Abstract
OBJECTIVE
The purpose of this study was to examine the predictors of intensification of antihyperglycemic therapy in patients with type 2 diabetes; its impact on A1C, body weight, symptoms of anxiety/depression, and health status; and patient characteristics associated with improvement in A1C.
RESEARCH DESIGN AND METHODS
We analyzed survey, medical record, and health plan administrative data collected in Translating Research into Action for Diabetes (TRIAD). We examined patients who were using diet/exercise or oral antihyperglycemic medications at baseline, had A1C >7.2%, and stayed with the same therapy or intensified therapy (initiated or increased the number of classes of oral antihyperglycemic medications or began insulin) over 18 months.
RESULTS
Of 1,093 patients, 520 intensified therapy with oral medications or insulin. Patients intensifying therapy were aged 58 ± 12 years, had diabetes duration of 11 ± 9 years, and had A1C of 9.1 ± 1.5%. Younger age and higher A1C were associated with therapy intensification. Compared with patients who did not intensify therapy, those who intensified therapy experienced a 0.49% reduction in A1C (P < 0.0001), a 3-pound increase in weight (P = 0.003), and no change in anxiety/depression (P = 0.5) or health status (P = 0.2). Among those who intensified therapy, improvement in A1C was associated with higher baseline A1C, older age, black race/ethnicity, lower income, and more physician visits.
CONCLUSIONS
Treatment intensification improved glycemic control with no worsening of anxiety/depression or health status, especially in elderly, lower-income, and minority patients with type 2 diabetes. Interventions are needed to overcome clinical inertia when patients might benefit from treatment intensification and improved glycemic control.
Over the past decade, the number of therapies available for the management of type 2 diabetes has increased dramatically. Much of the evidence demonstrating the efficacy of these therapies has come from randomized, controlled, clinical trials (1,2). The effectiveness of these therapies in real-world clinical settings has not been studied as thoroughly.
Clinical inertia, defined as the failure of health care providers to appropriately intensify medical management (3), and patient nonadherence, defined as the failure of patients to initiate or continuephysician-recommended changes in management, contribute to suboptimal glycemic control (4–6). A previous study of patients with poorly controlled diabetes showed that older patients and those of nonwhite race/ethnicity were less likely to have their treatment intensified than younger, white individuals (7). Physicians cite concerns about hypoglycemia, weight gain, and patient preferences as reasons for not intensifying therapy, and patients often express concerns about injections and the negative impact on quality of life as reasons for not intensifying therapy. Ideally, diabetes treatment regimens should be individually designed to prevent complications and comorbidities while respecting patient preferences and optimizing quality of life.
The current analyses were designed to assess the impact of changes in antihyperglycemic therapies on health outcomes in managed care patients with type 2 diabetes. Specifically, we assessed the predictors of intensification of antihyperglycemic therapy, its impact on A1C, body weight, symptoms of anxiety/depression, and health status, and patient characteristics associated with improvement in A1C.
RESEARCH DESIGN AND METHODS
Translating Research into Action for Diabetes (TRIAD) has been described in detail elsewhere (8). In brief, TRIAD studied a random sample of adults aged ≥18 years with diabetes from 10 heath plans and 68 provider groups serving ∼180,000 patients with diabetes. Institutional review boards at each participating site approved the study. All participants provided informed consent.
