Abstract
OBJECTIVE
The purpose of this study was to investigate whether pregnancy induces increased insulin production as a marker of improved β-cell function in women with long-term type 1 diabetes.
RESEARCH DESIGN AND METHODS
This was a prospective study of 90 consecutive pregnant women with type 1 diabetes. At 8, 14, 21, 27, and 33 weeks blood samples were drawn for measurements of A1C, C-peptide, and serum glucose. C-peptide (detection limit: 6 pmol/l) was considered stimulated at a corresponding serum glucose concentration ≥5.0 mmol/l. GAD antibody concentration was determined at 8 and 33 weeks in 35 women.
RESULTS
C-peptide concentrations gradually increased throughout pregnancy regardless of serum glucose concentrations in the 90 women with a median duration of diabetes of 17 years (range 1–36 years). Among 35 women with paired recordings of stimulated C-peptide, C-peptide production was detectable in 15 (43%) at 8 weeks and in 34 (97%) at 33 weeks (P < 0.0001), and median C-peptide gradually increased from 6 to 11 pmol/l (P = 0.0004) with a median change of 50% (range −50 to 3,271%) during pregnancy. GAD antibodies were present in 77% with no change from 8 to 33 weeks (P = 0.85). Multivariate regression analysis revealed a positive association between the absolute increase in C-peptide concentrations during pregnancy and decreased A1C from 8 to 33 weeks (P = 0.003).
CONCLUSIONS
A pregnancy-induced increase in C-peptide concentrations in women with long-term type 1 diabetes was demonstrated, even in women with undetectable C-peptide concentrations in early pregnancy. This increase is suggestive of improved β-cell function and was associated with improvement in glycemic control during pregnancy.
Previously it has been shown that individuals with long-term type 1 diabetes have scattered β-cells in the pancreas (1) that retain the ability to develop new β-cells, probably by neogenesis from exocrine duct cells (2,3).
Pregnancy is a unique event in which the fetus usually survives to full term without rejection by the maternal immune system, which apparently accepts the foreign fetus (4). During normal pregnancy, mild suppression of the maternal immune system occurs to tolerate the allogenic fetus (5). This requires substantial changes in the maternal immune system over a programmed period. Pregnancy is associated with partial suppression of the immune inflammatory system so that autoimmune diseases often go into remission during pregnancy (5).
During normal pregnancy, growth-promoting factors are expressed in increased amounts, and the pancreas responds to the additional demand forinsulin by an enlargement of existing islets of Langerhans and hyperplasia of the insulin-producing β-cells (6,7), up-regulation of insulin synthesis and secretion (6), and increased sensitivity of β-cells to glucose stimulation (6). Increased mitotic activity has been seen both in vivo and in vitro in islets exposed to growth hormone. Receptors for growth hormone are expressed in islet cells and are upregulated during pregnancy (8).
In small series of women with long-term type 1 diabetes, even with previously undetectable C-peptide secretion, occurrence of maternal insulin production in pregnancy has been observed (9–11). This phenomenon may be a consequence of rejuvenated β-cell function due to pregnancy-induced growth-promoting factors and suppression of the immune system (9), but improved metabolic control may also play a role in newly diagnosed type 1 diabetes (12) as well as in type 1 diabetes beyond the initial remission phase (10,13).
The aim of this study was to investigate whether pregnancy induces increased C-peptide concentration as a marker of possible β-cell regeneration in a large unselected cohort of women with long-term type 1 diabetes. In addition, possible factors related to increased C-peptide concentration were investigated.
RESEARCH DESIGN AND METHODS
As a part of another study, a total of 108 consecutive Danish-speaking Caucasian women with clinical type 1 diabetes for >1 year and with nonfasting C-peptide concentrations <600 pmol/l were investigated prospectively during pregnancy. Details on the materials and methods have been published previously (14). A total of 90 women with available measurements of C-peptide and serum glucose in both early and late pregnancy were included in the current study.
