Abstract
OBJECTIVE
Moderate alcohol consumption is associated with reduced incidence of type 2 diabetes and cardiovascular mortality and increases adiponectin concentrations, but effects might differ according to sex and beverage consumed.
RESEARCH DESIGN AND METHODS
A total of 72 healthy individuals (22–56 years) were enrolled in this randomized controlled crossover trial. After washout, two interventions for 3 weeks followed: ethanol (concentration 12.5%), beer (5.6%), or red wine (12.5%) equivalent to 30 g ethanol/day for men and 20 g/day for women or the same de-alcoholized beverages or water. Adiponectin was measured by sandwich enzyme-linked immunosorbent assay.
RESULTS
Among women, adiponectin significantly increased after consuming red wine (29.8%, P < 0.05) and increased among men after ethanol solution (17.4%, P < 0.05) and consuming beer (16.1%, P < 0.05). De-alcoholized beverages had no substantial effect on adiponectin concentrations.
CONCLUSIONS
Moderate amounts of ethanol-containing beverages increased adiponectin concentrations, but sex-specific effects might depend on type of beverage consumed.
Moderate alcohol intake is associated with lower risk for type 2 diabetes and fatal and nonfatal cardiovascular disease (CVD) (1,2). It has been suggested that alcohol in moderate doses—in addition to favorable changes of blood lipids, the hemostatic profile, and insulin resistance—exhibits anti-inflammatory mechanisms, thus potentially modulating atherosclerosis development (3). Adiponectin mightrepresent an important link between insulin resistance, type 2 diabetes, and atherosclerosis. Adiponectin improves insulin sensitivity and has several anti-inflammatory properties (4), and high concentrations of adiponectin were associated with lower risk of type 2 diabetes (5,6) and future cardiovascular events (7). Moderate alcohol consumption is associated with increased adiponectin concentrations in healthy individuals, in obese males, and in women with impaired glucose tolerance and type 2 diabetes (8–11). Recently, adiponectin was proposed to be the most important mediator between moderate alcohol consumption and lower incidence of type 2 diabetes among middle-aged women (12). However, neither of these studies assessed potential varying effects of different types of alcoholic beverages in a randomized setting in men and women; thus, they might be prone to selection bias because of personal preferences of study participants. Moreover, there is still controversial debate about potential favorable effects of nonalcoholic ingredients such as polyphenols.
We investigated the effect of short-term moderate consumption of either low-concentrated ethanol solution, red wine, and beer with or without alcohol on adiponectin in a randomized controlled crossover intervention trial.
RESEARCH DESIGN AND METHODS
A total of 72 nonsmoking healthy Caucasian men and women of German nationality, aged 22–56 years, were recruited. They were moderate alcohol consumers and had a family history free of alcohol dependencies. Liver disease was excluded measuring liver enzymes. All participants gave written informed consent to all procedures. The study was approved by the local ethics committee.
After a washout period of at least 2 weeks, participants were randomly allocated, stratified by age and sex, to the following interventions over 3 weeks: beer (5.6%), red wine (12.5%), or ethanol (concentration 12.5%), equivalent to 30 g ethanol/day for men and 20 g/day for women or the same amount of de-alcoholized beer or de-alcoholized red wine (same brand) or pure water (control group). After the second washout period of 3 weeks, a further intervention with the corresponding beverage followed (beer/de-alcoholized beer, wine/de-alcoholized wine, ethanol/water, and vice versa). The rationale for this study design was avoidance of selection bias caused by preferences in drinking behavior. All participants were asked not to change their dietary habits and habitual physical activity during the study period.
A baseline history of alcohol consumption, dietary habits, medical history, and sociodemographic parameters was obtained by standardized interview. At each visit, symptoms of concurrent inflammatory processes/infections including fever, cough, or antibiotic therapy were carefully assessed and, if present, the participant or the respective intervention period was excluded from analyses. Fasting blood was collected from the antecubital vein in a sitting position with minimal suction and short-term occlusion. Plasma and serum was stored within 90 min at −80°C until analysis. Laboratory analyses were blinded. Plasma adiponectin concentrations were measured before and after intervention by sandwich enzyme-linked immunosorbent assay (ELISA) (Quantikine; R&D Systems, Wiesbaden, Germany).
