood pressure <130/80 mmHg, 2) A1C <7%, 3) LDL cholesterol <2.6 mmol/l, 4) fasting plasma triglyceride <2 mmol/l, and 5) treatment with ACE inhibitors and/or ARBs.
CONCLUSIONS
In this multicenter, randomized translational study, we tested the superiority of protocol-driven care delivered by a diabetologist-nurse team over usual care in reducing death and ESRD. We observed that attainment of ≥3 treatment goals (blood pressure, A1C, LDL cholesterol, triglycerides, and use of ACE inhibitors or ARBs) rather than structured care was associated with improved clinical outcomes.
To date, most clinical studies are “mechanism research,” with the aim of understanding disease causality and efficacy of interventions under controlled environments. This type of research does not address issues such as barriers in translating such evidence to daily clinical practice. “Translational research” is more focused on outcomes of practical relevance to patients, health care providers, and health care systems. It examines the relationships between structural factors (e.g., practice structure and health care personnel), care processes (e.g., frequencies of measurements of A1C), and health outcomes in the real-life setting. Thus, whereas translational research has higher external validity (i.e., the possibility of generalizing study results), it has lower internal validity (i.e., the strength of the causal relationship between intervention and outcome of interest) than mechanism research. Nevertheless, these two types of research are complementary because once the evidence base of an intervention is established, there is a need to translate it to practice. With the growing popularity of chronic disease management, translation research is important in evaluating the effectiveness of these programs.
Several factors may explain our failure to prove that structured care was better than usual care in improving clinical outcomes. These include underpower of the study because of general improvement in the care standard, contamination by specialist care in the usual care group, and insufficient auditing to ensure adherence to the structured care protocol. When we first conceptualized the study in 2000, we used data available then to estimate sample size. In the Asian subgroup analysis of the Reduction of End Points in Type 2 Diabetes With the Angiotensin II Antagonist Losartan (RENAAL) study, which examined the renoprotective effects of losartan in type 2 diabetic patients with plasma creatinine (110–260 μmol/l) levels lower than those of the present cohort (150–350 μmol/l), the 3-year cumulative incidence of the primary end point of death and ESRD was 48% (9). In a subsequent proof-of-concept study involving patients with renal function comparable to that of the present cohort, the 2-year cumulative incidence of death and ESRD was 50% in the usual care group and 25% in the structured care group (6). Compared with these two studies (1997–2002), the 2-year cumulative incidence of the primary end point in the present cohort (2003–2007) was 24% in both the structured care and usual care groups. These findings suggest that increasing awareness of the beneficial effects of intensive risk factor control and inhibition of the renin-angiotensin system (10) might have led to improvement in the care standard and the reduced rate of clinical end points, at least in the Hong Kong setting.
Although this improvement in the care standard might have attenuated the benefits of structured care in our study, consistent with our predefined hypothesis, more patients in the structured care group attained ≥3 treatment goals (61%) than in the usual care group (28%). This difference was translated to 60–70% risk reduction in premature death and ESRD. In an observational study of 6,386 type 2 diabetic patients, attainment of ≥2 treatment goals (A1C, blood pressure, or LDL cholesterol) was associated with 30–50% risk reduction in new onset of CHD (11). These findings strongly support the need to attain multiple treatment targets to improve cardiorenal complications in type 2 diabetes. Because of the small sample size, we were unable to stratify patients by severity of renal function to perform subgroup analysis.
Although the structured care group was three times (61%) more likely than the usual care group (28%) to attain ≥3 treatment targets, there was considerable heterogeneity in event rates among hospitals, possibly due to different care systems. In two study sites, event rates were higher in the structured care group; in one, the result was due to a higher baseline plasma creatinine level in the structured care group, and in another, the usual care group was managed by the specialist team with regular review meetings. These findings have provided important insights into the potential effects of care organization on clinical outcomes. Thus, to fully realize the benefits of protocol-driven care, trained personnel or information technology is needed to ensure adherence to protocol and attainment of treatment targets through regular audits and feedback, a setup not dissimilar to a clinical trial setting (12). However, because of resource limitation, we were unable to implement these measures to complete the cycle of quality planning, assurance, and improvements (13).