At inclusion at median 8 (range 5–13) weeks, at the visits at 14 (12–16), 21 (20–23), 27 (25–29), and 33 (31–35) weeks, and once within 5 days postpartum, blood samples were drawn from an antecubital vein in the nonfasting statebetween breakfast and lunch and centrifuged at 3,000 g. Serum for C-peptide, placental growth hormone, and IGF-I measurements obtained during pregnancy was stored at −80°C for lateranalysis within one run of an assay. Postpartum blood samples for C-peptide and serum glucose were analyzed separately 6 months later. The differences in C-peptide concentrations and serum glucose levels between the two runs of assays were 0% for C-peptide and 2% for serum glucose, respectively, tested in 20 representative samples.
Serum glucose concentrations were measured by the glucose-hexokinase method (Roche Diagnostics, Mannheim, Germany) with an intra-assay coefficient of variation (CV) of 1.0% and an interassay CV of 1.7%. In 67% of the samples obtained during pregnancy, glucose concentrations were measured immediately; in the remaining samples measurement of serum glucose concentrations was obtained from thawed serum samples.
C-peptide was measured by the electrochemiluminescence immunoassay ECLIA (Modular 170, Roche Diagnostics). The concentrations in healthy fasting subjects were >180 pmol/l with an intra-assay CV of 1.9% and an interassay CV of 2.3%. C-peptide production was defined as concentrations above the detection limit of 6 pmol/l, whereas C-peptide concentrations ≤6 pmol/l were set to 6 pmol/l.
To secure measurements of C-peptide at serum glucose concentrations known to stimulate C-peptide production, the C-peptide data were post hoc divided into two groups, i.e., with corresponding plasma glucose concentrations greater than or less than 5.0 mmol/l (15). C-peptide concentrations with corresponding serum glucose concentrations ≥5.0 mmol/l are arbitrarily stated as “stimulated C-peptide” in the following. Thirty-five women had paired stimulated C-peptide concentrations at 8 and 33 weeks.
GAD antibody concentrations were determined in early and late pregnancy in the 35 women with paired stimulated C-peptide concentrations using a quantitative radioligand assay as described previously (16). The upper detection limit was 250 units/ml.
Placental growth hormone and IGF-I were analyzed as described previously (17). Serum creatinine was assayed on a routine basis, and the upper normal limit was 88 μmol/l (18).
All women visited our and/or their local diabetes clinic at 1- or 2-week intervals throughout pregnancy where weight, A1C, insulin dose, and blood pressure were recorded. The majority of women were treated with a basal bolus insulin regimen. All women registered their self-monitored plasma glucose (SMPG) readings in diabetes diaries, which were evaluated at each clinic visit. Routine self-monitoring was recommended at least seven times daily to obtain preprandial SMPG of 4.0–6.0 mmol/l, 90 min postprandial SMPG of 4.0–8.0 mmol/l, and pre-bedtime SMPG of 6.0–8.0 mmol/l (14).
A1C was measured by a latex immunoagglutination inhibition method with the same analyzer (DCA 2000, Bayer, England). Normal A1C range outside pregnancy was 4.7–6.3%, in early pregnancy was 4.5–5.7%, and in late pregnancy was 4.4–5.6% (19).
Selective serotonin reuptake inhibitors were given in 3 women and antihypertensive therapy, mainly with methyldopa, was given in 22 women during pregnancy. Thyroid dysfunction was treated with levothyroxine in 15 women and with thiamazole in 2 women, resulting in normal thyroid function in all 17 women during pregnancy.
All participants gave written informed consent. The research protocol was approved by the regional committees for ethics and science and by the Danish Data Protection Agency.
Statistics
Descriptive statistics are given as median (range) or number (percent). Categorical variables were compared by a χ2 test or Fisher's exact test as appropriate. Continuous variables were analyzed by Kruskal-Wallis or Mann-Whitney tests when appropriate.
Changes during pregnancy were tested by assessing the within-subject differences between continuous values at week 33 and week 8 using the Kruskal-Wallis test. McNemar's test was used for within-subject differences between frequencies.