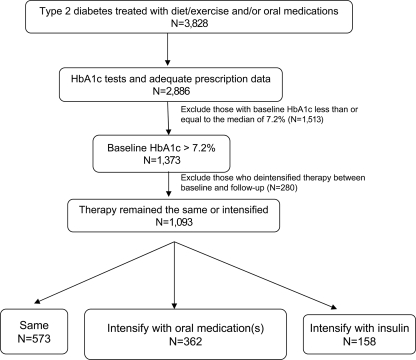
In 2000–2001, a baseline survey was administered (response rate 69%), and in 2002–2003, a follow-up survey was administered (response rate 80%), either by computer-assisted telephone interview or in writing by mail. Medical records and health plan administrative data for each participant were reviewed at each time point. We studied patients who had survey and medical record data at both baseline and follow-up, had type 2 diabetes (excluding those with age at diagnosis <30 years and treated with insulin only), and were using diet/exercise alone or in combination with oral antihyperglycemic medications at baseline (n = 3,828). We further limited the population to patients who had two A1C values reported in their medical records >180 days apart and had pharmacy usage as evidenced by at least one claim for a prescription in the administrative data at baseline and one at follow-up (n = 2,886). Characteristics of our study population were similar to those of the entire TRIAD population (data not shown). For the purpose of these analyses, we considered the threshold for optimal control to be the population median A1C of 7.2%. Anyone with A1C above this threshold was considered to have “suboptimally controlled” diabetes. We excluded 1,513 individuals who had A1C ≤7.2% at baseline (n = 1,373) (Fig. 1). Age, sex, race/ethnicity, educational level, income, duration of diabetes, BMI, Charlson index, and provider type were ascertained from the patient survey and had <15% missing data. We imputed missing values for these variables using the transcan function in S-PLUS (edition 6.1, Insightful, Seattle, WA). A sensitivity analysis that excluded patients with missing data yielded results similar to those presented (data not shown).
Figure 1.
Study population.
We used health plan administrative data to identify what type(s) of antihyperglycemic medication(s), if any, the patient possessed on the date of baseline and follow-up A1C testing. We classified a person as taking insulin if he or she filled a prescription for insulin both 0–3 months before the test date and 3–6 months before the test date. We created variables at baseline and follow-up to indicate the type of antihyperglycemic therapy the patient was using: diet/exercise only; one, two, three, or four classes of oral antihyperglycemic medications (secretogogues, biguanides, thiazolidinediones, or α-glucosidase inhibitors); or insulin. We then created a variable with three categories (deintensify, stay the same, and intensify) representing the change in therapy from baseline to follow-up. Deintensify was defined as decreasing the number of classes of oral antihyperglycemic medications and/or stopping insulin between baseline and follow-up (n = 280). Stay the same was defined as remaining with diet/exercise therapy only, the same number of classes of oral antihyperglycemic medications, or insulin at baseline and follow-up (n = 573). Intensify was defined as initiating or increasing the number of classes of oral antihyperglycemic medications or beginning insulin (n = 520). Individuals who deintensified therapy from baseline to follow-up were excluded from this analysis (Fig. 1).
We conducted bivariate analyses to compare patient characteristics among those who intensified therapy and those who stayed with the same therapy using t tests for continuous variables and χ2 tests for categorical variables. We also compared those who intensified with oral medication(s) and those who intensified with insulin. We used multivariable logistic regression analyses, which accounted for clustering of patients at the health plan/provider group level to examine factors associated with intensification of therapy. We included the followingvariables measured at baseline: age, sex, race/ethnicity, income, education, A1C, Charlson index, management of patient's diabetes by an endocrinologist, and provider communication. We also included the number of visits to the primary care physician (PCP), whether the patient reported being hospitalized, the development of complications (self-reported retinopathy, nephropathy, neuropathy, and cardiovascular disease), and the occurrence of symptoms of hyperglycemia (dry mouth, excessive thirst, and nocturia) between baseline and follow-up.
The outcomes of intensification of therapy were measured with the follow-up survey and medical record review. A1C was obtained from medical records; weight, anxiety/depression, and health status were obtained from self-report; and hypoglycemia was obtained from administrative claims data. Anxiety/depression and health status were measured with the EQ-5D, a generic measure of health status with three levels representing no problems, some problems, and extreme problems. For anxiety/depression, we created a dichotomous variable to indicate whether symptoms 1) stayed at none or some or got better or 2) got worse or remained extreme. We also recategorized health status into a dichotomous variable to indicate whether the follow-up measurement was better or the same as (greater than or equal to) the baseline measurement or whether the follow-up measurement was worse than (less than) the baseline measurement. Hypoglycemia was defined by the presence of at least one inpatient or outpatient claim in the administrative data (D-9-CM codes 250.8 [diabetes with other specified manifestations], 251.0 [hypoglycemic coma], 251.1 [other specified hypoglycemia], and 251.2 [hypoglycemia, unspecified]). We used t tests to compare values for continuous outcomes (A1C and weight) and χ2 tests to compare categorical outcomes (anxiety/depression, health status, and hypoglycemia).