There are also multiple barriers to translating evidence to practice at the levels of patients, care providers, and health care systems (14,15). In most clinical audits, <10% of type 2 diabetic patients attained ≥3 treatment goals of blood pressure, LDL cholesterol, and A1C (15). In addition, 20–50% of patients were noncompliant with life-saving drugs such as statins (16,17). Whereas clinical inertia of physicians might delay commencement or escalation of therapy (18), frequent testing of risk factors may not translate to changes in treatment regimens (19). Although use of e-mails and telephone calls improved doctors' adherence to some performance indexes (e.g., blood tests), the effects of these behavioral changes on clinical outcomes have not been explored (20). Conversely, in a 2-year randomized study, regular telephone calls by a pharmacist to reinforce compliance and ensure continuation of care reduced mortality and hospitalization by 30–50% in patients receiving ≥5 chronic medications including drugs for diabetes and CHD (17).
Thus, in the management of chronic diseases such as diabetes, an integrated approach including early diagnosis, risk stratification, use of protocols with predefined follow-up schedules, assessments, prompts for intervention further augmented by audits, and patient empowerment is needed to achieve desired outcomes (13). It has been suggested that if the specialist-led team care in the Steno-2 study can be translated to the primary care setting, such a model can be cost-saving (21). However, the challenge lies in identifying effective measures to ensure integration between different care providers with adequate communication and quality assurance. To this end, change in the practice environment to promote multidisciplinary care and self-management (22), a community-based shared care program (23), and pay-for-performance schemes (24) have improved adherence to treatment guidelines and metabolic control in type 2 diabetes.
In summary, type 2 diabetic patients with renal insufficiency receiving protocol-driven care delivered by a diabetes specialist team were more likely to attain multiple treatment targets, which was associated with a reduced risk of death and/or ESRD, than those receiving usual care. Clinical audits and regular feedback to doctors and patients may further improve compliance to structured care protocols and reduce the rate of clinical events.
Acknowledgments
This project was supported by the Hong Kong Government Health Services Research Committee (HSRC/HCPF s121012).
No potential conflicts of interest relevant to this article were reported.
We thank our research nurses, G. Cheung, Y.M. Lee, and D. Wong, for their assistance in coordinating the project and data entry. We thank the following diabetes nurses for their dedication in managing the patients: E. Kan (Alice Ho Miu Ling Nethersole Hospital); M.T. Chan (Caritas Medical Center); L.H. Lin and M.L. Tong (North District Hospital); J.J. Kwan, H. Tang, and Y.C. Han (Our Lady of Maryknoll Hospital); C.S. Law (Tseung Kwan O Hospital); C. Wong (Tung Wah Eastern Hospital); P.H. Mok (United Christian Hospital); A. Leung (Yan Chai Hospital); and M. Siu (The Princess Margaret Hospital).
APPENDIX
Other members of the SURE Study Group: Drs. Kin-Wah Chan and Chun-Hoi Chung (Princess Margaret Hospital); Grace Kum (United Christian Hospital), Lai-Ming Fung (Caritas Medical Center), and Tai-Pan Ip (Tung Wah Eastern Diabetes Center).
Footnotes
The sponsors did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Clinical trial reg. no. NCT00309127, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying editorial, p. 1132.