After logarithmic transformation to improve approximation of the normal distribution, correlation analyses were performed using Pearson's coefficient, denoted r. To determine factors associated with absolute change (after logarithmic transformation) in C-peptide concentrations from 8 to 33 weeks, univariate linear regression analyses were conducted with the following explanatory variables: maternal age, duration of diabetes, prepregnancy BMI, weight gain during pregnancy and decrease in A1C from 8 to 33 weeks, as well as insulin dose (international units per kilogram), A1C (percentage), placental growth hormone, IGF-I, and GAD antibody concentrations at 8 and 33 weeks. Significant variables were further tested in backward stepwise multivariate linear regression analysis. A variable remaining significantly associated with change in C-peptide concentration during pregnancy in this analysis was regarded as an independent factor.
Differences were considered to be statistically significant at two-sided P < 0.05. All statistical analyses were performed using SAS (version 9.1, SAS Institute, Cary, NC).
RESULTS
The 90 women were median 31 (range 21–42) years old with a duration of diabetes of 17 (1–36) years and a prepregnancy BMI of 24.3 (18.4–34.4) kg/m2. At inclusion, A1C was 6.6 (4.9–8.8)%, and insulin dose was 0.75 (0.33–1.17) IU/kg. At each blood sampling time, concentrations of both C-peptide and corresponding serum glucose were measured in 87–100% of women (Table 1).
Table 1.
C-peptide concentrations during pregnancy in 90 women with type 1 diabetes divided according to serum glucose values ≥5.0 mmol/l or <5.0 mmol/l at the time of sampling
| 8 weeks | 14 weeks | 21 weeks | 27 weeks | 33 weeks | Postpartum | |
|---|---|---|---|---|---|---|
| Women with blood sampling | 90 (100) | 85 (94) | 87 (97) | 85 (94) | 90 (100) | 78 (87) |
| Number of samples <5.0 mmol/l | 32 | 43 | 39 | 28 | 31 | 13 |
| Number of samples ≥5.0 mmol/l | 58 | 42 | 48 | 57 | 59 | 65 |
| C-peptide concentration (pmol/l) | 6 (6–325) | 7 (6–598) | 7 (6–434) | 9 (6–527) | 11 (6–472) | 6 (6–1,250) |
| C-peptide concentration (pmol/l) at serum glucose <5.0 mmol/l | 6 (6–194) | 7 (6–248) | 7 (6–120) | 8 (6–308) | 9 (6–164) | 6 (6–350) |
| C-peptide concentration (pmol/l) at serum glucose ≥5.0 mmol/l | 6 (6–325) | 7 (6–598) | 7 (6–434) | 9 (6–527) | 13 (6–472) | 6 (6–1,250) |
| Women with C-peptide production | 38 (42) | 47 (49) | 54 (62) | 79 (93) | 88 (98) | 28 (36) |
| Women with C-peptide production at serum glucose <5.0 mmol/l | 14 (44) | 25 (58) | 22 (56) | 23 (82) | 30 (97) | 4 (31) |
| Women with C-peptide production at serum glucose ≥5.0 mmol/l | 24 (41) | 22 (52) | 32 (67) | 56 (98) | 58 (98) | 24 (37) |
| Nonfasting serum glucose concentration <5.0 mmol/l | 3.7 (0.8–4.9) | 3.6 (1.8–4.9) | 3.4 (1.4–4.9) | 3.7 (1.8–4.9) | 3.5 (2.1–4.9) | 4.1 (2.0–4.8) |
| Nonfasting serum glucose concentration ≥5.0 mmol/l | 7.2 (5.0–14.5) | 8.1 (5.1–15.5) | 7.0 (5.1–15.5) | 6.6 (5.0–14.4) | 6.9 (5.0–18.3) | 8.4 (5.0–20.9) |
Data are given as n(%) or median (range). C-peptide production was defined as concentrations above the detection limit of 6 pmol/l. Concentrations at or below 6 were set to 6 pmol/l.