We performed multivariable regression analyses for the population thatintensified therapy with either oral medications or insulin to examine patient characteristics associated with improvement in A1C. The outcome for this regression model was the difference in A1C calculated as the follow-up A1C minus the baseline A1C. A negative regression coefficient represents a decrease or improvement in A1C over time and a positive regression coefficient indicates an increase or worsening in A1C over time. The multivariate model included the baseline measurement of A1C, age, sex, race/ethnicity, education, income, BMI, Charlson index, and management of the patient's diabetes by an endocrinologist. We also adjusted for the number of visits to the PCP from baseline to follow-up, whether the patient was hospitalized between baseline and follow-up, new onset of complications from baseline to follow-up, and health plan/provider group clustering. All analyses were performed with SAS (version 9.1.3 SP 4; SAS Institute, Cary, NC).
RESULTS
For the 1,093 patients with type 2 diabetes and A1C >7.2%, the mean baseline A1C was 8.9% and the range was 7.3–15.6%. During the 18-month follow-up period, 520 of the1,093 (48%) patients with suboptimally controlled type 2 diabetes intensified antihyperglycemic therapy by adding either new oral antihyperglycemic medications or insulin. The mean number of new oral antihyperglycemic medication classes added was 1.2 (range 1–3). Patients intensifying therapy were on average 58 ± 12 years of age, with diabetes duration of 11 ± 9 years and baseline A1C of 9.1 ± 1.5% (Table 1). Those who intensified therapy were younger and heavier, had higher A1C, had more visits to their PCP, and were more likely to have developed nephropathy between baseline and follow-up (Table 1). In adjusted analyses, only younger age (odds ratio [OR] 0.98 [95% CI 0.97–1.00]) and higher baseline A1C (1.21 [1.09–1.34]) predicted therapy intensification.
Table 1.
Characteristics of patients with type 2 diabetes treated with diet/exercise or oral medications at baseline and with A1C ≥7.2: TRIAD 2000–2002
| Total population | Stayed the same | Intensified therapy | P | Intensified with oral medication(s) | Intensified with insulin | P | |
|---|---|---|---|---|---|---|---|
| n | 1,093 | 573 | 520 | 362 | 158 | ||
| Age | 59 ± 12 | 61 ± 12 | 58 ± 12 | <0.0001 | 58 ± 12 | 57 ± 12 | 0.374 |
| Male sex | 556 (51) | 298 (52) | 258 (50) | 0.430 | 191 (53) | 67 (42) | 0.030 |
| Race/ethnicity | 0.665 | 0.584 | |||||
| Hispanic | 184 (17) | 102 (18) | 82 (16) | 57 (16) | 25 (16) | ||
| Black | 116 (11) | 65 (11) | 51 (10) | 32 (9) | 19 (12) | ||
| White | 367 (34) | 186 (32) | 181 (35) | 124 (34) | 57 (36) | ||
| Asian/Pacific Islander | 305 (28) | 161 (28) | 144 (28) | 107 (30) | 37 (23) | ||
| Other | 121 (11) | 59 (10) | 62 (12) | 42 (12) | 20 (13) | ||
| Income | 0.365 | 0.183 | |||||