References
- 1.Gaede P, Lund-Andersen H, Parving HH, et al. : Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580– 591 [DOI] [PubMed] [Google Scholar]
- 2.Gaede P, Vedel P, Larsen N, et al. : Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383– 393 [DOI] [PubMed] [Google Scholar]
- 3.So WY, Kong AP, Ma RC, et al. : Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care 2006; 29: 2046– 2052 [DOI] [PubMed] [Google Scholar]
- 4.Ritz E, Rychlik I, Locatelli F, et al. : End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis 1999; 34: 795– 808 [DOI] [PubMed] [Google Scholar]
- 5.So WY, Tong PC, Ko GT, et al. : Effects of protocol-driven care versus usual outpatient clinic care on survival rates in patients with type 2 diabetes. Am J Manag Care 2003; 9: 606– 615 [PubMed] [Google Scholar]
- 6.Leung WYS, So WY, Tong PCY, et al. : Effects of structured care by a pharmacist-diabetes specialist team in patients with type 2 diabetic nephropathy. Am J Med 2005; 118: 1414.e1421– 1414.e1427 [DOI] [PubMed] [Google Scholar]
- 7.Piwernetz K, Home PD, Snorgaard O, et al. : Monitoring the targets of the St. Vincent declaration and the implementation of quality management in diabetes care: the Diabetes Care initiative. Diabet Med 1993; 10: 371– 377 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization: Diabetes Mellitus: Report of a WHO Study Group Geneva, World Health Org., 1985(Tech. Rep. Ser., no. 727) [PubMed] [Google Scholar]
- 9.Chan JCN, Wat NMS, So WY, et al. : RAAS blockade and renal disease in type 2 diabetic patients: an Asian perspective from the RENAAL Study. Diabetes Care 2004; 27: 874– 879 [DOI] [PubMed] [Google Scholar]
- 10.Jafar TH, Stark PC, Schmid CH, et al. : Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139: 244– 252 [DOI] [PubMed] [Google Scholar]
- 11.Kong AP, Yang X, Ko GT, et al. : Effects of treatment targets on subsequent cardiovascular events in Chinese patients with type 2 diabetes. Diabetes Care 2007; 30: 953– 959 [DOI] [PubMed] [Google Scholar]
- 12.Leung WY, So WY, Tong PC, et al. : The renoprotective effects of structured care in a clinical trial setting in type 2 diabetic patients with nephropathy. Nephrol Dial Transplant 2004; 19: 2519– 2525 [DOI] [PubMed] [Google Scholar]
- 13.Ellrodt G, Cook DJ, Lee J, et al. : Evidence-based disease management. JAMA 1997; 258: 1687– 1692 [PubMed] [Google Scholar]
- 14.Grol R, Grimshaw J: From best evidence to best practice: effective implementation of change in patient's care. Lancet 2003; 362: 1225– 1230 [DOI] [PubMed] [Google Scholar]
- 15.Chan JC, Gagliardino JJ, Baik SH, et al. : Multi-faceted determinants for achieving glycemic control: The InternationalDiabetes Management Practice Study (IDMPS). Diabetes Care 2009; 32: 227– 233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho PM, Rumsfeld JS, Masoudi FA, et al. : Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006; 166: 1836– 1841 [DOI] [PubMed] [Google Scholar]
- 17.Wu JY, Leung WY, Chang S, et al. : Effectiveness of telephone counselling by a pharmacist in reducing mortality in patients receiving polypharmacy: randomised controlled trial. BMJ 2006; 333522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips LS, Branch WT, Cook CB, et al. : Clinical inertia. Ann Intern Med 2001; 135: 825– 834 [DOI] [PubMed] [Google Scholar]
- 19.Grant R, Adams AS, Trinacty CM, et al. : Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care 2007; 30: 807– 812 [DOI] [PubMed] [Google Scholar]
- 20.Montori VM, Dinneen SF, Gorman CA, et al. : Theimpact of planned care and a diabeteselectronic management system on community-based diabetes care: the Mayo Health System Diabetes Translation Project. Diabetes Care 2002; 25: 1952– 1957 [DOI] [PubMed] [Google Scholar]
- 21.Gaede P, Valentine WJ, Palmer AJ, et al. : Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care 2008; 31: 1510– 1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funnell MM, Anderson RM: Changing office practice and health care systems to facilitate diabetes self-management. Curr Diab Rep 2003; 3: 127– 133 [DOI] [PubMed] [Google Scholar]
- 23.Piatt G, Orchard T, Emerson S, et al. : Translating the chronic care model into the community: results from a randomized controlled trial of a multifaceted diabetes care intervention. Diabetes Care 2006; 29: 811– 817 [DOI] [PubMed] [Google Scholar]
- 24.Millett C, Gray J, Saxena S, et al. : Ethnic disparities in diabetes management and pay-for-performance in the UK: the Wandsworth Prospective Diabetes Study. PLoS Med 2007; 4e191. [DOI] [PMC free article] [PubMed] [Google Scholar]