At 8 weeks, the C-peptide concentration was detectable in serum in 44% of women with a corresponding serum glucose concentration <5.0 mmol/l and in 41% of women with stimulated C-peptide (corresponding serum glucose concentration ≥5.0 mmol/l). Throughout pregnancy, these proportions gradually increased (Table 1), and at 33 weeks, the C-peptide production was detectable in 97% of women with a corresponding serum glucose concentration <5.0 mmol/l and in 98% of women with stimulated C-peptide. Median serum glucose concentrations in the groups with concentrations less than and greater than 5.0 mmol/l, respectively, were comparable throughout pregnancy (Table 1), but the individual women's glucose concentrations varied at greater than and less than 5.0 mmol/l at the different sampling times. C-peptide and serum glucose concentrations did not correlate significantly at any sampling time.
Serum creatinine concentrations were within normal range in all but two women with concentrations of 89 and 95 μmol/l at inclusion, increasing to 101 and 125 μmol/l at 33 weeks. C-peptide increased from undetectable concentrations at inclusion to 10 pmol/l at 33 weeks in both women.
Table 2 shows data for 35 women with paired recordings of stimulated C-peptide concentrations at 8 and 33 weeks. The number of women with detectable C-peptide concentrations increased from 15 (43%) at 8 weeks to 34 (97%) at 33 weeks (P < 0.0001) at comparable serum glucose concentrations. From 8 to 33 weeks, the median C-peptide concentration increased from 6 to 11 pmol/l (P = 0.0004) with a median absolute change in C-peptide concentrations of 50% (range −50 to 3,271%).
Table 2.
Clinical data in 35 type 1 diabetic women who had paired values of stimulated C-peptide concentrations in both early and late pregnancy
| Women without C-peptide production in early pregnancy | Women with C-peptide production in early pregnancy | |
|---|---|---|
| n (%) | 20 (57) | 15 (43) |
| Age (years) | 32 (27–40)* | 30 (26–34) |
| Duration of diabetes (years) | 20 (6–28) | 16 (2–31) |
| Last A1C before pregnancy (%) | 7.5 (6.1–10.0) | 7.6 (6.0–9.3) |
| A1C at 8 weeks (%) | 7.1 (5.9–8.7) | 6.8 (5.6–8.8) |
| A1C at 33 weeks (%) | 6.0 (5.4–7.2) | 6.0 (5.5–7.1) |
| Median change in A1C from 8 to 33 weeks (%) | 0.8 (−0.2 to 2.1) | 0.6 (−0.5 to 2.9) |
| Insulin dose before pregnancy (IU/kg) | 0.75 (0.49–1.05) | 0.56 (0.32–1.17) |
| Insulin dose at 8 weeks (IU/kg) | 0.74 (0.45–1.04) | 0.77 (0.33–1.14) |
| Insulin dose at 33 weeks (IU/kg) | 1.04 (0.72–1.60) | 1.05 (0.52–1.46) |
| BMI before pregnancy (kg/m2) | 24.2 (18.4–34.4) | 23.5 (19.7–33.7) |
| Weight gain during pregnancy (kg) | 14.3 (5.6–26.8) | 15.0 (8.0–26.0) |
| C-peptide concentrations at 8 weeks (pmol/l) | 6 | 22 (7–325) |
| C-peptide concentrations at 33 weeks (pmol/l) | 10 (6–16) | 38 (8–472) |
| Median change in C-peptide concentrations from 8 to 33 weeks (%) | 67 (0–167)* | 30 (−50 to 3,271) |
| Serum glucose concentrations at 8 weeks (mmol/l) | 6.8 (5.5–13.3) | 7.7 (5.1–14.5) |
| Serum glucose concentrations at 33 weeks (mmol/l) | 6.7 (5.4–18.3) | 7.6 (5.1–12.8) |
| Placental growth hormone concentrations at 8 weeks (ng/ml) | 1.0 (0.1–6.9) | 1.3 (0.2–5.2) |
| Placental growth hormone concentrations at 33 weeks (ng/ml) | 36.6 (3.5–157) | 41 (8.2–87) |
| IGF-I concentrations at 8 weeks (ng/ml) | 158 (26–234) | 169 (100 to 268) |
| IGF-I concentrations at 33 weeks (ng/ml) | 235 (115–483) | 280 (144–331) |
| GAD antibody concentrations at 8 weeks (units/ml) | 20.5 (0–250) | 7 (0–250) |
| GAD antibody concentrations at 33 weeks (units/ml) | 15 (0–250) | 6 (0–129) |
Data are median (range) unless indicated otherwise.