| <15,000 USD | 212 (19) | 113 (20) | 99 (19) | 61 (17) | 38 (24) | ||
| 15,000–40,000 USD | 368 (34) | 205 (36) | 163 (31) | 112 (31) | 51 (32) | ||
| 40,000–75,000 USD | 309 (28) | 154 (27) | 155 (30) | 112 (31) | 43 (27) | ||
| >$75,000 | 204 (19) | 101 (18) | 103 (20) | 77 (21) | 26 (16) | ||
| Education | 0.910 | 0.129 | |||||
| Some high school or less | 180 (16) | 95 (17) | 85 (16) | 57 (16) | 28 (18) | ||
| High school graduate | 308 (28) | 157 (27) | 151 (29) | 98 (27) | 53 (34) | ||
| Some college | 376 (34) | 202 (35) | 174 (33) | 121 (33) | 53 (34) | ||
| ≥4 year college | 229 (21) | 119 (21) | 110 (21) | 86 (24) | 24 (15) | ||
| Diabetes duration (years) | 11 ± 10 | 11 ± 10 | 11 ± 9 | 0.787 | 9.7 ± 9.2 | 12.6 ± 9.6 | 0.001 |
| Baseline A1C | 8.9 ± 1.5 | 8.7 ± 1.4 | 9.1 ± 1.5 | <0.0001 | 9.0 ± 1.53 | 9.5 ± 1.5 | 0.002 |
| Charlson index | 2.1 ± 1.5 | 2.2 ± 1.4 | 2.2 ± 1.6 | 0.920 | 2.04 ± 1.66 | 2.43 ± 1.54 | 0.011 |
| Baseline weight (pounds) | 192 ± 49 | 188 ± 48 | 196 ± 49 | 0.008 | 193 ± 47 | 201 ± 54 | 0.106 |
| Baseline BMI (kg/m2) | 31.0 ± 7.1 | 30.5 ± 7.0 | 31.4 ± 7.2 | 0.037 | 30.9 ± 7.0 | 32.5 ± 7.5 | 0.021 |
| Baseline EQ-5D | 0.83 ± 0.17 | 0.84 ± 0.15 | 0.82 ± 0.18 | 0.030 | 0.83 ± 0.17 | 0.78 ± 21 | 0.019 |
| Baseline anxiety/depression | 0.030 | 0.082 | |||||
| None | 725 (72) | 394 (75) | 331 (69) | 239 (72) | 92 (63) | ||
| Moderate | 250 (25) | 122 (23) | 128 (27) | 83 (25) | 45 (31) | ||
| Extreme | 28 (3) | 9 (2) | 19 (4) | 10 (3) | 9 (6) | ||
| Smoking | 181 (17) | 85 (15) | 96 (18) | 0.107 | |||
| Diabetes care provided by an endocrinologist | 79 (7) | 40 (7) | 39 (8) | 0.741 | 24 (7) | 15 (10) | 0.254 |
| Provider communication | 0.747 | 0.651 | |||||
| 4–7 (poor) | 77 (8) | 37 (8) | 40 (9) | 25 (8) | 15 (11) | ||
| 8–11 | 354 (38) | 187 (39) | 167 (37) | 117 (38) | 50 (35) | ||
| 12 (good) | 503 (54) | 260 (54) | 243 (54) | 167 (54) | 76 (54) | ||
| Number of PCP visits | 5.5 ± 3.6 | 5.2 ± 3.5 | 5.8 ± 3.7 | 0.018 | 5.8 ± 3.5 | 7.2 ± 4.1 | 0.002 |
| Hospitalized | 201 (18) | 102 (18) | 99 (19) | 0.598 | 55 (15) | 44 (28) | 0.001 |
| New onset cardiovascular disease | 66 (6) | 33 (6) | 33 (6) | 0.684 | 18 (5) | 15 (10) | 0.152 |
| New onset retinopathy | 124 (11) | 64 (11) | 60 (12) | 0.848 | 35 (10) | 25 (16) | 0.043 |
| New onset nephropathy | 91 (8) | 38 (7) | 53 (10) | 0.033 | 27 (7) | 26 (16) | 0.002 |
| New onset neuropathy | 112 (10) | 61 (11) | 51 (10) | 0.648 | 36 (10) | 15 (10) | 0.874 |
| New onset of any complication | 332 (30) | 172 (30) | 160 (31) | 0.787 | 94 (26) | 66 (42) | 0.0003 |
| Dry mouth | 309 (30) | 163 (30) | 146 (29) | 0.757 | 97 (28) | 49 (31) | 0.452 |
| Excessive thirst | 294 (29) | 157 (29) | 137 (28) | 0.668 | 98 (29) | 39 (26) | 0.524 |
| Nocturia | 513 (48) | 279 (50) | 234 (46) | 0.220 | 166 (47) | 68 (43) | 0.406 |
Data are means ± SD or n(%). n = 1,093. PCP, primary care physician.