Statistical analysis
Each intervention group contained 12 males and 12 females undergoing two interventions in random sequence. Datasets of 16 intervention periods were removed before analyses because of concurrent infections or dropout, leaving 128 datasets for analyses. Numbers of excluded datasets were similar in all groups and did not substantially differ between males and females. ANOVA with mixed linear models was used to assess the effect of interventions on adiponectin (PROC MIXED in SAS; SAS Institute, Cary, NC). All tests performed were two-sided, and a P value <0.05 was considered statistically significant using SAS software, release 8.2 (SAS Institute).
RESULTS
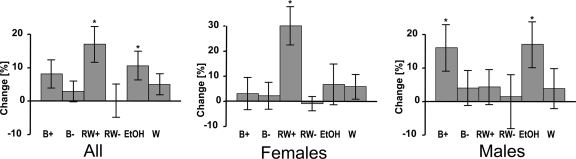
Compliance was excellent according to self-report and counting of empty bottles returned. One participant was excluded because of protocol violation. Baseline characteristics including age, BMI, and liver enzymes did not differ within sexes and between intervention groups. Adiponectin concentrations were substantially higher among females (means 7.6–8.8 μg/ml) than males (means 4.8–6.3 μg/ml) but also did not differ between the intervention groups within each sex. Among females, adiponectin significantly increased after intervention with red wine (29.8%, P < 0.05) and increased among men after ethanol solution (17.4%, P < 0.05) and beer consumption (16.1%, P < 0.04). De-alcoholized beverages had no substantial effect on adiponectin concentrations (Fig. 1).
Figure 1.
Mean percent change with standard deviation of adiponectin concentrations from baseline after intervention with beer (B+), de-alcoholized beer (B−), red wine (RW+), de-alcoholized red wine (RW−), enthanol solution (EtOH), and water (W) (*P < 0.05).
CONCLUSIONS
In this open randomized crossover intervention study, we found a substantial increase of plasma concentrations of adiponectin after consumption of moderate doses of alcoholic beverages for 3 weeks. Among women, this effect was statistically significant after intake of red wine and among men after intake of beer and ethanol solution.
This is the first study assessing effects of different alcohol-containing and corresponding de-alcoholized beverages in a randomized study in both sexes. We confirm and extend findings from other studies. Adiponectin exhibits a bundle of various favorable metabolic effects bolstering the hypothesis of its pivotal role in affecting risk of diabetes and CVD (4). We and others observed robust increasing effects of alcoholic beverages on adiponectin concentrations. This indicates that adiponectin might partly mediate beneficial effects of these beverages on the respective diseases (8–12). Most strikingly, effects differ with respect to sex and type of beverage. However, drinking preferences of participants might substantially affect our findings by incomplete adherence to the study protocol. Among females, 50% reported to drink preferably wine but only 11% preferred beer in daily life. Among males, first choice was beer for 33% and wine for 22%. All others denied strong preferences. Furthermore, experimental and clinical data suggest that sex hormones affect adiponectin concentrations, and among healthy males, effects of alcohol on sex hormones varied by drinking pattern and between beer and wine, indicating a potential explanation for our findings (13–15).
In summary, our results suggest that alcohol-containing beverages have robust increasing effects on adiponectin concentrations, but effects might differ between sexes, depending on type of beverage consumed.
Acknowledgments
This study was supported by a grant from the European Research Advisory Board (ERAB), Ligne, Belgium.
No potential conflicts of interest relevant to this article were reported.