*P < 0.05.
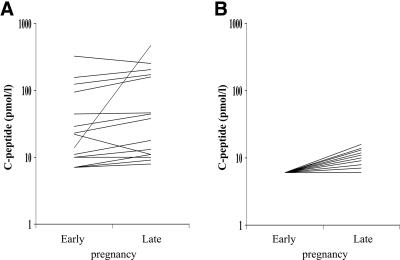
Twenty women had undetectable C-peptide concentrations in early pregnancy and were older (P = 0.04) with a tendency toward longer diabetes duration (P = 0.06) compared with 15 women with detectable C-peptide concentrations in early pregnancy (Table 2). The C-peptide concentrations in these women measured in late pregnancy did not reach the values of women with detectable values at inclusion (Fig. 1), but the percentage of absolute change in C-peptide concentrations was highest in the women with undetectable concentrations at inclusion. No differences were detected in the prevalence of diabetic retinopathy, hypoglycemia awareness, or severe hypoglycemia (requiring assistance from another individual) during pregnancy (data not shown).
Figure 1.
A: Paired recordings of stimulated C-peptide concentration in 15 pregnant women with type 1 diabetes and C-peptide production in early pregnancy. B: Paired recordings of stimulated C-peptide concentration in 20 pregnant women with type 1 diabetes without C-peptide production in early pregnancy. Detection limit was 6 pmol/l. C-peptide concentration is indicated on a logarithmic scale.
GAD antibodies were present in 27 (77%) of the 35 women. There was a tendency toward higher concentrations among women with undetectable C-peptide concentrations at baseline (Table 2), but this did not reach statistical significance (P = 0.14). A decrease in GAD antibody concentrations during pregnancy could not be detected (P = 0.85).
In univariate linear regression analyses, the absolute change in C-peptide concentrations from 8 to 33 weeks was positively associated with A1C at inclusion (r = 0.34; P = 0.047) and with a decrease in A1C from 8 to 33 weeks (r = 0.49; P = 0.003). No other significant associations were found. In multivariate linear regression analysis, a decrease in A1C from 8 to 33 weeks was the only variable associated with an absolute change in C-peptide concentrations (parameter estimate: β = 50 [95% CI 20–80], P = 0.003). This means that C-peptide concentration increases 50 pmol/l per percent decrease in A1C.
CONCLUSIONS
In this prospective study of 90 pregnant women with long-term type 1 diabetes, we demonstrated gradually increasing C-peptide concentrations in serum throughout pregnancy. Even in women with undetectable stimulated C-peptide concentrations in early pregnancy, a significant increase in stimulated C-peptide concentrations was obtained in late pregnancy. This increase was associated with an improvement in glycemic control during pregnancy.
These findings are in extension of preliminary observations of rejuvenation of β-cell function in early pregnancy in 10 women with long-term type 1 diabetes (9) and in 23 type 1 diabetic women during the entire pregnancy (11). In the current study, detectable C-peptide production was present in 42% of women in early pregnancy, which is comparable with recent findings in nonpregnant type 1 diabetic subjects with comparable duration of diabetes and good metabolic control (20).
C-peptide does not cross the placenta in either direction and, consequently, the C-peptide concentrations detected in the current study originate from the mother and not from the developing fetus. Likewise it is not likely that the increase seen in C-peptide concentrations is due to cross-reactivity with other pregnancy-related hormones (9). To avoid overestimation of the increase in C-peptide concentrations in the subset of women with C-peptide concentrations below the detection limit in early pregnancy, these concentrations were all set to 6 pmol/l (detection limit).