In subanalyses comparing characteristics of individuals who intensified therapy with insulin versus oral antihyperglycemic medication(s), we found that those who intensified therapy with insulin were more likely to be women, to be heavier, to have longer duration of diabetes, and to have higher A1C (Table 1). They were also more likely to be “sicker” as evidenced by a higher Charlson index, more visits to the PCP, more hospitalizations, and more frequent development of complications between baseline and follow-up (Table 1). In adjusted analyses, only higher baseline A1C (OR 1.26 [95% CI 1.01–1.58]), higher Charlson index (1.17 [1.03–1.32]), and greater number of follow-up visits to the PCP (1.08 [1.01–1.16]) predicted intensification of therapy with insulin.
In unadjusted analyses, those who intensified therapy had a 0.48% reduction in A1C, a 3-pound weight gain, and no significant change in anxiety/depression or health status compared with those who remained with the same therapy (Table 2). Of those who intensified therapy, 4% had at least one hypoglycemic event compared with 3% of those who remained with the same therapy, and 21% achieved A1C <7% compared with 17% of those who remained with the same therapy (Table 2).
Table 2.
Unadjusted outcomes associated with intensification of therapy for patients with type 2 diabetes treated with diet/exercise or oral medications at baseline and with A1C ≥7.2: TRIAD 2000–2002
| Change from 2000 to 2002 |
||||
|---|---|---|---|---|
| Stay the same | Intensify with oral medication or insulin | Intensify with oral medication(s) | Intensify with insulin | |
| A1C (%) | −0.36 | −0.84* | −0.73 | −1.10† |
| Weight (pounds) | −3.20 | 0.06* | −0.47 | 1.22 |
| Increase in anxiety/depression | 9 | 8 | 8 | 7 |
| Decrease in health status | 33 | 36 | 35 | 39 |
| Met goal A1C of ≤7% | 17 | 21 | 22 | 19 |
| At least one hypoglycemic event | 3 | 3 | 2 | 6† |
Data are percent unless otherwise indicated.n = 1,093.
*P < 0.01 vs. stay the same.
†P < 0.05 vs. intensify with oral medication.
In subanalyses of those who intensified therapy with insulin versus oral antihyperglycemic medication(s), we found that those who intensified therapy with insulin had a 0.37% greater reduction in A1C and no significant change in weight, anxiety/depression, or health status (Table 2). Of those intensifying therapy with insulin, 6% had at least one hypoglycemic event compared with 2% of those who intensified therapy with oral medications. Of those who intensified therapy with insulin, 19% achieved A1C <7% compared with 22% of those who intensified therapy with oral medications (Table 2).
In adjusted analyses of those who intensified therapy with either oral antihyperglycemic medications or insulin, black race/ethnicity versus white, lower income (<$15,000 vs. ≥$75,000), higher baseline A1C, and greater number of follow-up visits to the PCP were associated with a decrease in A1C from baseline to follow-up (Table 3). Younger age (<50 vs. ≥70 years and 50–70 vs. ≥70 years) and receiving diabetes care from an endocrinologist were associated with an increase in A1C from baseline to follow-up (Table 3).
Table 3.