We thank Sascha Wunderlich and Professor Back from the Institute of Brewery Technology (Technical University of Munich, Weihenstephan, Germany) for providing us with the beer and Euresis (Aachen, Germany) for providing us with the wine.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ: Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care 2005; 28: 719– 725 [DOI] [PubMed] [Google Scholar]
- 2.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G: Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006; 166: 2437– 2445 [DOI] [PubMed] [Google Scholar]
- 3.Imhof A, Koenig W: Alcohol inflammation and coronary heart disease. Addict Biol 2003; 8: 271– 277 [DOI] [PubMed] [Google Scholar]
- 4.Aldhahi W, Hamdy O: Adipokines, inflammation, and the endothelium in diabetes. Curr Diab Rep 2003; 3: 293– 298 [DOI] [PubMed] [Google Scholar]
- 5.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF: Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003; 361: 226– 228 [DOI] [PubMed] [Google Scholar]
- 6.Duncan BB, Schmidt MI: The epidemiology of low-grade chronic systemic inflammation and type 2 diabetes. Diabetes Technol Ther 2006; 8: 7– 17 [DOI] [PubMed] [Google Scholar]
- 7.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB: Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004; 291: 1730– 1737 [DOI] [PubMed] [Google Scholar]
- 8.Sierksma A, Patel H, Ouchi N, Kihara S, Funahashi T, Heine RJ, Grobbee DE, Kluft C, Hendriks HF: Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-alpha, and insulin sensitivity. Diabetes Care 2004; 27: 184– 189 [DOI] [PubMed] [Google Scholar]
- 9.Beulens JW, van Beers RM, Stolk RP, Schaafsma G, Hendriks HF: The effect of moderate alcohol consumption on fat distribution and adipocytokines. Obesity (Silver Spring) 2006; 14: 60– 66 [DOI] [PubMed] [Google Scholar]
- 10.Beulens JW, de Zoete EC, Kok FJ, Schaafsma G, Hendriks HF: Effect of moderate alcohol consumption on adipokines and insulin sensitivity in lean and overweight men: a diet intervention study. Eur J Clin Nutr 2007; 62: 1098– 105 [DOI] [PubMed] [Google Scholar]
- 11.Englund Ogge L, Brohall G, Behre CJ, Schmidt C, Fagerberg B: Alcohol consumption in relation to metabolic regulation, inflammation, and adiponectin in 64-year-old Caucasian women: a population-based study with a focus on impaired glucose regulation. Diabetes Care 2006; 29: 908– 913 [DOI] [PubMed] [Google Scholar]
- 12.Beulens JW, Rimm EB, Hu FB, Hendriks HF, Mukamal KJ: Alcohol consumption, mediating biomarkers and risk of type 2 diabetes among middle-aged women. Diabetes Care 2008; 31: 2050– 2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y: Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 2002; 51: 2734– 2741 [DOI] [PubMed] [Google Scholar]
- 14.Rinaldi S, Peeters PH, Bezemer ID, Dossus L, Biessy C, Sacerdote C, Berrino F, Panico S, Palli D, Tumino R, Khaw KT, Bingham S, Allen NE, Key T, Jensen MK, Overvad K, Olsen A, Tjonneland A, Amiano P, Ardanaz E, Agudo A, Martinez-Garcia C, Quiros JR, Tormo MJ, Nagel G, Linseisen J, Boeing H, Schulz M, Grobbee DE, Bueno-de-Mesquita HB, Koliva M, Kyriazi G, Thrichopoulou A, Boutron-Ruault MC, Clavel-Chapelon F, Ferrari P, Slimani N, Saracci R, Riboli E, Kaaks R: Relationship of alcohol intake and sex steroid concentrations in blood in pre- and post-menopausal women: the European Prospective Investigation into Cancer and Nutrition. Cancer Causes Control 2006; 17: 1033– 1043 [DOI] [PubMed] [Google Scholar]
- 15.Couwenbergs CJ: Acute effects of drinking beer or wine on the steroid hormones of healthy men. J Steroid Biochem 1988; 31: 467– 473 [DOI] [PubMed] [Google Scholar]