Serum glucose concentrations were measured immediately in the majority of samples. However, for practical reasons, in a minority of samples, serum glucose concentrations were assayed on stored samples, which might reveal slightly lower concentrations due to intermediate glucose metabolism.
For practical reasons standardized sampling either in the fasting state or after a standardized meal was not performed. To compensate for this weakness of the study, a post hoc analysis of women with paired C-peptide measurements at serum glucose concentrations known to stimulate C-peptide production (15) was performed, and a significant increase in C-peptide concentration during pregnancy was demonstrated in this subgroup. Surprisingly, the C-peptide concentrations measured at serum glucose concentrations <5 mmol/l were comparable with the concentrations measured at stimulated serum glucose concentrations. The explanation for this finding remains speculative. The duration of the low serum glucose concentrations is unknown, and it is not known whether the relatively immature and stressed β-cells of these pregnant women are able to produce significant differences in C-peptide concentrations with the relatively small difference in serum glucose concentrations detected in this study.
In normal rodents, the number of insulin-producing β-cells increases during pregnancy, and the sensitivity to secretagogues is increased in the islet β-cells (7). Another recent study of pregnant, nondiabetic mice showed a 2-fold increase in β-cell mass during pregnancy (21). In human studies, the area of the pancreatic endocrine tissue doubles during normal pregnancy, and hyperplasia of the β-cells is demonstrated (7). The demonstrated increase in C-peptide production in the current study may thus reflect pregnancy-induced hyperplasia of the existing β-cells. Such a mechanism is supported by the rapid decline in the circulating C-peptide concentration postpartum. However, β-cell neoplasia may also be present, which is supported by the autopsy finding of insulin-positive β-cells in 88% of patients with long-standing type 1 diabetes (2). In that study, the histological investigations suggested that new β-cells are formed through differentiation of progenitor cells as duct cells or putative stem cells (2). In healthy pregnant women a similar increase in C-peptide concentration during pregnancy has been reported (7,10,11).
The mechanisms underlying the increased C-peptide production demonstrated in the present study remain unknown. Menin, a protein previously characterized as an endocrine tumor suppressor and transcriptional regulator, controls islet growth in pregnant, nondiabetic mice (21). Further studies of possible growth promoters during human pregnancy are warranted.
In the current study, the increased C-peptide concentrations were associated with improved metabolic control during pregnancy. This finding is in accordance with previous findings of an association between better metabolic control and the presence of C-peptide production in pregnant (11) and nonpregnant patients with type 1 diabetes (10,12,13,22). It is not known whether improved metabolic control results in C-peptide production or, vice versa, whether the increased C-peptide production results in improved metabolic control. However, a prospective randomized trial demonstrated that improved metabolic control leads to increased C-peptide concentrations in patients with short-term type 1 diabetes (13), and in the current study the increased C-peptide concentrations were not associated with a reduced insulin dose in late pregnancy, leading us to suggest that the improved metabolic control facilitated C-peptide production. In postmortum analysis of pancreatic tissue from patients with type 1 diabetes, the number of β-cells was independent of duration of diabetes but was higher in patients with lower blood glucose concentrations (2). This finding may indicate that obtaining and maintaining good metabolic control in pregnancy plays a pivotal role in the regeneration of insulin-producing β-cells during pregnancy.
C-peptide concentrations may be elevated in renal disease (23). It is unlikely that this confounds the present results as the renal clearance is generally increased during pregnancy (24), which may indeed underestimate the C-peptide concentrations. Notably, serum creatinine remained within the normal range throughout pregnancy in the vast majority of our patients (18).
During normal pregnancy mild maternal immunosuppression occurs to tolerate the allogenic fetus (5). This may result in metabolically important regeneration of β-cell function. We could not demonstrate a relation between changes in GAD antibody concentration and regeneration of β-cell function during pregnancy, in agreement with Novak et al. (10), who did not detect any association between C-peptide positivity during pregnancy and the presence of antibodies against GAD, insulinoma antigen 2, or insulin (10). A possible role of pregnancy-induced changes in other aspects of the immune system cannot be ruled out.