Characteristics associated with a change in A1C from baseline to follow-up in patients with type 2 diabetes treated with diet/exercise or oral medications at baseline and with A1C ≥7.2: TRIAD 2000–2002
| Estimate | P | |
|---|---|---|
| Age-group (referent = ≥70 years) | ||
| <50 years | 0.73 | 0.004 |
| 50–70 years | 0.31 | 0.021 |
| Sex (referent = male) | −0.02 | 0.878 |
| Race/ethnicity (referent = white) | ||
| Hispanic | 0.23 | 0.313 |
| Black | −0.48 | 0.031 |
| Asian/Pacific Islander | −0.16 | 0.247 |
| Other | 0.30 | 0.109 |
| Income (referent = >75,000 USD) | ||
| <15,000 USD | −0.65 | 0.005 |
| 15,000–40,000 USD | 0.26 | 0.103 |
| 40,000–75,000 USD | 0.05 | 0.799 |
| Education (referent = ≥4 year college) | ||
| Some high school or less | 0.27 | 0.347 |
| High school graduate | −0.1 | 0.543 |
| Some college | −0.16 | 0.396 |
| Baseline A1C | −0.58 | <0.0001 |
| Intensification with insulin (referent = oral medication) | 0.03 | 0.820 |
| BMI (kg/m2) | −0.011 | 0.345 |
| Charlson index | 0.04 | 0.205 |
| Diabetes care provided by an endocrinologist | 0.44 | 0.011 |
| Number of PCP visits | −0.05 | 0.004 |
| Hospitalized | −0.26 | 0.055 |
| New onset of any complication | 0.18 | 0.185 |
n = 520. Results are adjusted for all variables presented in the table.
CONCLUSIONS
We examined the characteristics of managed care patients with suboptimally controlled type 2 diabetes who intensified antihyperglycemic therapy over an 18-month period and the associated changes in glycemic control, weight, anxiety/depression, quality of life, and hypoglycemia. We found that 48% of patients using diet/exercise therapy alone or oral antihyperglycemic medications and with baseline A1C >7.2% intensified therapy with either oral medications or insulin: 70% added one or more classes of oral antihyperglycemic medications and 30% began insulin. Patients who intensified therapy with oral antihyperglycemic medications or insulin were younger and had higher baseline A1C than those who remained with the same therapy.
Compared with those who intensified therapy with oral antihyperglycemic medications, those who intensified therapy with insulin had higher baseline A1C, higher Charlson index, and a greater number of follow-up visits to the PCP. Compared with patients who remained with the same therapy, those who intensified therapy experienced a reduction in A1C, a slight increase in weight, no significant change in anxiety/depression or health status, and no significant increase in hypoglycemia. Despite initiating oral medications or insulin, only about one-fifth of patients achieved an A1C <7%. Karter et al. (9) studied patients with A1C >8% who initiated new diabetes therapies and found that 18% achieved an A1C <7% in one of the populations (Kaiser Permanente) included in the present study. Our findings are consistent with a subsequent study by Karter et al. (10) in the same population that reported a reduction in A1C with treatment intensification. In that study, A1C reduction was similar across classes of antihyperglycemic medications (sulfonylurea, metformin, thiazolidinedione, or insulin), and treatment effects did not differ by age, race, diabetes duration, obesity, or level of renal function.
We also examined the characteristics of patients whose A1C levels improved after intensification of antihyperglycemic therapy. We found that among those who intensified therapy, improvement in A1C was associated with higher baseline A1C, older age, black race/ethnicity, lower income, and greater number of visits to the PCP. Rodondi et al. (7) showed that in a routine clinical setting, minorities are less likely to get appropriate intensification of therapy. Recently, Schmittdiel et al. (11) demonstrated that clinical inertia is as important a barrier to improved glycemic control as is medication nonadherence. The UK Prospective Diabetes Study (UKPDS), in the context of a clinical trial, demonstrated that Afro-Caribbean patients and Asians of Indian origin were as likely as Caucasians to achieve good A1C control (12). The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) (13) and Action to Control Cardiovascular Risk in Diabetes(ACCORD) (14) studies have similarly demonstrated that older individuals (aged >65 years) and minorities are as likely to achieve glycemic goals as younger individuals and white individuals and that with improvement in A1C there is equal improvement in outcomes (13,14). Our study suggests that if treatment is intensified, outcomes are better, especially in elderly, minority, and lower income groups. This finding highlights the need for providers to overcome clinical inertia in routine clinical practice.
In light of the ACCORD study, which randomly assigned patients to an A1C goal of 6.0 or 7.0–7.9% and found harm associated with intensive glucose lowering (14), glycemic goals need to be reassessed. The UKPDS supports an A1C goal of <7.0% for most patients with type 2 diabetes, although this goal may be inappropriate for individuals with multiple comorbidities or poor functional status (15). The American Geriatric Society recommends that glycemic goals be established on the basis of patient preferences, life expectancy, and comorbidities and that the decision to intensify therapy needs to be modified by the patients' expectations (16).
There were a number of limitations to our study. First, we assessed antihyperglycemic treatment using only pharmacy claims and utilization data. Because patients without pharmacy benefits lack financial incentives to use health plan pharmacies, some utilization data may not be captured. We minimized this potential bias by excluding from the analyses anyone with no pharmacy claims or utilization at either baseline or follow-up. Second, we defined intensification of therapy on the basis of adding classes of medications and not by increasing the dose of the same oral antidiabetic medication. As a result, the observed differences between subjects with intensified versus nonintensified therapy are likely to be conservative. Third, we did not assess medication adherence, which may have resulted in a conservative bias if patients filled prescriptions for new therapies but failed to take the medications. Moreover, a portion of patients may have been prescribed new therapies but never picked them up from the pharmacy (i.e., primary nonadherence). Reliance on pharmacy utilization data and claims precludes identifying this less common type of nonadherence (∼5% of new prescriptions), a patient factor that is typically misclassified as clinical inertia. Fourth, we were unable to evaluate baseline status and synchronize the time of initiation of new therapies (i.e., new user cohort design) (17). Although our design had a less stringent data collection schedule, our findings should simulate observations made in usual care, e.g., during primary care visits. Fifth, many of our analyses were descriptive and not adjusted for potential confounders. Finally, our patients were all enrolled in managed care health plans. Consequently, the results may not be generalizable to uninsured patients with diabetes or those in other systems of care.
Failure to appropriately intensify therapy, or clinical inertia, remains an important barrier to optimal glycemic control. Although it is important to individualize treatment, our study clearly indicates that intensifying therapy lowers A1C without substantial weight gain, worsening anxiety/depression or health status, or hypoglycemia. Intensification is especially effective in improving glycemic control in elderly, lower income, and minority patients, groups who may be less likely to have therapy intensified and who experience a disproportionate burden of diabetes and its complications (18–21). More research is needed to define and compare the glucose-lowering and side effect profiles of individual therapeutic medications, and careful consideration is needed regarding the risks of intensification in elderly patients. Interventions should be implemented to overcome clinical inertia when patients might benefit from treatment intensification and improved glycemic control.
Acknowledgments
This study was jointly funded by Program Announcement 04005 from the Centers for Disease Control andPrevention (Division of Diabetes Translation) and the National Institute of Diabetes andDigestive and Kidney Diseases. sanofi-aventis supported these analyses through a Research Services Contract to the University of Michigan.
L.N.M., D.B., J.B.H., and W.H.H. received research grant support for this study from sanofi-aventis. No other potential conflicts of interest relevant to this article were reported.