Increased mitotic activity has been seen both in vivo and in vitro in islets exposed to growth hormone (8), suggesting that this hormone may have played a role in the increasing C-peptide concentrations during pregnancy. Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet β-cells from pancreatic duct cells and an increase in functional β-cell mass (3). In the current study we demonstrated a significant increase in placental growth hormone and IGF-I during pregnancy (17), but associations between these growth promotors and increased C-peptide production during pregnancy could not be demonstrated. However, because of the relatively small number of pregnant women with paired recordings of stimulated C-peptide and the small number of growth promoters examined, an association between pregnancy-related growth promoters and increased C-peptide production could not be excluded.
In the current study, C-peptide concentrations decreased rapidly postpartum. This may imply that the increasing C-peptide concentrations demonstrated during pregnancy may be generated by placenta-derived growth promoters or hormonal factors that are not lasting after pregnancy. In favor of this hypothesis is the finding in mice of a pregnancy-stimulated proliferation of maternal rodent pancreatic islet β-cells with a prompt decline in the proliferation within 4 days postpartum (21). Alternatively, the increasing C-peptide concentrations during pregnancy may be ascribed to interference with other substances. However, the C-peptide assay used in our study measures C-peptide accurately and specifically (25).
In summary, a pregnancy-induced increase in C-peptide concentrations in women with long-term type 1 diabetes was demonstrated, even in women with undetectable C-peptide concentrations in early pregnancy.
Acknowledgments
P.D. has received funds for research and fees for consulting and speaking from Novo Nordisk. E.R.M. has received fees for speaking from Novartis and Novo Nordisk and funds for research and fees for consulting from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Our gratitude goes to Lois Jovanovic, Sansum Diabetes Research Institute (Santa Barbara, CA), for inspiring scientific discussions during the conduction of this study. We are indebted to nurses E. Stage and C. Barfred, to laboratory technicians K.M. Larsen and M. Wahl, for careful handling of patients and data, and to laboratory technicians A. von der Lieth and C.G.F. Leth for the hormone measurements.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Lohr M, Kloppel G: Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia 1987; 30: 757– 762 [DOI] [PubMed] [Google Scholar]
- 2.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC: Sustained β cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 2005; 48: 2221– 2228 [DOI] [PubMed] [Google Scholar]
- 3.Suarez-Pinzon WL, Lakey JR, Brand SJ, Rabinovitch A: Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet β-cells from pancreatic duct cells and an increase in functional β-cell mass. J Clin Endocrinol Metab 2005; 90: 3401– 3409 [DOI] [PubMed] [Google Scholar]
- 4.Poole JA, Claman HN: Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol 2004; 26: 161– 170 [DOI] [PubMed] [Google Scholar]
- 5.Wilder RL: Hormones, pregnancy, and autoimmune diseases. Ann NY Acad Sci 1998; 840: 45– 50 [DOI] [PubMed] [Google Scholar]
- 6.Sorenson RL, Brelje TC: Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 1997; 29: 301– 307 [DOI] [PubMed] [Google Scholar]
- 7.Van Assche FA, Aerts L, de Prins F: A morphological study of the endocrine pancreas in human pregnancy. Br J Obstet Gynaecol 1978; 85: 818– 820 [DOI] [PubMed] [Google Scholar]
- 8.Nielsen JH, Svensson C, Galsgaard ED, Moldrup A, Billestrup N: β Cell proliferation and growth factors. J Mol Med 1999; 77: 62– 66 [DOI] [PubMed] [Google Scholar]