Members of the Translating Research into Action for Diabetes (TRIAD) Study Group contributed to this study. The authors acknowledge the participation of our health plan partners.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding organizations.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, Levy JC: Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007; 357: 1716– 1730 [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Zinman B, Viberti G: Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427– 2443 [DOI] [PubMed] [Google Scholar]
- 3.Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, Miller CD, Ziemer DC, Barnes CS: Clinical inertia. Ann Intern Med 2001; 135: 825– 834 [DOI] [PubMed] [Google Scholar]
- 4.Sclar DA, Robison LM, Skaer TL, Dickson WM, Kozma CM, Reeder CE: Sulfonylurea pharmacotherapy regimen adherence in a medicaid population: influence of age, gender, and race. Diabetes Educ 1999; 25: 531– 538 [DOI] [PubMed] [Google Scholar]
- 5.Osterberg L, Blaschke T: Adherence to medication. N Engl J Med 2005; 353: 487– 497 [DOI] [PubMed] [Google Scholar]
- 6.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ: Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006; 166: 1836– 1841 [DOI] [PubMed] [Google Scholar]
- 7.Rodondi N, Peng T, Karter AJ, Bauer DC, Vittinghoff E, Tang S, Pettitt D, Kerr EA, Selby JV: Therapy modifications in response to poorly controlled hypertension, dyslipidemia, and diabetes mellitus. Ann Intern Med 2006; 144: 475– 484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Translating Research into Action for Diabetes (TRIAD) study: a multicenter study of diabetes in managed care. Diabetes Care 2002; 25: 386– 389 [DOI] [PubMed] [Google Scholar]
- 9.Karter AJ, Moffet HH, Liu J, Parker MM, Ahmed AT, Ferrara A, Selby JV: Achieving good glycemic control: initiation of new antihyperglycemic therapies in patients with type 2 diabetes from the Kaiser Permanente Northern California Diabetes Registry. Am J Manag Care 2005; 11: 262– 270 [PMC free article] [PubMed] [Google Scholar]
- 10.Karter AJ, Moffet HH, Liu J, Parker MM, Ahmed AT, Go AS, Selby JV: Glycemic response to newly initiated diabetes therapies. Am J Manag Care 2007; 13: 598– 606 [PMC free article] [PubMed] [Google Scholar]
- 11.Schmittdiel JA, Uratsu CS, Karter AJ, Heisler M, Subramanian U, Mangione CM, Selby JV: Why don't diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med 2008; 23: 588– 594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis TM, Cull CA, Holman RR: Relationship between ethnicity and glycemic control, lipid profiles, and blood pressure during the first 9 years of type 2 diabetes: UK Prospective Diabetes Study (UKPDS 55). Diabetes Care 2001; 24: 1167– 1174 [DOI] [PubMed] [Google Scholar]
- 13.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560– 2572 [DOI] [PubMed] [Google Scholar]
- 14.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545– 2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B: Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2006; 29: 1963– 1972 [DOI] [PubMed] [Google Scholar]
- 16.Brown AF, Mangione CM, Saliba D, Sarkisian CA: Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51: S265– S280 [DOI] [PubMed] [Google Scholar]
- 17.Ray WA: Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003; 158: 915– 920 [DOI] [PubMed] [Google Scholar]
- 18.Brown AF, Gregg EW, Stevens MR, Karter AJ, Weinberger M, Safford MM, Gary TL, Caputo DA, Waitzfelder B, Kim C, Beckles GL: Race, ethnicity, socioeconomic position, and quality of care for adults with diabetes enrolled in managed care: the Translating Research into Action for Diabetes (TRIAD) study. Diabetes Care 2005; 28: 2864– 2870 [DOI] [PubMed] [Google Scholar]
- 19.McEwen LN, Kim C, Karter AJ, Haan MN, Ghosh D, Lantz PM, Mangione CM, Thompson TJ, Herman WH: Risk factors for mortality among patients with diabetes: the Translating Research into Action for Diabetes (TRIAD) Study. Diabetes Care 2007; 30: 1736– 1741 [DOI] [PubMed] [Google Scholar]
- 20.Selby JV, Swain BE, Gerzoff RB, Karter AJ, Waitzfelder BE, Brown AF, Ackermann RT, Duru OK, Ferrara A, Herman W, Marrero DG, Caputo D, Narayan KM: Understanding the gap between good processes of diabetes care and poor intermediate outcomes: Translating Research into Action for Diabetes (TRIAD). Med Care 2007; 45: 1144– 1153 [DOI] [PubMed] [Google Scholar]
- 21.Tseng CW, Tierney EF, Gerzoff RB, Dudley RA, Waitzfelder B, Ackermann RT, Karter AJ, Piette J, Crosson JC, Ngo-Metzger Q, Chung R, Mangione CM: Race/ethnicity and economic differences in cost-related medication underuse among insured adults with diabetes: the Translating Research into Action for Diabetes Study. Diabetes Care 2008; 31: 261– 266 [DOI] [PubMed] [Google Scholar]