- 9.Ilic S, Jovanovic L, Wollitzer AO: Is the paradoxical first trimester drop in insulin requirement due to an increase in C-peptide concentration in pregnant type Idiabetic women? Diabetologia 2005; 43: 1329– 1330 [DOI] [PubMed] [Google Scholar]
- 10.Novak EJ, Ortqvist E, Nord E, Edwall L, Hampe CS, Bekris L, Persson BE, Lernmark A: Stability of disease-associated antibody titers in pregnant women with type 1 diabetes with or without residual β-cell function. Diabetes Care 2005; 23: 1019– 1021 [DOI] [PubMed] [Google Scholar]
- 11.Pirttiaho HI, Hartikainen-Sorri AL, Kaapa P, Kaila JM, Puukka R: Maternal residual beta-cell function and the outcome of diabetic pregnancy. J Perinat Med 1987; 15: 83– 89 [DOI] [PubMed] [Google Scholar]
- 12.Shah SC, Malone JI, Simpson NE: A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med 1989; 320: 550– 554 [DOI] [PubMed] [Google Scholar]
- 13.Madsbad S, Krarup T, Reguer L, Faber OK, Binder C: Effect of strict blood glucose control on residual B-cell function in insulin-dependent diabetics. Diabetologia 1981; 20: 530– 534 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen LR, Pedersen-Bjergaard U, Thorsteinsson B, Johansen M, Damm P, Mathiesen ER: Hypoglycemia in pregnant women with type 1 diabetes: predictors and role of metabolic control. Diabetes Care 2008; 31: 9– 14 [DOI] [PubMed] [Google Scholar]
- 15.Howell SL, Pickup J, Williams G: Textbook of Diabetes, vol.1, 2nd ed.Oxford, UK, Blackwell Science, 1997, p. 8.1– 8.14 [Google Scholar]
- 16.Petersen JS, Dyrberg T, Karlsen AE, Molvig J, Michelsen B, Nerup J, Mandrup-Poulsen T: Glutamic acid decarboxylase (GAD65) autoantibodies in prediction of β-cell function and remission in recent-onset IDDM after cyclosporin treatment: the Canadian-European Randomized Control Trial Group. Diabetes 1994; 43: 1291– 1296 [DOI] [PubMed] [Google Scholar]
- 17.Ringholm NL, Juul A, Pedersen-Bjergaard U, Thorsteinsson B, Damm P, Mathiesen ER: Lower levels of circulating IGF-I in type 1 diabetic women with frequent severe hypoglycaemia during pregnancy. Diabet Med 2008; 25: 826– 833 [DOI] [PubMed] [Google Scholar]
- 18.Nielsen LR, Damm P, Mathiesen ER: Improved pregnancy outcome in type 1 diabetic women with microalbuminuria or diabetic nephropathy: effect of intensified antihypertensive therapy? Diabetes Care 2009; 32: 38– 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen LR, Ekbom P, Damm P, Glumer C, Frandsen MM, Jensen DM, Mathiesen ER: HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 2004; 27: 1200– 1201 [DOI] [PubMed] [Google Scholar]
- 20.Hedman CA, Frystyk J, Lindstrom T, Chen JW, Flyvbjerg A, Orskov H, Arnqvist HJ: Residual β-cell function more than glycemic control determines abnormalities of the insulin-like growth factor system in type 1 diabetes. J Clin Endocrinol Metab 2004; 89: 6305– 6309 [DOI] [PubMed] [Google Scholar]
- 21.Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK: Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science 2007; 318: 806– 809 [DOI] [PubMed] [Google Scholar]
- 22.Madsbad S: Prevalence of residual B cell function and its metabolic consequences in type 1 (insulin-dependent) diabetes. Diabetologia 1983; 24: 141– 147 [DOI] [PubMed] [Google Scholar]
- 23.Clark PM: Assays for insulin, proinsulin(s) and C-peptide. Ann Clin Biochem 1999; 36 ( Pt 5): 541– 564 [DOI] [PubMed] [Google Scholar]
- 24.Davison JM, Dunlop W, Ezimokhai M: 24-Hour creatinine clearance during the third trimester of normal pregnancy. Br J Obstet Gynaecol 1980; 87: 106– 109 [DOI] [PubMed] [Google Scholar]
- 25.Department of Clinical Biochemistry, Rigshospitalet Denmark Validation report on proinsulin - C-peptide measurements. Copenhagen, Denmark, Department of Clinical Biochemistry, Rigshospitalet Denmark, 2005p. 1– 9 [Google Scholar]